Effects of Kelulut Honey on Oestrus Cycle Regulation and Histomorphological Changes in Letrozole-Induced Polycystic Ovary Syndrome Rats: A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Honey Sample
2.2. Animal Preparation
2.3. Animal Treatment
2.4. Determination of Fasting Blood Glucose
2.5. Determination of Oestrous Cycle
2.6. Histomorphological Analysis of the Ovaries
2.7. Statistical Analysis
3. Results
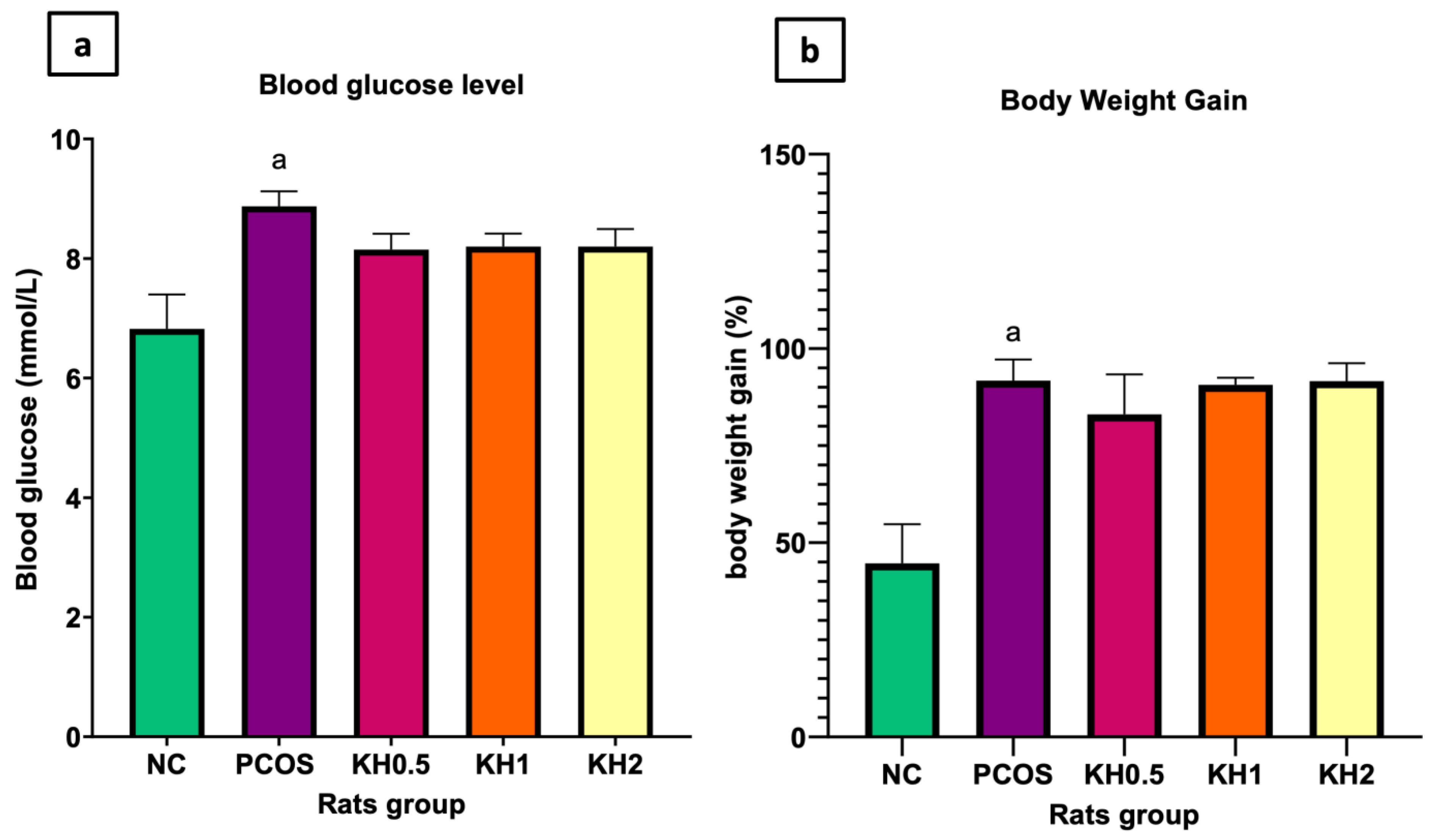
3.1. Effects of Kelulut Honey on the Blood Glucose Levels and Body Weight Gain
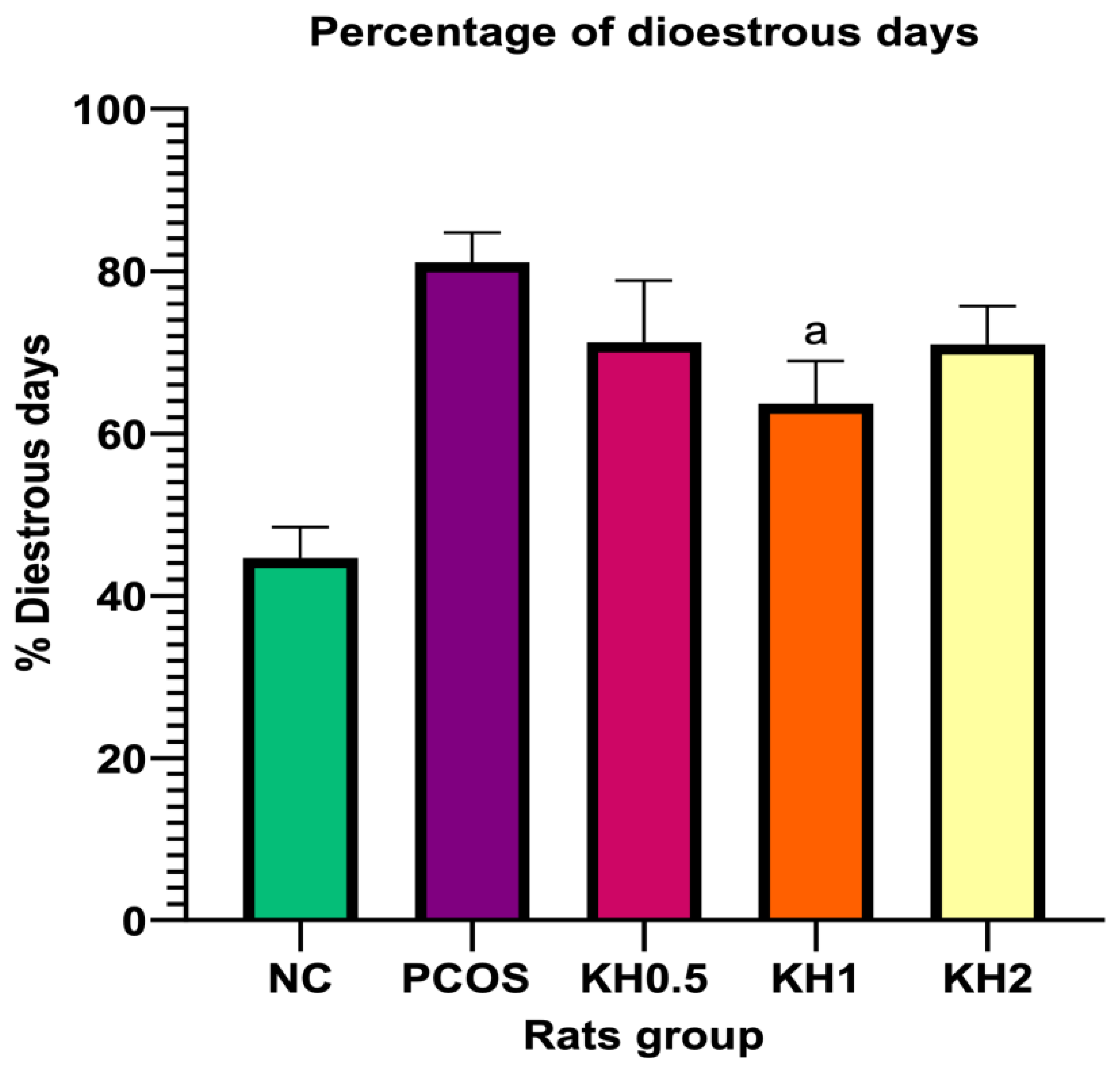
3.2. Effects of Kelulut Honey on the Oestrus Cycle
3.3. Effects of Kelulut Honey on the Ovarian Histomorphological Changes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Effects of Letrozole Treatment on the Percentage of Dioestrous Days, Blood Glucose and Ovarian Histomorphological Parameters


Appendix B
Rat Vaginal Epithelium Staining in Different Stages of Estrus Cycle

References
- Teede, H.; Deeks, A.; Moran, L. Polycystic ovary syndrome: A complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010, 8, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escobar-Morreale, H.F. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nat. Rev. Endocrinol. 2018, 14, 270. [Google Scholar] [CrossRef] [PubMed]
- McCartney, C.R.; Marshall, J.C. Polycystic Ovary Syndrome. N. Engl. J. Med. 2016, 375, 54–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balen, A.H.; Morley, L.C.; Misso, M.; Franks, S.; Legro, R.S.; Wijeyaratne, C.N.; Stener-Victorin, E.; Fauser, B.C.; Norman, R.J.; Teede, H. The management of anovulatory infertility in women with polycystic ovary syndrome: An analysis of the evidence to support the development of global WHO guidance. Hum. Reprod. Update 2016, 22, 687–708. [Google Scholar] [CrossRef]
- Dennett, C.C.; Simon, J. The role of polycystic ovary syndrome in reproductive and metabolic health: Overview and approaches for treatment. Diabetes Spectr. A Publ. Am. Diabetes Assoc. 2015, 28, 116–120. [Google Scholar] [CrossRef] [Green Version]
- Kyrou, I.; Karteris, E.; Robbins, T.; Chatha, K.; Drenos, F.; Randeva, H.S. Polycystic ovary syndrome (PCOS) and COVID-19: An overlooked female patient population at potentially higher risk during the COVID-19 pandemic. BMC Med. 2020, 18, 220. [Google Scholar] [CrossRef]
- Rosenfield, R.L. Current concepts of polycystic ovary syndrome pathogenesis. Curr. Opin. Pediatrics 2020, 32, 698–706. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Oberfield, S.E.; Stener-Victorin, E.; Marshall, J.C.; Laven, J.S.; Legro, R.S. Scientific Statement on the Diagnostic Criteria, Epidemiology, Pathophysiology, and Molecular Genetics of Polycystic Ovary Syndrome. Endocr. Rev. 2015, 36, 487–525. [Google Scholar] [CrossRef] [Green Version]
- Azziz, R. Androgen Excess Disorders in Women; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Mancini, A.; Bruno, C.; Vergani, E.; d’Abate, C.; Giacchi, E.; Silvestrini, A. Oxidative Stress and Low-Grade Inflammation in Polycystic Ovary Syndrome: Controversies and New Insights. Int. J. Mol. Sci. 2021, 22, 1667. [Google Scholar] [CrossRef]
- Khashchenko, E.; Vysokikh, M.; Uvarova, E.; Krechetova, L.; Vtorushina, V.; Ivanets, T.; Volodina, M.; Tarasova, N.; Sukhanova, I.; Sukhikh, G. Activation of Systemic Inflammation and Oxidative Stress in Adolescent Girls with Polycystic Ovary Syndrome in Combination with Metabolic Disorders and Excessive Body Weight. J. Clin. Med. 2020, 9, 1399. [Google Scholar] [CrossRef]
- Murri, M.; Luque-Ramírez, M.; Insenser, M.; Ojeda-Ojeda, M.; Escobar-Morreale, H.F. Circulating markers of oxidative stress and polycystic ovary syndrome (PCOS): A systematic review and meta-analysis. Hum. Reprod. Update 2013, 19, 268–288. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J.; Network, I.P. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum. Reprod. 2018, 33, 1602–1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.H.; Yang, H.; Lee, S.R.; Kwon, S.W.; Hong, E.J.; Lee, H.W. Welsh Onion Root (Allium fistulosum) Restores Ovarian Functions from Letrozole Induced-Polycystic Ovary Syndrome. Nutrients 2018, 10, 1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgante, G.; Massaro, M.G.; Di Sabatino, A.; Cappelli, V.; De Leo, V. Therapeutic approach for metabolic disorders and infertility in women with PCOS. Gynecol. Endocrinol. 2018, 34, 4–9. [Google Scholar] [CrossRef]
- Domecq, J.P.; Prutsky, G.; Mullan, R.J.; Sundaresh, V.; Wang, A.T.; Erwin, P.J.; Welt, C.; Ehrmann, D.; Montori, V.M.; Murad, M.H. Adverse effects of the common treatments for polycystic ovary syndrome: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2013, 98, 4646–4654. [Google Scholar] [CrossRef] [Green Version]
- Ismail, N.H.; Ibrahim, S.F.; Jaffar, F.H.F.; Mokhtar, M.H.; Chin, K.Y.; Osman, K. Augmentation of the Female Reproductive System Using Honey: A Mini Systematic Review. Molecules 2021, 26, 649. [Google Scholar] [CrossRef]
- Mohd Kamal, D.A.; Ibrahim, S.F.; Kamal, H.; Kashim, M.I.A.M.; Mokhtar, M.H. Physicochemical and Medicinal Properties of Tualang, Gelam and Kelulut Honeys: A Comprehensive Review. Nutrients 2021, 13, 197. [Google Scholar] [CrossRef]
- Ranneh, Y.; Ali, F.; Zarei, M.; Akim, A.M.; Hamid, H.A.; Khazaai, H. Malaysian stingless bee and Tualang honeys: A comparative characterization of total antioxidant capacity and phenolic profile using liquid chromatography-mass spectrometry. LWT 2018, 89, 1–9. [Google Scholar] [CrossRef]
- Abd Jalil, M.A.; Kasmuri, A.R.; Hadi, H. Stingless Bee Honey, the Natural Wound Healer: A Review. Ski. Pharmacol. Physiol. 2017, 30, 66–75. [Google Scholar] [CrossRef]
- Shamsudin, S.; Selamat, J.; Abdul Shomad, M.; Ab Aziz, M.F.; Haque Akanda, M.J. Antioxidant Properties and Characterization of Heterotrigona itama Honey from Various Botanical Origins according to Their Polyphenol Compounds. J. Food Q. 2022, 2022, 2893401. [Google Scholar] [CrossRef]
- Al-Hatamleh, M.A.I.; Boer, J.C.; Wilson, K.L.; Plebanski, M.; Mohamud, R.; Mustafa, M.Z. Antioxidant-Based Medicinal Properties of Stingless Bee Products: Recent Progress and Future Directions. Biomolecules 2020, 10, 923. [Google Scholar] [CrossRef] [PubMed]
- Karim, N. Antioxidant Properties of Stingless Bee Honey and Its Effect on the Viability of Lymphoblastoid Cell Line. Med. Health 2019, 14, 91–105. [Google Scholar] [CrossRef]
- Shamsudin, S.; Selamat, J.; Sanny, M.; A.R., S.B.; Jambari, N.N.; Khatib, A. A Comparative Characterization of Physicochemical and Antioxidants Properties of Processed Heterotrigona itama Honey from Different Origins and Classification by Chemometrics Analysis. Molecules 2019, 24, 3898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fletcher, M.T.; Hungerford, N.L.; Webber, D.; Carpinelli de Jesus, M.; Zhang, J.; Stone, I.S.J.; Blanchfield, J.T.; Zawawi, N. Stingless bee honey, a novel source of trehalulose: A biologically active disaccharide with health benefits. Sci. Rep. 2020, 10, 12128. [Google Scholar] [CrossRef]
- Budin, S.B.; Jubaidi, F.F.; Mohd Noor Azam, S.N.F.; Mohamed Yusof, N.L.; Taib, I.S.; Mohamed, J. Kelulut Honey Supplementation Prevents Sperm And Testicular Oxidative Damage In Streptozotocin-Induced Diabetic Rats. J. Teknol. 2017, 79, 3. [Google Scholar] [CrossRef] [Green Version]
- Kafali, H.; Iriadam, M.; Ozardali, I.; Demir, N. Letrozole-induced polycystic ovaries in the rat: A new model for cystic ovarian disease. Arch. Med. Res. 2004, 35, 103–108. [Google Scholar] [CrossRef]
- Mannerås, L.; Cajander, S.; Holmäng, A.; Seleskovic, Z.; Lystig, T.; Loönn, M.; Stener-Victorin, E. A New Rat Model Exhibiting Both Ovarian and Metabolic Characteristics of Polycystic Ovary Syndrome. Endocrinology 2007, 148, 3781–3791. [Google Scholar] [CrossRef] [Green Version]
- Sahlan, M.; Rahmawati, O.; Pratami, D.K.; Raffiudin, R.; Mukti, R.R.; Hermasyah, H. The Effects of stingless bee (Tetragonula biroi) honey on streptozotocin-induced diabetes mellitus in rats. Saudi J. Biol. Sci. 2020, 27, 2025–2030. [Google Scholar] [CrossRef]
- Kamal, D.A.M.; Ibrahim, S.F.; Mokhtar, M.H. Effects of Testosterone on the Expression of Connexin 26 and Connexin 43 in the Uterus of Rats During Early Pregnancy. In Vivo 2020, 34, 1863–1870. [Google Scholar] [CrossRef]
- Ajayi, A.F.; Akhigbe, R.E. Staging of the estrous cycle and induction of estrus in experimental rodents: An update. Fertil. Res. Pract. 2020, 6, 5. [Google Scholar] [CrossRef] [Green Version]
- Gozukara, I.O.; Pınar, N.; Ozcan, O.; Ozgur, T.; Dokuyucu, R.; Kurt, R.K.; Kucur, S.K.; Aksoy, A.N. Effect of colchicine on polycystic ovary syndrome: An experimental study. Arch. Gynecol. Obs. 2016, 293, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Osuka, S.; Nakanishi, N.; Murase, T.; Nakamura, T.; Goto, M.; Iwase, A.; Kikkawa, F. Animal models of polycystic ovary syndrome: A review of hormone-induced rodent models focused on hypothalamus-pituitary-ovary axis and neuropeptides. Reprod Med. Biol. 2019, 18, 151–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ndeingang, E.C.; Defo Deeh, P.B.; Watcho, P.; Kamanyi, A. Phyllanthus muellerianus (Euphorbiaceae) Restores Ovarian Functions in Letrozole-Induced Polycystic Ovarian Syndrome in Rats. Evid.-Based Complementary Altern. Med. 2019, 2019, 2965821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Dun, J.; Yang, J.; Zhang, J.; Lin, Q.; Huang, M.; Ji, F.; Huang, L.; You, X.; Lin, Y. Letrozole Rat Model Mimics Human Polycystic Ovarian Syndrome and Changes in Insulin Signal Pathways. Med. Sci. Monit. 2020, 26, e923073. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, Y.F.; Alorabi, M.; Abdelzaher, W.Y.; Toni, N.D.M.; Thabet, K.; Hegazy, A.; Bahaa, H.A.; Batiha, G.E.-S.; Welson, N.N.; Morsy, M.A.; et al. Diacerein ameliorates letrozole-induced polycystic ovarian syndrome in rats. Biomed. Pharmacother. 2022, 149, 112870. [Google Scholar] [CrossRef]
- Boudreaux, M.Y.; Talbott, E.O.; Kip, K.E.; Brooks, M.M.; Witchel, S.F. Risk of T2DM and impaired fasting glucose among PCOS subjects: Results of an 8-year follow-up. Curr. Diabetes Rep. 2006, 6, 77–83. [Google Scholar] [CrossRef]
- Flannery, C.A.; Rackow, B.; Cong, X.; Duran, E.; Selen, D.J.; Burgert, T.S. Polycystic ovary syndrome in adolescence: Impaired glucose tolerance occurs across the spectrum of BMI. Pediatr Diabetes 2013, 14, 42–49. [Google Scholar] [CrossRef] [Green Version]
- Rashid, M.R.; Nor Aripin, K.N.; Syed Mohideen, F.B.; Baharom, N.; Omar, K.; Md Taujuddin, N.M.S.; Mohd Yusof, H.H.; Addnan, F.H. The Effect of Kelulut Honey on Fasting Blood Glucose and Metabolic Parameters in Patients with Impaired Fasting Glucose. J. Nutr. Metab. 2019, 2019, 3176018. [Google Scholar] [CrossRef]
- Atangwho, I.J.; Ibeneme, C.E.; Egbung, G.E.; Ibeneme, E.; Eno, M.A.; Nwankpa, P. Effect of long-term feeding of the Obudu natural honey and table sugar-sweetened diets on obesity and pro-inflammatory biomarkers in rats. BMC Nutr. 2020, 6, 3. [Google Scholar] [CrossRef]
- Mohd Rafie, A.Z.; Syahir, A.; Wan Ahmad, W.A.N.; Mustafa, M.Z.; Mariatulqabtiah, A.R. Supplementation of Stingless Bee Honey from Heterotrigona itama Improves Antiobesity Parameters in High-Fat Diet Induced Obese Rat Model. Evid.-Based Complementary Altern. Med. 2018, 2018, 6371582. [Google Scholar] [CrossRef] [Green Version]
- Ramli, N.Z.; Chin, K.-Y.; Zarkasi, K.A.; Ahmad, F. The Beneficial Effects of Stingless Bee Honey from Heterotrigona itama against Metabolic Changes in Rats Fed with High-Carbohydrate and High-Fat Diet. Int. J. Environ. Res. Public Health 2019, 16, 4987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohaei, S.; Amani, R.; Tarrahi, M.J.; Ghasemi-Tehrani, H. The effects of curcumin supplementation on glycemic status, lipid profile and hs-CRP levels in overweight/obese women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled clinical trial. Complement. Med. 2019, 47, 102201. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; Hanson, P.; Weickert, M.O.; Franks, S. Obesity and Polycystic Ovary Syndrome: Implications for Pathogenesis and Novel Management Strategies. Clin. Med. Insights Reprod Health 2019, 13, 1179558119874042. [Google Scholar] [CrossRef] [Green Version]
- Rackow, B.W.; Brink, H.V.; Hammers, L.; Flannery, C.A.; Lujan, M.E.; Burgert, T.S. Ovarian morphology by transabdominal ultrasound correlates with reproductive and metabolic disturbance in adolescents with PCOS. J. Adolesc. Health 2018, 62, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.J.; Cook-Andersen, H. Disordered follicle development. Mol. Cell. Endocrinol. 2013, 373, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Jahan, S.; Abid, A.; Khalid, S.; Afsar, T.; Qurat Ul, A.; Shaheen, G.; Almajwal, A.; Razak, S. Therapeutic potentials of Quercetin in management of polycystic ovarian syndrome using Letrozole induced rat model: A histological and a biochemical study. J. Ovarian Res. 2018, 11, 26. [Google Scholar] [CrossRef] [Green Version]
- Rajan, R.K.; Siva Selva Kumar, M.; Balaji, B. Soy isoflavones exert beneficial effects on letrozole-induced rat polycystic ovary syndrome (PCOS) model through anti-androgenic mechanism. Pharm. Biol. 2017, 55, 242–251. [Google Scholar] [CrossRef] [Green Version]
- Mehraban, M.; Jelodar, G.; Rahmanifar, F. A combination of spearmint and flaxseed extract improved endocrine and histomorphology of ovary in experimental PCOS. J. Ovarian Res. 2020, 13, 32. [Google Scholar] [CrossRef]
- Franks, S.; Hardy, K. Androgen Action in the Ovary. Front. Endocrinol. 2018, 9, 452. [Google Scholar] [CrossRef]
- Dos Santos, A.C.; Viana, D.C.; Oliveira, G.B.; Silva, R.S.; Oliveira, M.F.; Assis-Neto, A.C. Follicular development and morphological changes in the vaginal epithelium during the estrous cycle of Galea spixii. Microsc. Res. Tech. 2017, 80, 167–176. [Google Scholar] [CrossRef]
- Rezvanfar, M.; Rezvanfar, M.; Ahmadi, A.; Saadi, H.S.; Baeeri, M.; Abdollahi, M. Mechanistic links between oxidative/nitrosative stress and tumor necrosis factor alpha in letrozole-induced murine polycystic ovary::Biochemical and pathological evidences for beneficial effect of pioglitazone. Hum. Exp. Toxicol. 2012, 31, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Arentz, S.; Abbott, J.A.; Smith, C.A.; Bensoussan, A. Herbal medicine for the management of polycystic ovary syndrome (PCOS) and associated oligo/amenorrhoea and hyperandrogenism; a review of the laboratory evidence for effects with corroborative clinical findings. BMC Complement. Altern. Med. 2014, 14, 511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arentz, S.; Smith, C.A.; Abbott, J.; Bensoussan, A. Nutritional supplements and herbal medicines for women with polycystic ovary syndrome; a systematic review and meta-analysis. BMC Complement. Altern. Med. 2017, 17, 500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaid, S.S.M.; Ruslee, S.S.; Mokhtar, M.H. Protective Roles of Honey in Reproductive Health: A Review. Molecules 2021, 26, 3322. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamal, D.A.M.; Ibrahim, S.F.; Ugusman, A.; Mokhtar, M.H. Effects of Kelulut Honey on Oestrus Cycle Regulation and Histomorphological Changes in Letrozole-Induced Polycystic Ovary Syndrome Rats: A Preliminary Study. Life 2022, 12, 890. https://doi.org/10.3390/life12060890
Kamal DAM, Ibrahim SF, Ugusman A, Mokhtar MH. Effects of Kelulut Honey on Oestrus Cycle Regulation and Histomorphological Changes in Letrozole-Induced Polycystic Ovary Syndrome Rats: A Preliminary Study. Life. 2022; 12(6):890. https://doi.org/10.3390/life12060890
Chicago/Turabian StyleKamal, Datu Agasi Mohd, Siti Fatimah Ibrahim, Azizah Ugusman, and Mohd Helmy Mokhtar. 2022. "Effects of Kelulut Honey on Oestrus Cycle Regulation and Histomorphological Changes in Letrozole-Induced Polycystic Ovary Syndrome Rats: A Preliminary Study" Life 12, no. 6: 890. https://doi.org/10.3390/life12060890
APA StyleKamal, D. A. M., Ibrahim, S. F., Ugusman, A., & Mokhtar, M. H. (2022). Effects of Kelulut Honey on Oestrus Cycle Regulation and Histomorphological Changes in Letrozole-Induced Polycystic Ovary Syndrome Rats: A Preliminary Study. Life, 12(6), 890. https://doi.org/10.3390/life12060890





