Nontarget and Out-of-Field Doses from Electron Beam Radiotherapy
Abstract
:1. Introduction
2. Materials and Methods

3. Results
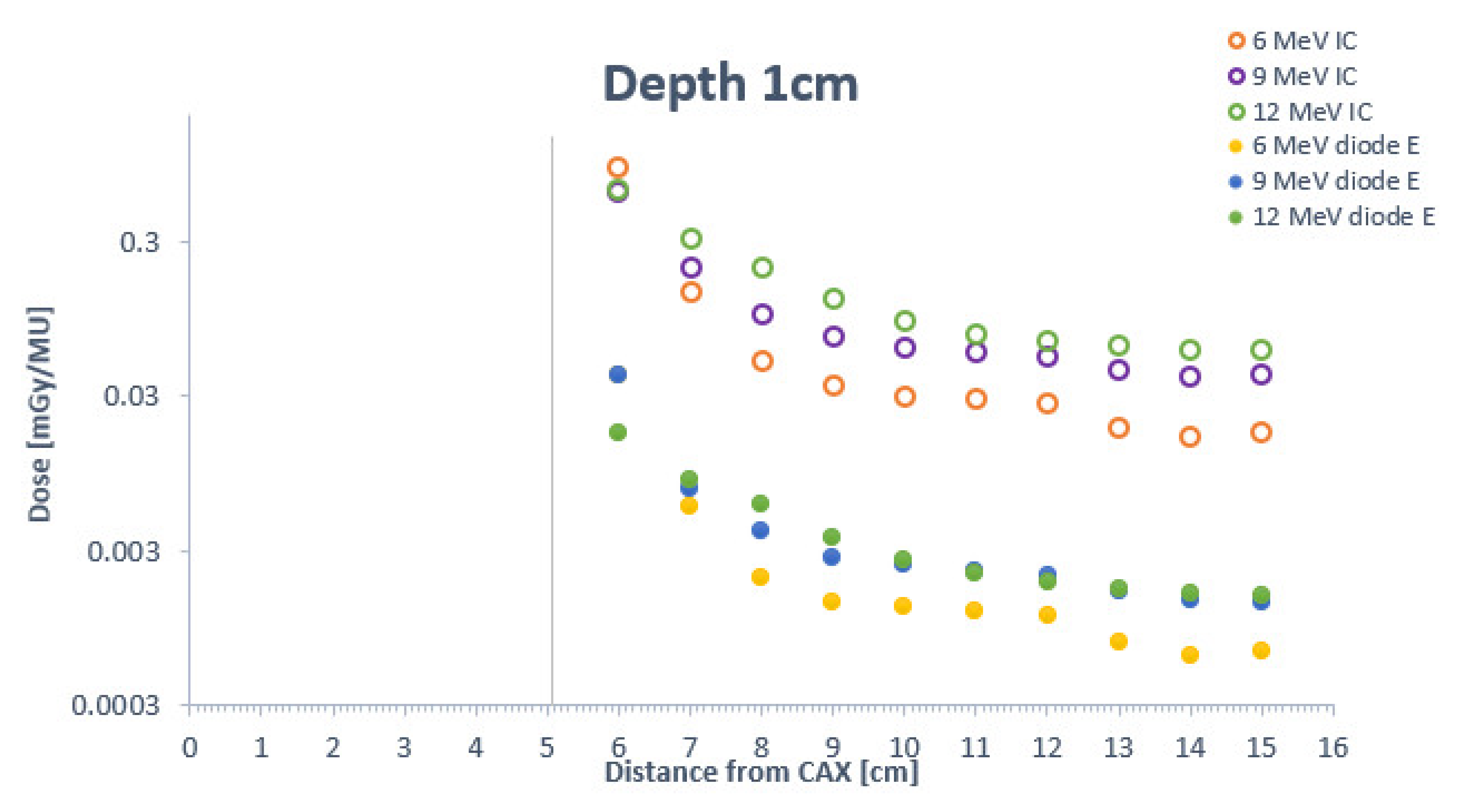

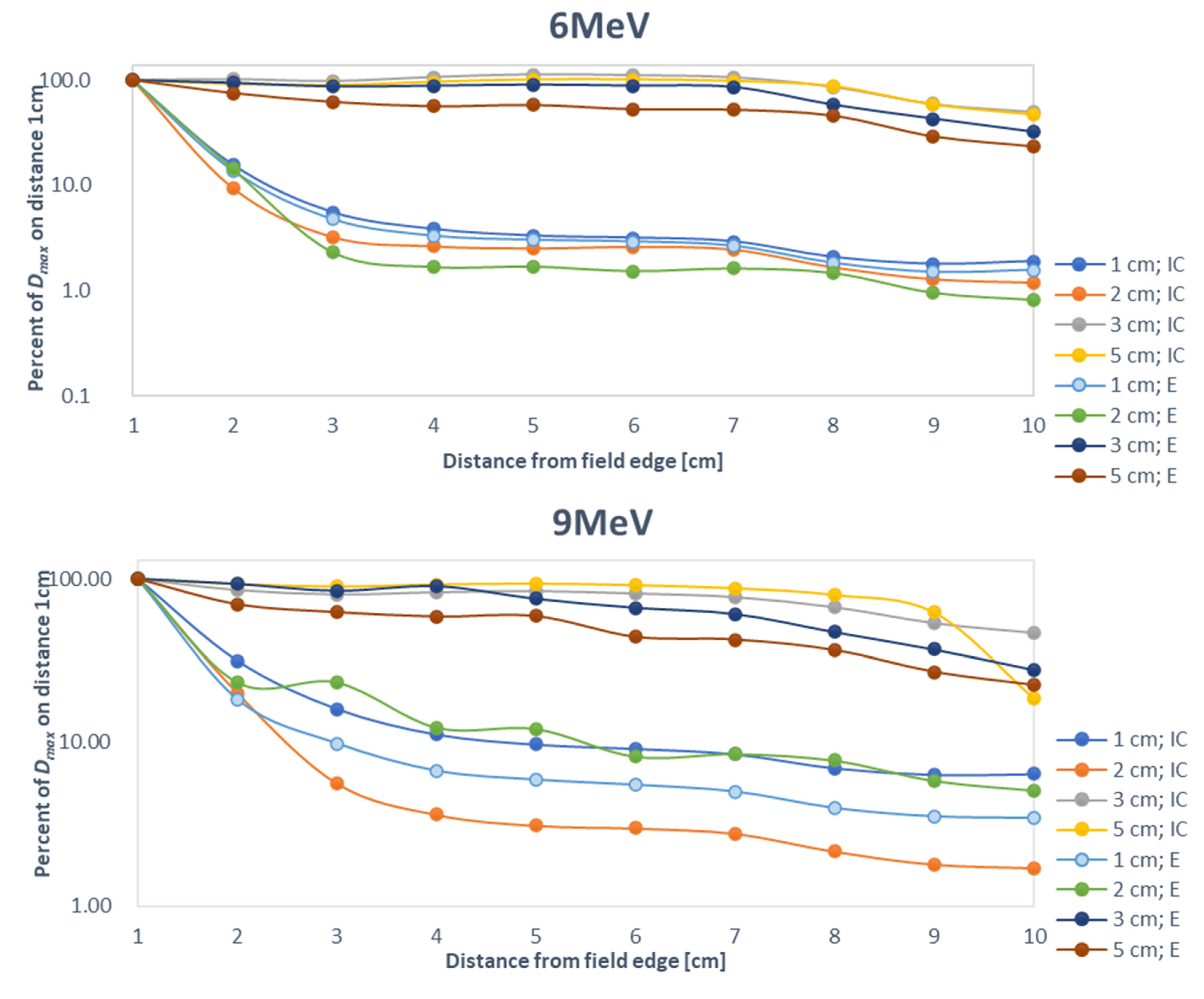
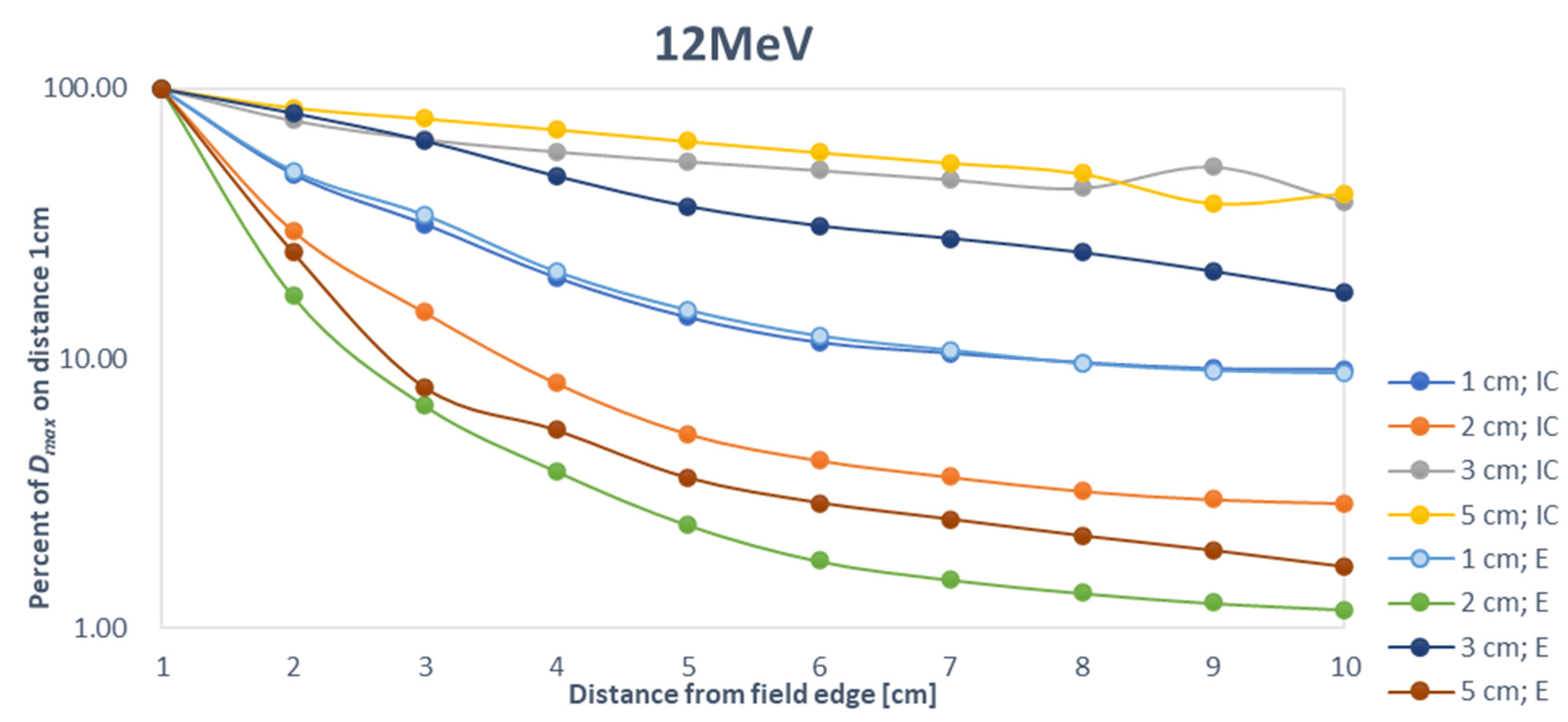
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Athiyaman, H.; Mayilvaganan, A.; Chougule, A.; Joan, M.; Kumar, H.S. Estimation of radiation-induced second cancer risk associated with the institutional field matching craniospinal irradiation technique: A comparative treatment planning study. Rep. Pract. Oncol. Radiother. 2019, 24, 409–420. [Google Scholar] [CrossRef] [PubMed]
- König, L.; Haering, P.; Lang, C.; Splinter, M.; von Nettelbladt, B.; Weykamp, F.; Hoegen, P.; Lischalk, J.W.; Herfarth, K.; Debus, J.; et al. Secondary malignancy risk following proton vs. X-ray treatment of mediastinal malignant lymphoma: A comparative modeling study of thoracic organ-specific cancer risk. Front. Oncol. 2020, 10, 989. [Google Scholar] [CrossRef] [PubMed]
- Podgoršak, E.B.; International Atomic Energy Agency. Radiation Oncology Physics: A Handbook for Teachers and Students; International Atomic Energy Agency: Vienna, Austria, 2005; 657p. [Google Scholar]
- Hogstrom, K.R.; Almond, P.R. Review of electron beam therapy physics. Phys. Med. Biol. 2006, 51, R455–R489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruszyna-Mochalska, M.; Skrobala, A.; Romanski, P.; Ryczkowski, A.; Suchorska, W.; Kulcenty, K.; Piotrowski, I.; Borowicz, D.; Graczyk, K.; Matuszak, N.; et al. Influence of specific treatment parameters on nontarget and out-of-field doses in a phantom model of prostate SBRT with CyberKnife and TrueBeam. Life 2022, 12, 628. [Google Scholar] [CrossRef]
- Kruszyna, M.; Adamczyk, S.; Skrobała, A.; Skórska, M.; Suchorska, W.; Zaleska, K.; Kowalik, A.; Jackowiak, W.; Malicki, J. Low dose out-of-field radiotherapy, part 1: Measurement of scattered doses. Cancer Radiothér. 2017, 21, 345–351. [Google Scholar] [CrossRef]
- Zhu, T.C.; Das, I.J.; Bjarngard, B.E. Characteristics of bremsstrahlung in electron beams. Med. Phys. 2001, 28, 1352–1358. [Google Scholar] [CrossRef]
- Gerbi, B.J.; Antolak, J.A.; Deibel, F.C.; Followill, D.S.; Herman, M.G.; Higgins, P.D.; Huq, M.S.; Mihailidis, D.N.; Yorke, E.D.; Hogstrom, K.R.; et al. Recommendations for clinical electron beam dosimetry: Supplement to the recommendations of Task Group 25. Med. Phys. 2009, 36, 3239. [Google Scholar] [CrossRef]
- Alabdoaburas, M.M.; Mege, J.-P.; Chavaudra, J.; Bezin, J.V.; Veres, A.; de Vathaire, F.; Lefkopoulos, D.; Diallo, I. Experimental assessment of out-of-field dose components in high energy electron beams used in external beam radiotherapy. J. Appl. Clin. Med. Phys. 2015, 16, 435–448. [Google Scholar] [CrossRef] [Green Version]
- Shimozato, T.; Okudaira, K.; Fuse, H.; Tabushi, K. Monte Carlo simulation and measurement of radiation leakage from applicators used in external electron radiotherapy. Phys. Med. 2013, 29, 388–396. [Google Scholar] [CrossRef] [Green Version]
- Kry, S.F.; Vassiliev, O.N.; Mohan, R. Out-of-field photon dose following removal of the flattening filter from a medical accelerator. Phys. Med. Biol. 2010, 55, 2155–2166. [Google Scholar] [CrossRef]
- Van Battum, L.J.; van der Zee, W.; Huizenga, H. Scattered radiation from applicators in clinical electron beams. Phys. Med. Biol. 2003, 48, 2493–2507. [Google Scholar] [CrossRef]
- Perec, A.; Kubo, H. Radiation leakage through electron applicators on Clinac-1800 accelerators. Med. Phys. 1990, 17, 715–719. [Google Scholar] [CrossRef]
- Bordy, J.M.; Bessieres, I.; D’Agostino, E.; Domingo, C.; D’Errico, F.; di Fulvio, A.; Knežević, Ž.; Miljanić, S.; Olko, P.; Ostrowsky, A.; et al. Radiotherapy out-of-field dosimetry: Experimental and computational results for photons in a water tank. Radiat. Meas. 2013, 57, 29–34. [Google Scholar] [CrossRef]
- Harrison, R. Out-of-field doses in radiotherapy: Input to epidemiological studies and dose-risk models. Phys. Med. 2017, 42, 239–246. [Google Scholar] [CrossRef]
- Sedlmayer, F.; Reitsamer, R.; Fussl, C.; Ziegler, I.; Zehentmayr, F.; Deutschmann, H.; Kopp, P.; Fastner, G. Boost IORT in breast cancer: Body of evidence. Int. J. Breast Cancer 2014, 2014, 472516. [Google Scholar] [CrossRef]
- Favaudon, V.; Caplier, L.; Monceau, V.; Pouzoulet, F.; Sayarath, M.; Fouillade, C.; Poupon, M.F.; Brito, I.; Hupé, P.; Bourhis, J.; et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumour tissue in mice. Sci. Transl. Med. 2014, 6, 245ra93. [Google Scholar] [CrossRef]
- Matuszak, N.; Suchorska, W.M.; Milecki, P.; Kruszyna-Mochalska, M.; Misiarz, A.; Pracz, J.; Malicki, J. FLASH radiotherapy: An emerging approach in radiation therapy. Rep. Pract. Oncol. Radiother. 2022, 27, 343–351. [Google Scholar] [CrossRef]
- Bayatiani, M.; Fallahi, F.; Aliasgharzadeh, A.; Ghorbani, M.; Khajetash, B.; Seif, F. A comparison of symmetry and flatness measurements in small electron fields by different dosimeters in electron beam radiotherapy. Rep. Pract. Oncol. Radiother. 2021, 26, 50–58. [Google Scholar] [CrossRef]
- Skrobala, A.; Adamczyk, S.; Kruszyna-Mochalska, M.; Skórska, M.; Konefał, A.; Suchorska, W.; Zaleska, K.; Kowalik, A.; Jackowiak, W.; Malicki, J. Low dose out-of-field radiotherapy, part 2: Calculating the mean photon energy values for the out-of-field photon energy spectrum from scattered radiation using Monte Carlo methods. Cancer Radiothér. 2017, 21, 352–357. [Google Scholar] [CrossRef]
- Cardenas, C.E.; Nitsch, P.L.; Kudchadker, R.J.; Howell, R.M.; Kry, S.F. Out-of-field doses and neutron dose equivalents for electron beams from modern Varian and Elekta linear accelerators. J. Appl. Clin. Med. Phys. 2016, 17, 442–455. [Google Scholar] [CrossRef]
- Kry, S.F.; Bednarz, B.; Howell, R.M.; Dauer, L.; Followill, D.; Klein, E.; Paganetti, H.; Wang, B.; Wuu, C.-S.; Xu, X.G. AAPM TG 158: Measurement and calculation of doses outside the treated volume from external-beam radiation therapy. Med. Phys. 2017, 44, e391–e429. [Google Scholar] [CrossRef]
- Bieda, M.R.; Antolak, J.A.; Hogstrom, K.R. The effect of scattering foil parameters on electron-beam Monte Carlo calculations. Med. Phys. 2001, 28, 2527–2534. [Google Scholar] [CrossRef]
- Chow, J.C.; Grigorov, G.N. Peripheral dose outside applicators in electron beams. Phys. Med. Biol. 2006, 51, N231–N240. [Google Scholar] [CrossRef]
- Jabbari, N.; Hashemi-Malayeri, B.; Farajollahi, A.R.; Kazemnejad, A. Monte Carlo calculation of scattered radiation from applicators in low energy clinical electron beams. Nukleonika 2007, 52, 97–103. [Google Scholar]
- Verhaegen, F.; Symonds-Tayler, R.; Liu, H.H.; Nahum, A.E. Backscatter towards the monitor ion chamber in high-energy photon and electron beams: Charge integration versus Monte Carlo simulation. Phys. Med. Biol. 2000, 45, 3159–3170. [Google Scholar] [CrossRef]
- Haghparast, A.; Amiri, F.; Yarahmadi, M.; Rezaei, M. The peripheral dose outside the applicator in electron beams of an Elekta linear accelerator. Australas. Phys. Eng. Sci. Med. 2018, 41, 647–655. [Google Scholar] [CrossRef]
- Hogstrom, K.R. Electron beam therapy: Dosimetry, planning, and techniques. In Principles and Practice of Radiation Oncology; Perez, C.A., Brady, L.W., Halperin, E.C., Schmidt-Ullrich, R.K., Eds.; Lippincott, Williams & Wilkins: Baltimore, MD, USA, 2004; pp. 252–282. [Google Scholar]
- Lax, I.; Brahme, A. Collimation of high energy electron beams. Acta Radiol. Oncol. 1980, 19, 199–207. [Google Scholar] [CrossRef]
- Van Battum, L.J.; Huizenga, H. On the initial angular variances of clinical electron beams. Phys. Med. Biol. 1999, 44, 2803–2820. [Google Scholar] [CrossRef] [PubMed]
- Yeboah, C.; Karotki, A.; Hunt, D.; Holly, R. Quantification and reduction of peripheral dose from leakage radiation on Siemens primus accelerators in electron therapy mode. J. Appl. Clin. Med. Phys. 2010, 11, 3105. [Google Scholar] [CrossRef] [PubMed]
- Yani, S.; Budiansah, I.; Rhani, M.F.; Haryanto, F. Monte Carlo model and output factors of Elekta infinity™ 6 and 10 MV photon beam. Rep. Pract. Oncol. Radiother. 2020, 25, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Kaderka, R.; Schardt, D.; Durante, M.; Berger, T.; Ramm, U.; Licher, J.; La Tessa, C. Out-of-field dose measurements in a water phantom using different radiotherapy modalities. Phys. Med. Biol. 2012, 57, 5059–5074. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.; Heins, D.; Zhao, X.; Sanders, M.; Zhang, R. Measurement and modeling of out-of-field doses from various advanced post-mastectomy radiotherapy techniques. Phys. Med. Biol. 2017, 62, 9039–9053. [Google Scholar] [CrossRef]
| 6 MeV | 9 MeV | 12 MeV | ||||
|---|---|---|---|---|---|---|
| Depth | Distance from Field Edge | |||||
| 1 cm | 10 cm | 1 cm | 10 cm | 1 cm | 10 cm | |
| 1 cm | 4.5% | 3.8% | 6.3% | 3.4% | 2.7% | 2.5% |
| 5 cm | 11.5% | 6.0% | 13.7% | 5.5% | 3.9% | 4.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matuszak, N.; Kruszyna-Mochalska, M.; Skrobala, A.; Ryczkowski, A.; Romanski, P.; Piotrowski, I.; Kulcenty, K.; Suchorska, W.M.; Malicki, J. Nontarget and Out-of-Field Doses from Electron Beam Radiotherapy. Life 2022, 12, 858. https://doi.org/10.3390/life12060858
Matuszak N, Kruszyna-Mochalska M, Skrobala A, Ryczkowski A, Romanski P, Piotrowski I, Kulcenty K, Suchorska WM, Malicki J. Nontarget and Out-of-Field Doses from Electron Beam Radiotherapy. Life. 2022; 12(6):858. https://doi.org/10.3390/life12060858
Chicago/Turabian StyleMatuszak, Natalia, Marta Kruszyna-Mochalska, Agnieszka Skrobala, Adam Ryczkowski, Piotr Romanski, Igor Piotrowski, Katarzyna Kulcenty, Wiktoria Maria Suchorska, and Julian Malicki. 2022. "Nontarget and Out-of-Field Doses from Electron Beam Radiotherapy" Life 12, no. 6: 858. https://doi.org/10.3390/life12060858
APA StyleMatuszak, N., Kruszyna-Mochalska, M., Skrobala, A., Ryczkowski, A., Romanski, P., Piotrowski, I., Kulcenty, K., Suchorska, W. M., & Malicki, J. (2022). Nontarget and Out-of-Field Doses from Electron Beam Radiotherapy. Life, 12(6), 858. https://doi.org/10.3390/life12060858







