Therapeutic Effect and Optimal Electrode Placement of Transcutaneous Neuromuscular Electrical Stimulation in Patients with Post-Stroke Dysphagia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
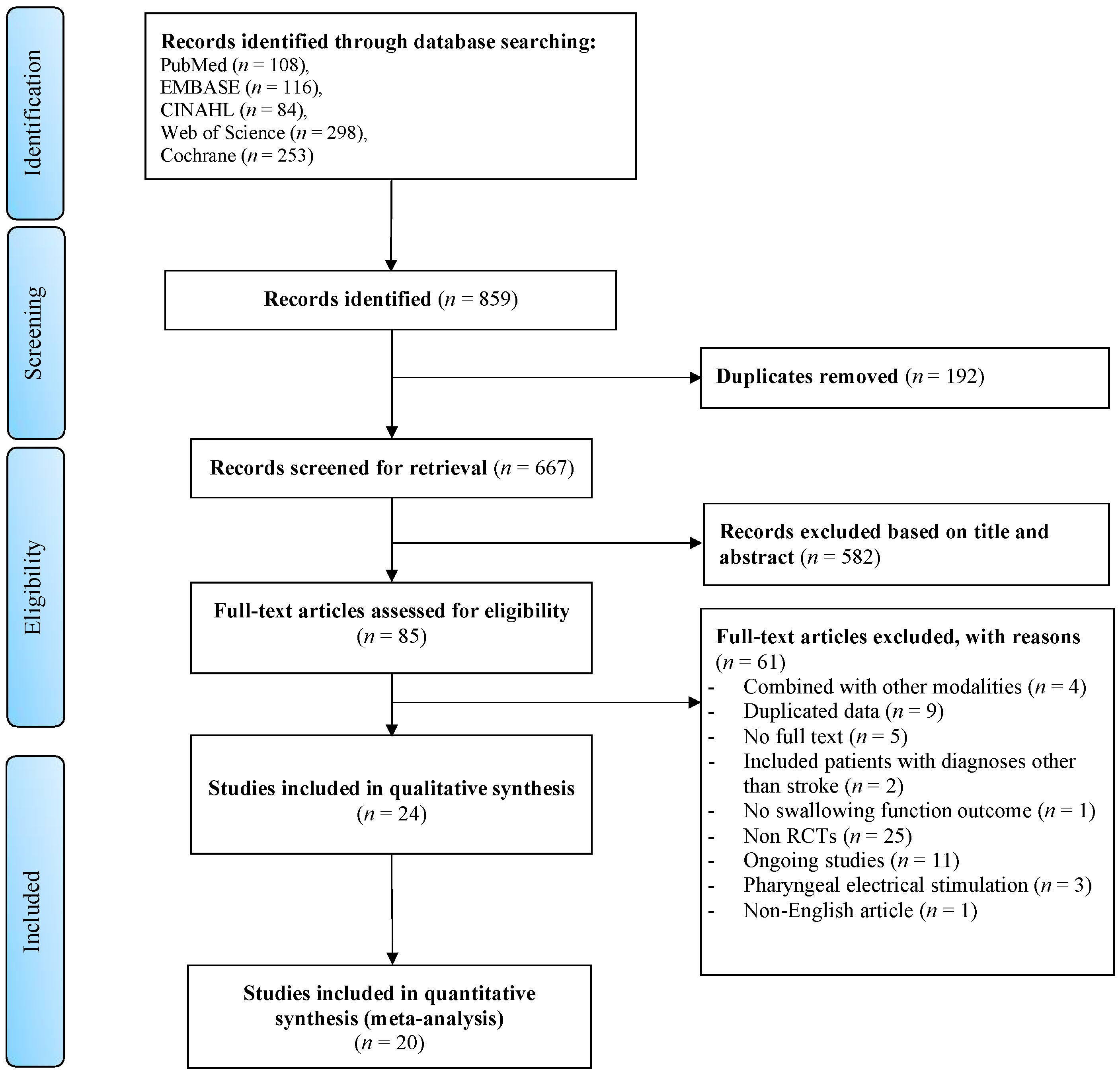
2.2. Search Strategy and Screening Process
2.3. Assessment of the Risk of Bias
2.4. Data Extraction
2.5. Statistical Analysis
3. Results
3.1. Risk of Bias Assessment
3.2. Characteristics of Studies
3.3. Meta-Analysis
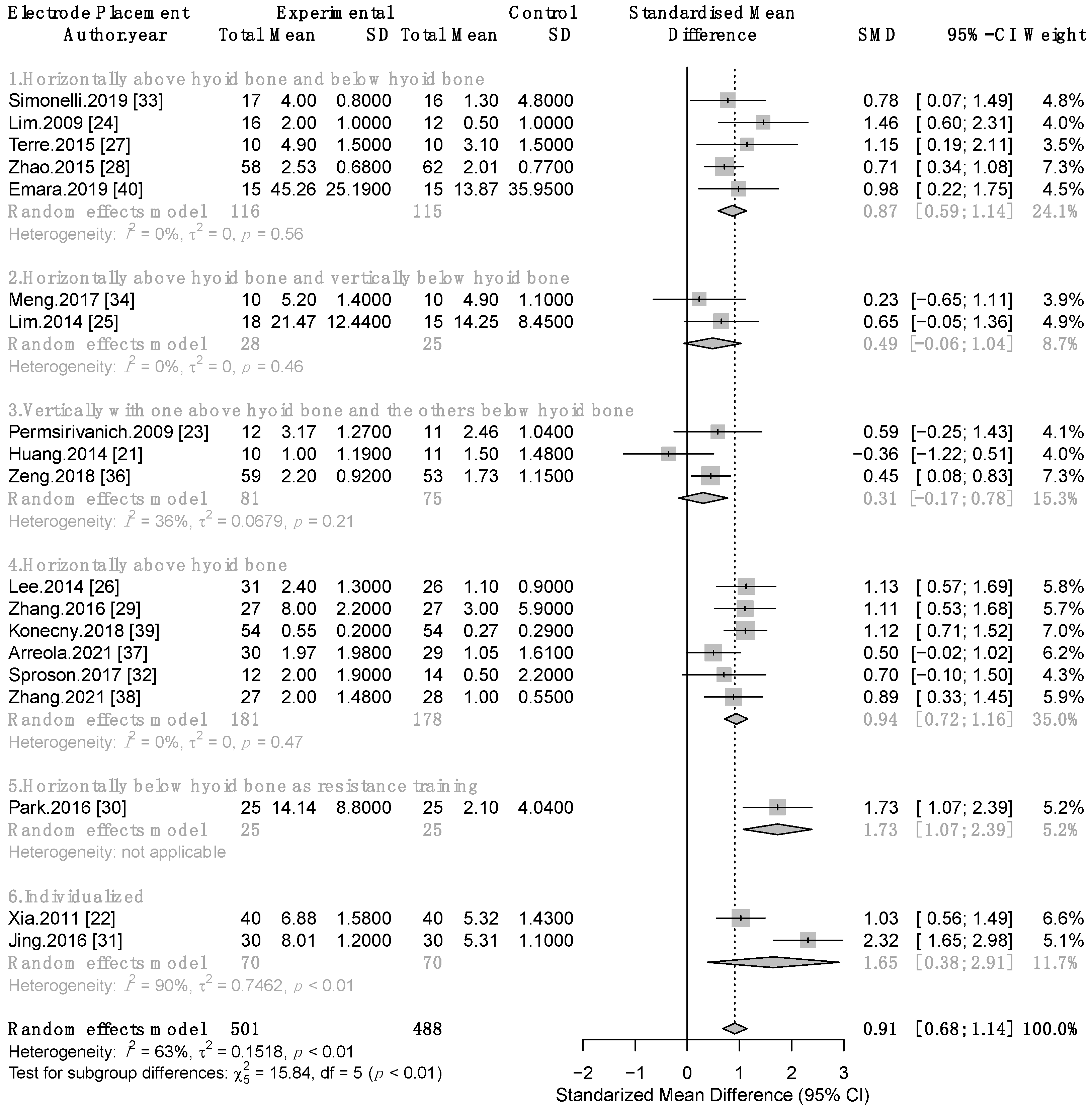
3.3.1. Therapeutic Effect of Combined TNMES and CSTs on Swallowing Function
3.3.2. Therapeutic Effect of TNMES Alone Compared to CSTs on Swallowing Function
3.3.3. Therapeutic Effect of TNMES Combined CSTs on Patients’ Quality of Life
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Banda, K.J.; Chu, H.; Kang, X.L.; Liu, D.; Pien, L.C.; Jen, H.J.; Hsiao, S.S.; Chou, K.R. Prevalence of dysphagia and risk of pneumonia and mortality in acute stroke patients: A meta-analysis. BMC Geriatr. 2022, 22, 420. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.L.; Roffe, C.; Beavan, J.; Blackett, B.; Fairfield, C.A.; Hamdy, S.; Havard, D.; McFarlane, M.; McLauglin, C.; Randall, M.; et al. Post-stroke dysphagia: A review and design considerations for future trials. Int. J. Stroke 2016, 11, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, S.; Aziz, Q.; Rothwell, J.C.; Power, M.; Singh, K.D.; Nicholson, D.A.; Tallis, R.C.; Thompson, D.G. Recovery of swallowing after dysphagic stroke relates to functional reorganization in the intact motor cortex. Gastroenterology 1998, 115, 1104–1112. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Hsu, T.-W.; Leong, C.-P.; Hsieh, H.-C.; Lin, W.-C. Clinical Effects and Differences in Neural Function Connectivity Revealed by MRI in Subacute Hemispheric and Brainstem Infarction Patients with Dysphagia After Swallowing Therapy. Front. Neurosci. 2018, 12, 488. [Google Scholar] [CrossRef] [Green Version]
- Carnaby, G.D.; Harenberg, L. What is “usual care” in dysphagia rehabilitation: A survey of USA dysphagia practice patterns. Dysphagia 2013, 28, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Chang, K.H.; Chen, H.C.; Liang, W.M.; Wang, Y.H.; Lin, Y.N. The effects of surface neuromuscular electrical stimulation on post-stroke dysphagia: A systemic review and meta-analysis. Clin. Rehabil. 2016, 30, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, X.; Qiao, J.; Song, G.; Xu, Y.; Zhang, Y.; Xu, D.; Gao, W.; Li, Y.; Xu, C. Effects of Transcutaneous Neuromuscular Electrical Stimulation on Swallowing Disorders: A Systematic Review and Meta-Analysis. Am. J. Phys. Med. Rehabil. 2020, 99, 701–711. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence (NICE). Transcutaneous Neuromuscular Electrical Stimulation for Oropharyngeal Dysphagia in Adults. 2018 NICE International Guidance No IPG634. Available online: https://www.nice.org.uk/guidance/ipg634/chapter/1-Recommendations (accessed on 22 November 2021).
- Chen, Y.-S. Effects of electrical stimulation on peripheral nerve regeneration. BioMedicine 2011, 1, 33–36. [Google Scholar] [CrossRef]
- Chang, M.C.; Park, S.; Cho, J.Y.; Lee, B.J.; Hwang, J.M.; Kim, K.; Park, D. Comparison of three different types of exercises for selective contractions of supra- and infrahyoid muscles. Sci. Rep. 2021, 11, 7131. [Google Scholar] [CrossRef]
- Humbert, I.A.; Poletto, C.J.; Saxon, K.G.; Kearney, P.R.; Crujido, L.; Wright-Harp, W.; Payne, J.; Jeffries, N.; Sonies, B.C.; Ludlow, C.L. The effect of surface electrical stimulation on hyolaryngeal movement in normal individuals at rest and during swallowing. J. Appl. Physiol. 2006, 101, 1657–1663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paik, N.J.; Kim, S.J.; Lee, H.J.; Jeon, J.Y.; Lim, J.Y.; Han, T.R. Movement of the hyoid bone and the epiglottis during swallowing in patients with dysphagia from different etiologies. J. Electromyogr. Kinesiol. 2008, 18, 329–335. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef] [Green Version]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Taylor & Francis: Milton Park, UK, 2013. [Google Scholar]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Chu, H. Quantifying publication bias in meta-analysis. Biometrics 2018, 74, 785–794. [Google Scholar] [CrossRef]
- Bülow, M.; Speyer, R.; Baijens, L.; Woisard, V.; Ekberg, O.; Bülow, M.; Speyer, R.; Baijens, L.; Woisard, V.; Ekberg, O. Neuromuscular electrical stimulation (NMES) in stroke patients with oral and pharyngeal dysfunction. Dysphagia 2008, 23, 302–309. [Google Scholar] [CrossRef]
- Huang, K.-L.; Liu, T.-Y.; Huang, Y.-C.; Leong, C.-P.; Lin, W.-C.; Pong, Y.-P. Functional outcome in acute stroke patients with oropharyngeal Dysphagia after swallowing therapy. J. Stroke Cerebrovasc. Dis. 2014, 23, 2547–2553. [Google Scholar] [CrossRef] [Green Version]
- Xia, W.; Zheng, C.; Lei, Q.; Tang, Z.; Hua, Q.; Zhang, Y.; Zhu, S. Treatment of post-stroke dysphagia by vitalstim therapy coupled with conventional swallowing training. Hua Zhong Ke Ji Da Xue Xue Bao. Yi Xue Ying De Wen Ban/J. Huazhong Univ. Sci. Technology. Med. Sci. 2011, 31, 73–76. [Google Scholar] [CrossRef]
- Permsirivanich, W.; Tipchatyotin, S.; Wongchai, M.; Leelamanit, V.; Setthawatcharawanich, S.; Sathirapanya, P.; Phabphal, K.; Juntawises, U.; Boonmeeprakob, A. Comparing the effects of rehabilitation swallowing therapy vs. neuromuscular electrical stimulation therapy among stroke patients with persistent pharyngeal dysphagia: A randomized controlled study. Chotmaihet Thangphaet/J. Med. Assoc. Thail. 2009, 92, 259–265. [Google Scholar]
- Lim, K.B.; Lee, H.J.; Lim, S.S.; Choi, Y.I. Neuromuscular electrical and thermal-tactile stimulation for dysphagia caused by stroke: A randomized controlled trial. J. Rehabil. Med. 2009, 41, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.B.; Lee, H.J.; Yoo, J.; Kwon, Y.G. Effect of Low-Frequency rTMS and NMES on Subacute Unilateral Hemispheric Stroke With Dysphagia. Ann. Rehabil. Med. 2014, 38, 592–602. [Google Scholar] [CrossRef]
- Lee, K.W.; Kim, S.B.; Lee, J.H.; Lee, S.J.; Ri, J.W.; Park, J.G. The effect of early neuromuscular electrical stimulation therapy in acute/subacute ischemic stroke patients with Dysphagia. Ann. Rehabil. Med. 2014, 38, 153–159. [Google Scholar] [CrossRef]
- Terre, R.; Mearin, F. A randomized controlled study of neuromuscular electrical stimulation in oropharyngeal dysphagia secondary to acquired brain injury. Eur. J. Neurol. 2015, 22, 687-e44. [Google Scholar] [CrossRef]
- Zhao, J.W.; Wang, Z.Y.; Cao, W.Z.; Zhang, Y.W.; Song, S.C.; Kang, W.G.; Yang, J.H. Therapeutic efficacy of swallowing neuromuscular electrical stimulation combined with acupuncture for post-stroke dysphagia. World J. Acupunct. Moxibustion 2015, 25, 19–23. [Google Scholar] [CrossRef]
- Zhang, M.; Tao, T.; Zhang, Z.-B.; Zhu, X.; Fan, W.-G.; Pu, L.-J.; Chu, L.; Yue, S.-W. Effectiveness of Neuromuscular Electrical Stimulation on Patients With Dysphagia With Medullary Infarction. Arch. Phys. Med. Rehabil. 2016, 97, 355–362. [Google Scholar] [CrossRef]
- Park, J.S.; Oh, D.H.; Hwang, N.K.; Lee, J.H. Effects of neuromuscular electrical stimulation combined with effortful swallowing on post-stroke oropharyngeal dysphagia: A randomised controlled trial. J. Oral Rehabil. 2016, 43, 426–434. [Google Scholar] [CrossRef]
- Jing, Q.; Yang, X.; Reng, Q. Effect of neuromuscular electrical stimulation in patients with post-stroke dysphagia. Med. Sci. Technol. 2016, 57, 1–5. [Google Scholar] [CrossRef]
- Sproson, L.; Pownall, S.; Enderby, P.; Freeman, J. Combined electrical stimulation and exercise for swallow rehabilitation post-stroke: A pilot randomized control trial. Int. J. Lang. Commun. Disord. 2018, 53, 405–417. [Google Scholar] [CrossRef] [Green Version]
- Simonelli, M.; Ruoppolo, G.; Iosa, M.; Morone, G.; Fusco, A.; Grasso, M.G.; Gallo, A.; Paolucci, S. A stimulus for eating. The use of neuromuscular transcutaneous electrical stimulation in patients affected by severe dysphagia after subacute stroke: A pilot randomized controlled trial. NeuroRehabilitation 2019, 44, 103–110. [Google Scholar] [CrossRef]
- Meng, P.; Zhang, S.; Wang, Q.; Wang, P.; Han, C.; Gao, J.; Yue, S. The effect of surface neuromuscular electrical stimulation on patients with post-stroke dysphagia. J. Back Musculoskelet. Rehabil. 2018, 31, 363–370. [Google Scholar] [CrossRef]
- Guillén-Solà, A.; Messagi Sartor, M.; Bofill Soler, N.; Duarte, E.; Barrera, M.C.; Marco, E. Respiratory muscle strength training and neuromuscular electrical stimulation in subacute dysphagic stroke patients: A randomized controlled trial. Clin. Rehabil. 2017, 31, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Yip, J.; Cui, H.; Guan, L.; Zhu, H.; Zhang, W.; Du, H.; Geng, X. Efficacy of neuromuscular electrical stimulation in improving the negative psychological state in patients with cerebral infarction and dysphagia. Neurol. Res. 2018, 40, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Arreola, V.; Ortega, O.; Alvarez-Berdugo, D.; Rofes, L.; Tomsen, N.; Cabib, C.; Muriana, D.; Palomera, E.; Clave, P. Effect of Transcutaneous Electrical Stimulation in Chronic Poststroke Patients with Oropharyngeal Dysphagia: 1-Year Results of a Randomized Controlled Trial. Neurorehabilit. Neural Repair 2021, 35, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, S. Effects of Synchronized Neuromuscular Electrical Stimulation (NMES) on the Submental Muscles During Ingestion of a Specified Volume of Soft Food in Patients with Mild-to-Moderate Dysphagia Following Stroke. Med. Sci. Monit. 2021, 27, e928988-1. [Google Scholar] [CrossRef]
- Konecny, P.; Elfmark, M.; Rosolova, M.; Bastlova, P.; Lerchova, I.; Muckova, A. Electrical Stimulation of the Suprahyoid Muscles in Post Stroke Patients with Dysphagia. Ceska a Slovenska Neurologie a Neurochirurgie 2017, 80, 578–581. [Google Scholar] [CrossRef] [Green Version]
- Emara, T. Effect of transcutaneous electrical nerve stimulation and conventional therapy in post-stroke dysphagic patients: A randomized controlled trial. Biosci. Res. 2019, 16, 11–16. [Google Scholar]
- Huh, J.W.; Park, E.; Min, Y.S.; Kim, A.R.; Yang, W.J.; Oh, H.M.; Nam, T.W.; Jung, T.D. Optimal placement of electrodes for treatment of post-stroke dysphagia by neuromuscular electrical stimulation combined with effortful swallowing. Singap. Med. J. 2020, 61, 487–491. [Google Scholar] [CrossRef]
- Oh, D.-H.; Park, J.-S.; Kim, H.-J.; Chang, M.-Y.; Hwang, N.-K. The effect of neuromuscular electrical stimulation with different electrode positions on swallowing in stroke patients with oropharyngeal dysphagia: A randomized trial. J. Back Musculoskelet. Rehabil. 2020, 33, 637–644. [Google Scholar] [CrossRef]
- Lee, K.W.; Kim, S.B.; Lee, J.H.; Lee, S.J.; Park, J.G.; Jang, K.W. Effects of Neuromuscular Electrical Stimulation for Masseter Muscle on Oral Dysfunction After Stroke. Ann. Rehabil. Med. 2019, 43, 11–18. [Google Scholar] [CrossRef]
- Rofes, L.; Arreola, V.; López, I.; Martin, A.; Sebastián, M.; Ciurana, A.; Clavé, P. Effect of surface sensory and motor electrical stimulation on chronic poststroke oropharyngeal dysfunction. Neurogastroenterol. Motil. 2013, 25, 888-e701. [Google Scholar] [CrossRef] [PubMed]
- Pearson, W.G., Jr.; Langmore, S.E.; Yu, L.B.; Zumwalt, A.C. Structural analysis of muscles elevating the hyolaryngeal complex. Dysphagia 2012, 27, 445–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| No | Author, Year | Country | Sample Size | Time Since Stroke | Interventional and Control Groups (TNMES Protocol) | Electrode Placements | Outcomes and Results |
|---|---|---|---|---|---|---|---|
| 1 | Bulow, 2008 [20] | France, The Netherlands, Sweden | 25 (12/13) | >3 months | Intervention: TNMES PD (pulse duration): 700 μs, F (frequency): 80 Hz, I (intensity): 4.5–25 mA (mean: 13 mA); 60 min/day, 5 days/week for 3 weeks Control: CSTs | Placement 1 | OMFT − VFSS − |
| 2 | Permsirivanich, 2009 [23] | Thailand | 23 (12/11) | >2 weeks | Intervention: TNMES + CSTs PD: 700 μs, F: 80 Hz; 60 min/day, 5 days/week for 4 weeks Control: CSTs | Placement 3 | FOIS + |
| 3 | Lim, 2009 [24] | Korea | 28 (16/12) | Intervention: TNMES + CSTs TNMES: PD: 700 μs, F: 80 Hz, I: approximately 7 mA; 60 min/day, 5 days/week for 4 weeks Control: CSTs | Placement 1 | VFSS + | |
| 4 | Xia, 2011 [22] | China | 120 (40/40/40) | Subacute | Intervention 1 (I1): TNMES + CSTs Intervention 2 (I2): TNMES TNMES: PD: 700 μs, F: 80 Hz, I: 0–25 mA; 30 min sessions twice a day, 5 days/week for 4 weeks Control (C): CSTs | Electrodes were individualized according to VFSS scores, tolerance and condition of patients | SSA + VFSS + SWALQOL + I2 vs. C − |
| 5 | Lim, 2014 [25] | Korea | 33 (18/15) | <3 months | Intervention: TNMES (2 weeks) + CSTs (4 weeks) TNMES: PD: 300 μs, F:80 Hz, I: 7–9 mA; 30 min/day, 5 days/week for 2 weeks Control: CSTs for 4 weeks | Placement 2 | VFSS + |
| 6 | Huang, 2014 [21] | Taiwan | 29 (10/11/8) | <3 months | Intervention 1 (I1): TNMES + CSTs Intervention 2 (I2): TNMES TNMES: PD: 700 μs, F: 80 Hz, I: 0–25 mA; 60 min/day, 3 days/week, totally 10 sessions Control: CSTs | Placement 3 | FOIS − VFSS − |
| 7 | Lee, 2014 [26] | Korea | 57 (31/26) | Within 10 days | Intervention: TNMES + CSTs TNMES: PD: 700 μs, F: 80 Hz; 30 min/day, 5 days/week + CSTs: 60 min/day, 5 days/week for 3 weeks Control: CSTs | Placement 4 | FOIS + |
| 8 | Terre, 2015 [27] | Spain | 20 (10/10) | 1–6 months | Intervention: TNMES + CSTs TNMES: PD: 300 μs, F: 80 Hz, I: 7–19.4 mA, 60 min/day, 5 days per week for 4 weeks Control: Sham TNMES + CSTs | Placement 1 | FOIS + |
| 9 | Zhao, 2015 [28] | China | 120 (58/62) | Intervention: TNMES + CSTs TNMES: F: 50–100 Hz, 30 min, twice a day for 2 weeks Control: CSTs | Placement 1 | WST + | |
| 10 | Zhang, 2016 [29] | China | 54 (27/27) | <1 month | Intervention: TNMES + CSTs TNMES: PD: 100 μs, F: 120 Hz, I: 2–60 mA, 20 min/session, twice a day, 5 days/week for 4 weeks Control: CSTs | Placement 4 | SSA + WST + FOIS + SWALQOL + |
| 11 | Park, 2016 [30] | Korea | 50 (25/25) | >6 months | Intervention: TNMES + CSTs TNMES: PD: 700 μs, F: 80 Hz, I: 9–14 mA, 30 min/day, 5 days/week for 6 weeks Patients performed effortful swallow to elevate the hyoid during stimulation Control: Sham TNMES + CSTs | The electrodes were located in the infrahyoid region to target the sternohyoid, omohyoid, and sternothyroid muscles | VFSS + |
| 12 | Jing, 2016 [31] | China | 60 (30/30) | Within 1–3 days | Intervention: TNMES + CSTs TNMES: PD: 700 μs, F: 80 Hz, I: 6–21 mA, in 10 consecutive days Control: CSTs | Electrodes was individualized according to the result of dysphagia evaluation | SFS + |
| 13 | Sproson, 2017 [32] | The UK | 26 (12/14) | >1 month | Intervention: TNMES + CSTs TNMES: F: 30 Hz, 30 min/day, 5 days/week for 4 weeks Control: CSTs | Placement 4 | FOIS − VFSS − SWALQOL − |
| 14 | Simonelli, 2019 [33] | Italy | 33 (17/16) | <3 months | Intervention: TNMES + CSTs TNMES: PD: 300 μs, F: 80 Hz, I: 7.8–12.5 mA, 30 min/day, 5 days/week for 8 weeks Control: CSTs | Placement 1 | FOIS + |
| 15 | Meng, 2017 [34] | China | 20 (10/10) | <6 months | Intervention: TNMES + CSTs TNMES: F: 80 Hz, I: 0–25 mA, 30 min/day, 5 days/week for 2 weeks Control: CSTs | Placement 2 | VFSS + WST + |
| 16 | Guillen, 2017 [35] | Spain | 41 (20/21) | Within 1–3 weeks | Intervention: TNMES + CSTs TNMES: F: 80 Hz, 40 min/day, 5 days/week for 3 weeks Control: CSTs | Placement 4 | V-VST + |
| 17 | Zeng, 2018 [36] | China | 112 (59/53) | Intervention: TNMES + CSTs TNMES: PD: 800 μs, I: 28 mA, 20 min session, once-daily for 12 days followed by a 2-day break, then continue another 12-day course of treatment Control: CSTs | Placement 3 | WST + | |
| 18 | Arreola, 2021 [37] | Spain | 59 (30/29) | >3 months | Intervention: TNMES + CSTs TNMES: PD: 700 μs, F: 80 Hz, I: 11.86 ± 5.11 mA, 1 h session twice a day for the first week and once a day for the second week (5 days/week) Control: CSTs | Placement 4 | VFSS + |
| 19 | Zhang, 2021 [38] | China | 55 (27/28) | 1–3 months | Intervention: TNMES + CSTs TNMES: PD: 300 μs, F: 80 Hz, I: 6.3–13.2 mA, 30 min/day, 5 days/week for 6 weeks Control: CSTs | Placement 4 | VFSS + |
| 20 | Konecny, 2018 [39] | Czech Republic | 108 (54/54) | Early stage after stroke | Intervention: TNMES + CSTs TNMES: PD: 300 μs, F: 60 Hz, 20 min/day, 5 days/week for 4 weeks Control: CSTs | Placement 4 | VFSS + |
| 21 | Emara, 2019 [40] | Egypt | 30 (15/15) | 1–3 months | Intervention: TNMES + CSTs TNMES: PD: 300 μs, F: 80 Hz, I: 2.5–25 mA, 30 min/day, 3 days/week for 3 weeks Control: Sham TNMES + CSTs | Placement 1 | MASA + |
| 22 | Huh, 2020 [41] | Korea | 31 (10/11/10) | Intervention: 3 groups based on electrode placement:I1: Placement 1 I2: Placement 2 I3: Placement 3 PD: 300 μs, F: 80 Hz, I: 0–25 mA, 20 min/day, 5 days/week for 4 weeks | FDS and DOSS in I1 improved significantly compared to other groups | ||
| 23 | Oh, 2019 [42] | Korea | 26 (14/12) | <6 months | Intervention: 2 groups based on electrode placement: I1: 2 pairs of electrodes targeted suprahyoid muscles. I2: 2 pairs of electrodes targeted infrahyoid muscles. PD: 700 μs, F: 80 Hz, I: 9–14 mA, 30 min/day, 5 days/week for 4 weeks Patients performed effortful swallow to elevate the hyoid during stimulation | Significant improvement in PAS scores favoring I1 | |
| 24 | Lee, 2019 [43] | Korea | 40 (20/20) | Subacute | Intervention: 2 groups based on electrode placement: I1: 1 pair of electrodes targeted masseter and the other pair targeted suprahyoid muscles. I2: 2 pairs of electrodes targeted suprahyoid muscles. PD: 300 μs, F: 80 Hz, I: 9–14 mA, 20 min/session, twice a day, 5 days/week for 2 weeks Both groups received CSTs | FDS and pharyngeal FDS improved in both groups. I1 improved oral FDS. No significant differences between groups | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doan, T.-N.; Ho, W.-C.; Wang, L.-H.; Chang, F.-C.; Tran, T.T.Q.; Chou, L.-W. Therapeutic Effect and Optimal Electrode Placement of Transcutaneous Neuromuscular Electrical Stimulation in Patients with Post-Stroke Dysphagia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Life 2022, 12, 875. https://doi.org/10.3390/life12060875
Doan T-N, Ho W-C, Wang L-H, Chang F-C, Tran TTQ, Chou L-W. Therapeutic Effect and Optimal Electrode Placement of Transcutaneous Neuromuscular Electrical Stimulation in Patients with Post-Stroke Dysphagia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Life. 2022; 12(6):875. https://doi.org/10.3390/life12060875
Chicago/Turabian StyleDoan, Thanh-Nhan, Wen-Chao Ho, Liang-Hui Wang, Fei-Chun Chang, Trang Thi Quynh Tran, and Li-Wei Chou. 2022. "Therapeutic Effect and Optimal Electrode Placement of Transcutaneous Neuromuscular Electrical Stimulation in Patients with Post-Stroke Dysphagia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Life 12, no. 6: 875. https://doi.org/10.3390/life12060875
APA StyleDoan, T.-N., Ho, W.-C., Wang, L.-H., Chang, F.-C., Tran, T. T. Q., & Chou, L.-W. (2022). Therapeutic Effect and Optimal Electrode Placement of Transcutaneous Neuromuscular Electrical Stimulation in Patients with Post-Stroke Dysphagia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Life, 12(6), 875. https://doi.org/10.3390/life12060875








