COVID-19 on Chest CT: Translating Known Microscopic Findings to Imaging Observations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. CT Protocol
2.3. Chest CT Evaluation
3. Results
3.1. Patient Characteristics and General Results
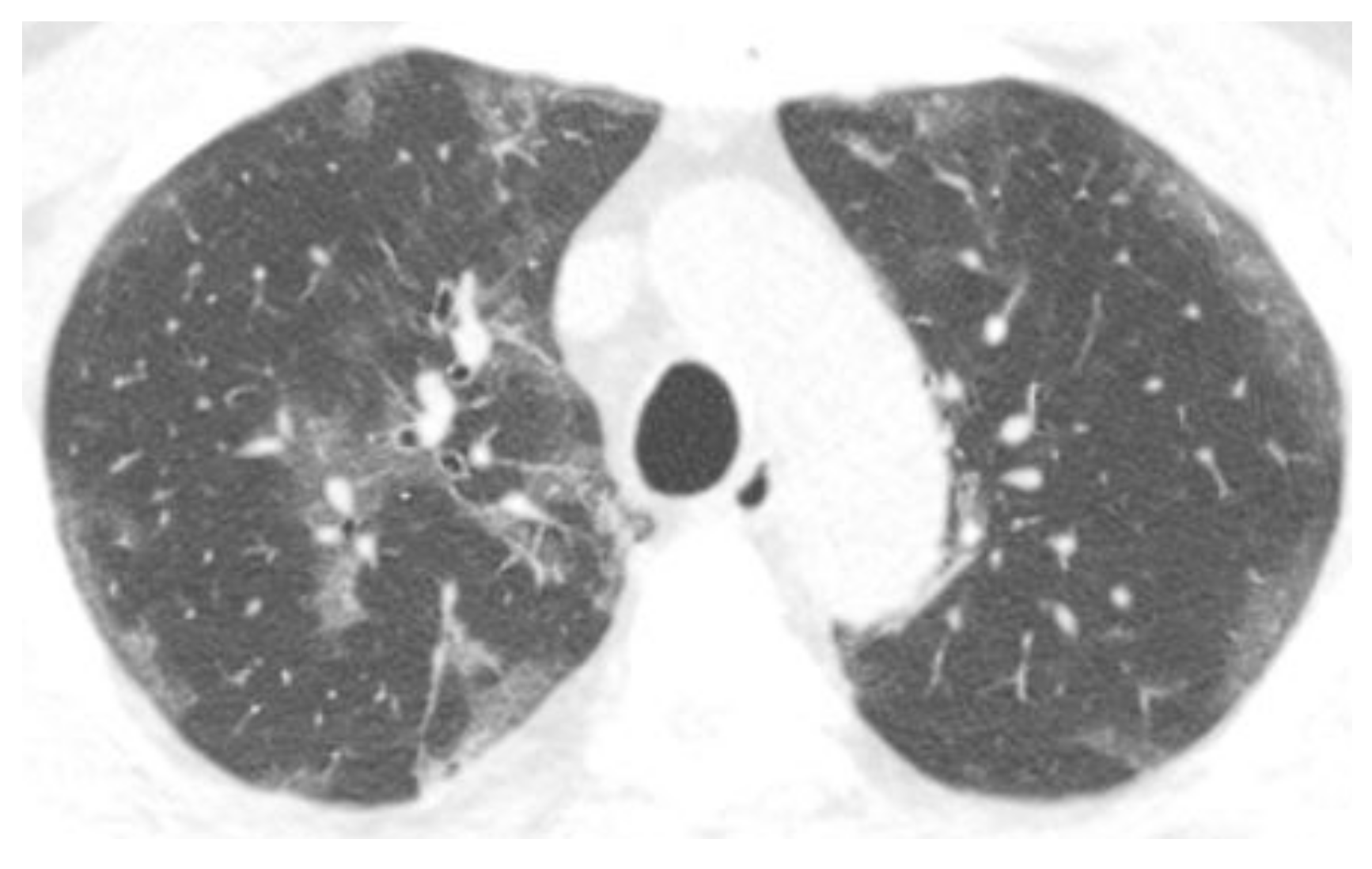
3.2. Chest CT Scans Performed in the Acute Phase (0–4 Days)
3.3. Chest CT Scans Were Performed in the Subacute Phase (5–12 Days)
3.4. Chest CT Scans Were Performed in the Persistent Phase (13–28 Days)
3.5. Chest CT Scans Performed in the Chronic Phase (>28 Days)
4. Discussion
4.1. Endothelial Dysfunction
4.2. Epithelial Dysfunction
4.3. Fibrosis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kwee, T.C.; Kwee, R.M. Chest CT in COVID-19: What the Radiologist Needs to Know. Radiographics 2020, 40, 1848–1865. [Google Scholar] [CrossRef]
- Helms, J.; Jacquard, C.; Severac, F.; Leonard-Lorant, I.; Ohana, M.; Delabranche, X.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Fagot Gandet, F.; et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020, 46, 1089–1098. [Google Scholar] [CrossRef]
- Poyiadji, N.; Cormier, P.; Patel, P.Y.; Hadied, M.O.; Bhargava, P.; Khanna, K.; Nadig, J.; Keimig, T.; Spizarny, D.; Reeser, N.; et al. Acute pulmonary embolism, and COVID-19. Radiology 2020, 297, E335–E338. [Google Scholar] [CrossRef]
- Bompard, F.; Monnier, H.; Saab, I.; Tordjman, M.; Abdoul, H.; Fournier, L.; Sanchez, O.; Lorut, C.; Chassagnon, G.; Revel, M.P. Pulmonary embolism in patients with COVID-19 pneumonia. Eur. Respir. J. 2020, 56, 2001365. [Google Scholar] [CrossRef]
- Grillet, F.; Behr, J.; Calame, P.; Aubry, S.; Delabrousse, E. Acute Pulmonary Embolism Associated with COVID-19 Pneumonia Detected with Pulmonary CT Angiography. Radiology 2020, 296, E186–E188. [Google Scholar] [CrossRef] [Green Version]
- Kaminetzky, M.; Moore, W.; Fansiwala, K.; Babb, J.S.; Kaminetzky, D.; Horwitz, L.I.; McGuinness, G.; Knoll, A.; Ko, J.P. Pulmonary embolism on CTPA in COVID-19 patients. Radiol. Cardiothorac. Imaging 2020, 2, e200308. [Google Scholar] [CrossRef] [PubMed]
- Capaccione, K.M.; Li, G.; Salvatore, M.M. Pulmonary embolism rate in patients infected with SARS-CoV-2. Blood Res. 2020, 55, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Al-Tawfiq, J.A.; Memish, Z.A. Diagnosis of SARS-CoV-2 infection based on CT scan vs RT-PCR: Reflecting on the experience fMERS-CoVSCoV. J. Hosp. Infect. 2020, 105, 154–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Michele, S.; Sun, Y.; Yilmaz, M.M.; Katsyv, I.; Salvatore, M.; Dzierba, A.L.; Marboe, C.C.; Brodie, D.; Patel, N.M.; Garcia, C.K.; et al. Forty Postmortem Examinations in COVID-19 Patients: Two Distinct Pathologic Phenotypes and Correlation with Clinical and Radiologic Findings. Am. J. Clin. Pathol. 2020, 154, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Elsoukkary, S.S.; Mostyka, M.; Dillard, A.; Berman, D.R.; Ma, L.X.; Chadburn, A.; Yantiss, R.K.; Jessurun, J.; Seshan, S.V.; Borczuk, A.C.; et al. Autopsy Findings in 32 Patients with COVID-19: A Single-Institution Experience. Pathobiology 2021, 88, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Hooper, J.E.; Padera, R.F.; Dolhnikoff, M.; da Silva, L.F.F.; Duarte-Neto, A.N.; Kapp, M.E.; Lacy, J.M.; Mauad, T.; Saldiva, P.H.N.; Rapkiewicz, A.V.; et al. A Postmortem Portrait of the Coronavirus Disease 2019 (COVID-19) Pandemic: A Large Multi-Institutional Autopsy Survey Study. Arch. Pathol. Lab Med. 2021, 145, 529–535. [Google Scholar] [CrossRef]
- Menter, T.; Haslbauer, J.D.; Nienhold, R.; Savic, S.; Hopfer, H.; Deigendesch, N.; Frank, S.; Turek, D.; Willi, N.; Pargger, H.; et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology 2020, 77, 198–209. [Google Scholar] [CrossRef]
- Schaller, T.; Hirschbühl, K.; Burkhardt, K.; Braun, G.; Trepel, M.; Märkl, B.; Claus, R. Postmortem Examination of Patients With COVID-19. JAMA 2020, 323, 2518–2520. [Google Scholar] [CrossRef]
- Doherty, S. Pulmonary embolism. An update. Aust. Fam. Physician 2017, 46, 816–820. [Google Scholar]
- Iba, T.; Connors, J.M.; Levy, J.H. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflamm. Res. 2020, 69, 1181–1189. [Google Scholar] [CrossRef]
- Salvatore, M.M. Pulmonary infarcts in COVID-19. Clin. Imaging 2021, 80, 158–159. [Google Scholar] [CrossRef]
- Mulay, A.; Konda, B.; Garcia, G., Jr.; Yao, C.; Beil, S.; Villalba, J.M.; Koziol, C.; Sen, C.; Purkayastha, A.; Kolls, J.K.; et al. SARS-CoV-2 infection of primary human lung epithelium for COVID-19 modeling and drug discovery. Cell Rep. 2021, 35, 109055. [Google Scholar] [CrossRef]
- Matthay, M.A.; Zimmerman, G.A. Acute lung injury and the acute respiratory distress syndrome: Four decades of inquiry into pathogenesis and rational management. Am. J. Respir. Cell Mol. Biol. 2005, 33, 319–327. [Google Scholar] [CrossRef]
- Battista, G.; Sassi, C.; Zompatori, M.; Palmarini, D.; Canini, R. Ground-glass opacity: Interpretation of high-resolution CT findings. Radiol. Med. 2003, 106, 425–442, quiz 443–444. [Google Scholar]
- Snijder, J.; Peraza, J.; Padilla, M.; Capaccione, K.; Salvatore, M.M. Pulmonary fibrosis: A disease of alveolar collapse and collagen deposition. Expert Rev. Respir. Med. 2019, 13, 615–619. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Mosleh, W.; Chen, K.; Pfau, S.E.; Vashist, A. Endotheliitis and Endothelial Dysfunction in Patients with COVID-19: Its Role in Thrombosis and Adverse Outcomes. J. Clin. Med. 2020, 9, 1862. [Google Scholar] [CrossRef]
- Moore, A.J.E.; Wachsmann, J.; Chamarthy, M.R.; Panjikaran, L.; Tanabe, Y.; Rajiah, P. Imaging of acute pulmonary embolism: An update. Cardiovasc. Diagn. Ther. 2018, 8, 225–243. [Google Scholar] [CrossRef]
- Price, L.C.; Ridge, C.; Wells, A.U. Pulmonary vascular involvement in COVID-19 pneumonitis: Is this the first and final insult? Respirology 2021, 26, 832–834. [Google Scholar] [CrossRef]
- Suh, Y.J.; Hong, H.; Ohana, M.; Bompard, F.; Revel, M.-P.; Valle, C.; Gervaise, A.; Poissy, J.; Susen, S.; Hékimian, G.; et al. Pulmonary Embolism and Deep Vein Thrombosis in COVID-19: A Systematic Review and Meta-Analysis. Radiology 2021, 298, E70–E80. [Google Scholar] [CrossRef]
- Tatco, V.; Piedad, H. The validity of hyperdense lumen sign in non-contrast chest CT scans in the detection of pulmonary thromboembolism. Int. J. Cardiovasc. Imaging 2011, 27, 433–440. [Google Scholar] [CrossRef]
- Hassan, H.G.E.M.A.; Khater, N.H.; Elia, R.Z. Added value of hyperdense lumen sign in the prediction of acute central and peripheral pulmonary embolism on non-contrast CT chest. Egypt J. Radiol. Nucl. Med. 2021, 52, 84. [Google Scholar] [CrossRef]
- Swensen, S.J.; McLeod, R.A.; Stephens, D.H. CT of extracranial hemorrhage and hematomas. AJR 1984, 143, 907–912. [Google Scholar] [CrossRef]
- Kanne, J.P.; Gotway, M.B.; Thoongsuwan, N.; Stern, E.J. Six cases of acute central pulmonary embolism revealed on unenhanced multidetector CT of the chest. AJR 2003, 180, 1661–1664. [Google Scholar] [CrossRef]
- Izadi, M.; Salehnia, N.; Rad, M.P.; Reihani, H.; Shafiee. Diagnostic Value of Pulmonary Artery Hypodense and Hyperdense Luminal Sign in Non-Contrast Thoracic CT scan for Detection of Pulmonary Embolism. J. Radiol. Clin. Imaging 2021, 4, 132–140. [Google Scholar] [CrossRef]
- Thompson, B.T.; Chambers, R.C.; Liu, K.D. Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2017, 377, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Desplechain, C.; Foliguet, B.; Barrat, E.; Grignon, G.; Touati, F. Les pores de Kohn des alvéoles pulmonaires [The pores of Kohn in pulmonary alveoli]. Bull. Eur. Physiopathol. Respir. 1983, 19, 59–68. [Google Scholar] [PubMed]
- Schaible, J.; Meiler, S.; Poschenrieder, F.; Scharf, G.; Zeman, F.; Knobloch, C.; Rennert, J.; Pregler, B.; Kleine, H.; Menzel, C.; et al. CT Features of COVID-19 Pneumonia Differ Depending on the Severity and Duration of Disease. Rofo 2021, 193, 672–682. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, W.; Wen, Y.; Zheng, Y.; Lv, F.; Xiao, K. COVID-19 pneumonia: CT findings of 122 patients and differentiation from influenza pneumonia. Eur. Radiol. 2020, 30, 5463–5469. [Google Scholar] [CrossRef]
- Wang, S.; Li, Z.; Wang, X.; Zhang, S.; Gao, P.; Shi, Z. The Role of Pulmonary Surfactants in the Treatment of Acute Respiratory Distress Syndrome in COVID-19. Front. Pharmacol. 2021, 12, 698905. [Google Scholar] [CrossRef]
- Aisa, T.; Hassan, T.; Khan, E.; Algrni, K.; Malik, M.A. Efficacy and feasibility of awake proning in patients with COVID-19-related acute hypoxemic respiratory failure: An observational, prospective study. Ir. J. Med. Sci. 2022, 14, 1–5. [Google Scholar] [CrossRef]
- McGroder, C.F.; Zhang, D.; Choudhury, M.A.; Salvatore, M.M.; D’Souza, B.M.; Hoffman, E.A.; Wei, Y.; Baldwin, M.R.; Garcia, C.K. Pulmonary fibrosis 4 months after COVID-19 is associated with severity of illness and blood leucocyte telomere length. Thorax. 2021, 76, 1242–1245. [Google Scholar] [CrossRef]
- Doran, A.C.; Yurdagul, A.; Tabas, I. Efferocytosis in health and disease. Nat. Rev. Immunol. 2020, 20, 254–267. [Google Scholar] [CrossRef]
- Dos-Santos, D.; Salina, A.C.G.; Rodrigues, T.S.; Fortes-Rocha, M.; Freitas-Filho, E.G.; Alzamora-Terrel, D.L.; de Lima, M.H.F.; Nascimento, D.C.; Castro, I.M.S.; Silva, C.M.; et al. Efferocytosis of SARS-CoV-2-infected dying cells impairs macrophage anti-inflammatory programming and continual clearance of apoptotic cells. medRxiv 2021. preprint. [Google Scholar] [CrossRef]
- Galindo, J.L.; Jiménez, L.F.; Lutz, J.R.; Izquierdo, M.A.; Rivillas, V.L.; Carrillo, J.A. Spontaneous pneumothorax, with or without pulmonary cysts, in patients with COVID-19 pneumonia. J. Infect. Dev. Ctries 2021, 15, 1404–1407. [Google Scholar] [CrossRef]
- Castiglioni, M.; Pelosi, G.; Meroni, A.; Tagliabue, M.; Uslenghi, E.; Salaris, D.; Incarbone, M. Surgical Resections of Superinfected Pneumatoceles in a COVID-19 Patient. Ann. Thorac. Surg. 2021, 111, e23–e25. [Google Scholar] [CrossRef]
- Gosangi, B.; Rubinowitz, A.N.; Irugu, D.; Gange, C.; Bader, A.; Cortopassi, I. COVID-19 ARDS: A review of imaging features and overview of mechanical ventilation and its complications. Emerg. Radiol. 2022, 29, 23–34. [Google Scholar] [CrossRef]
- Capaccione, K.M.; D’souza, B.; Leb, J.; Luk, L.; Duong, J.; Tsai, W.Y.; Navot, B.; Dumeer, S.; Mohammed, A.; Salvatore, M.M. Pneumothorax rate in intubated patients with COVID-19. Acute Crit. Care 2021, 36, 81–84. [Google Scholar] [CrossRef]




| Acute (0–4 Days) | Subacute (5–12 Days) | Persistent (13–28 Days) | Chronic (>28 Days) | Total (0–249 Days) | |
|---|---|---|---|---|---|
| Number of CT scans | 31 | 63 | 74 | 64 | 232 |
| Demographics | |||||
| Average age (range) | 60 (23–97) | 62 (27–88) | 62 (28–87) | 61 (25–85) | 61 (23–97) |
| % male | 18/31 (58%) | 30/63 (48%) | 49/74 (66%) | 32/64 (50%) | 129/232 (56%) |
| The extent of disease (0–16) | 7.7 | 9.8 | 10.8 | 11.0 | 10.2 |
| Endothelial disease | |||||
| Contrast | 19/31 (61%) | 50/63 (79%) | 64/74 (86%) | 36/64 (56%) | 169/232 (73%) |
| Pulmonary embolism | 3/19 (16%) | 7/50 (14%) | 21/64 (33%) | 4/36 (11%) | 35/169 (21%) |
| Hyperdense vessel | 3/12 (25%) | 5/13 (38%) | 4/10 (40%) | 9/28 (32%) | 21/63 (33%) |
| Hampton’s hump | 7/31 (23%) | 5/63 (8%) | 3/74 (4%) | 5/64 (8%) | 20/232 (9%) |
| Effusion | 2/31 (6%) | 7/63 (11%) | 11/74 (15%) | 15/64 (23%) | 35/232 (15%) |
| Epithelial disease | |||||
| Central consolidation | 17/31 (55%) | 49/63 (78%) | 63/74 (85%) | 51/64 (80%) | 180/232 (78%) |
| Round consolidation | 12/31 (39%) | 22/63 (35%) | 19/74 (26%) | 11/64 (17%) | 64/232 (28%) |
| Peripheral opacity | 17/31 (55%) | 38/63 (60%) | 37/74 (50%) | 17/64 (27%) | 109/232 (47%) |
| Atelectasis | 0/31 (0%) | 4/63 (6%) | 1/74 (1%) | 7/64 (11%) | 12/232 (5%) |
| Scarring | |||||
| Fibrosis | 1/31 (3%) | 3/63 (5%) | 7/74 (10%) | 14/64 (22%) | 25/232 (11%) |
| Pneumothorax | 0/31 (0%) | 0/63 (0%) | 0/74 (0%) | 6/64 (9%) | 6/232 (3%) |
| Cysts | 0/31 (0%) | 0/63 (0%) | 2/74 (3%) | 11/64 (17%) | 13/232 (6%) |
| Characteristics | Fibrosis (n = 25) | No Fibrosis (n = 207) | p-Value |
|---|---|---|---|
| Average age | 64 | 61 | 0.42 |
| % male | 17/25 (68%) | 112/207 (54%) | 0.19 |
| The average extent of disease | 11.8 | 10.0 | 0.04 |
| Pulmonary embolism | 1/15 (7%) | 34/154 (22%) | 0.19 |
| Hyperdense vessel | 6/25 (24%) | 21/55 (38%) | 0.21 |
| Peripheral opacity | 5/25 (20%) | 104/207 (50%) | 0.004 |
| Hampton’s hump | 1/25 (4%) | 19/207 (9%) | 0.39 |
| Central consolidation | 22/25 (88%) | 158/207 (76%) | 0.19 |
| Round consolidation | 5/25 (20%) | 59/207 (29%) | 0.19 |
| Effusion | 4/25 (16%) | 31/207 (15%) | 0.89 |
| Pneumothorax | 4/25 (16%) | 2/207 (1%) | <0.00001 |
| Cysts | 4/25 (16%) | 9/207 (4%) | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dsouza, B.; Capaccione, K.M.; Soleiman, A.; Leb, J.; Salvatore, M. COVID-19 on Chest CT: Translating Known Microscopic Findings to Imaging Observations. Life 2022, 12, 855. https://doi.org/10.3390/life12060855
Dsouza B, Capaccione KM, Soleiman A, Leb J, Salvatore M. COVID-19 on Chest CT: Translating Known Microscopic Findings to Imaging Observations. Life. 2022; 12(6):855. https://doi.org/10.3390/life12060855
Chicago/Turabian StyleDsouza, Belinda, Kathleen M. Capaccione, Aron Soleiman, Jay Leb, and Mary Salvatore. 2022. "COVID-19 on Chest CT: Translating Known Microscopic Findings to Imaging Observations" Life 12, no. 6: 855. https://doi.org/10.3390/life12060855
APA StyleDsouza, B., Capaccione, K. M., Soleiman, A., Leb, J., & Salvatore, M. (2022). COVID-19 on Chest CT: Translating Known Microscopic Findings to Imaging Observations. Life, 12(6), 855. https://doi.org/10.3390/life12060855






