Bone Tissue Engineering through 3D Bioprinting of Bioceramic Scaffolds: A Review and Update
Abstract
:1. Introduction
2. Bioceramic 3D Printing Overview
2.1. Biomaterials
2.2. Bioceramic Scaffolds
2.3. 3D Printing Manufacturing Technologies
2.3.1. Inkjet 3D Printing Technology
2.3.2. Selective Laser Sintering Technology
2.3.3. Direct-Ink-Writing 3D Printing Technology
2.3.4. SLA Printing Technology
2.3.5. Fused Deposition Modeling (FDM)
3. Improvements in 3D Printing Technology for Preparing Bioceramic Scaffolds
3.1. Improvements in Material Components
3.1.1. Carrying Active Ingredients
3.1.2. Doping with Trace Elements
3.1.3. Surface Functional Modification
3.2. Improvement in the Material Structure
3.2.1. Optimizing the Porous Structure
3.2.2. Construction of Micro-Nano Structures
3.2.3. Constructing a Bionic Structure
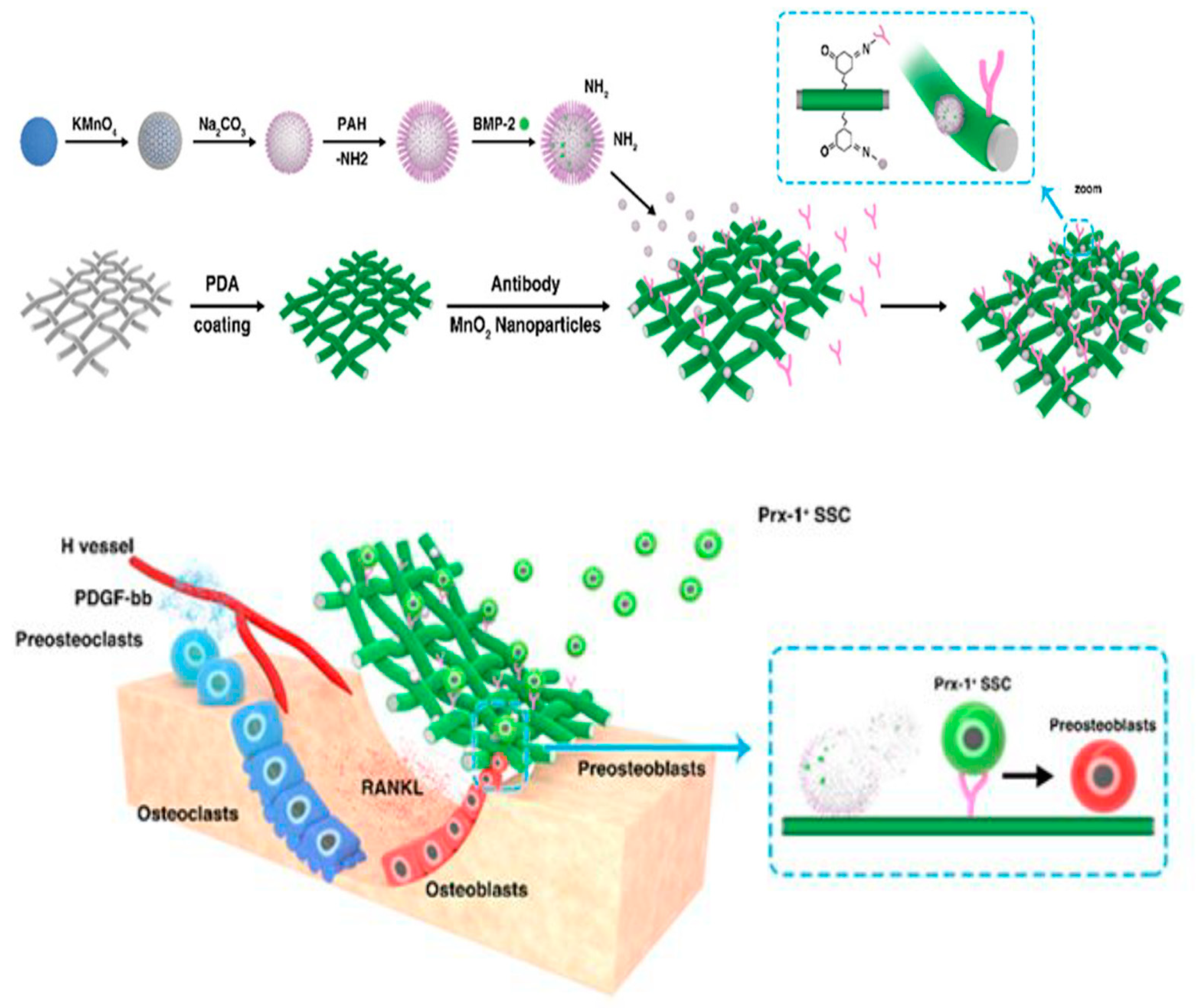
4. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ehrler, D.M.; Vaccaro, A.R. The use of allograft bone in lumbar spine surgery. Clin. Orthop. Relat. Res. 2000, 371, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Keskin, D.; Gundoğdu, C.; Atac, A.C. Experimental comparison of bovine-derived xenograft, xenograft-autologous bone marrow, and autogenous bone graft for the treatment of bony defects in the rabbit ulna. Med. Princ. Pract. 2007, 16, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18.1–18.27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- Krishna, B.V.; Bose, S.; Bandyopadhyay, A. Low stiffness porous Ti structures for load-bearing implants. Acta Biomater. 2007, 3, 997–1006. [Google Scholar] [CrossRef]
- Nagels, J.; Stokdijk, M.; Rozing, P.M. Stress shielding and bone resorption in shoulder arthroplasty. J. Shoulder Elb. Surg. 2003, 12, 35–39. [Google Scholar] [CrossRef]
- Sundelacruz, S.; Kaplan, D.L. Stem cell- and scaffold-based tissue engineering approaches to osteochondral regenerative medicine. Semin. Cell Dev. Biol. 2009, 20, 646–655. [Google Scholar] [CrossRef] [Green Version]
- Fröhlich, M.; Grayson, W.L.; Wan, L.Q.; Marolt, D.; Drobnic, M.; Vunjak-Novakovic, G. Tissue engineered bone grafts: Biological requirements, tissue culture and clinical relevance. Curr. Stem Cell Res. Ther. 2008, 3, 254–264. [Google Scholar] [CrossRef] [Green Version]
- Mobini, S.; Khanmohammadi, M.; Heidari-Vala, H.; Samadikuchaksaraei, A.; Moshiri, A.; Kazemnejad, S. Tissue Engineering and Regenerative Medicine in Iran: Current State of Research and Future Outlook. Mol. Biotechnol. 2015, 57, 589–605. [Google Scholar] [CrossRef]
- Reznikov, N.; Bilton, M.; Lari, L.; Stevens, M.M.; Kröger, R. Fractal-like hierarchical organization of bone begins at the nanoscale. Science 2018, 360, eaao2189. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Khalaf, A.T.; Ye, P.; Fan, W.; Su, J.; Chen, W.; Hu, H.; Menhas, R.; Wang, L.; Oglah, Z. Therapeutic Benefits of Pomegranate Flower Extract: A Novel Effect That Reduces Oxidative Stress and Significantly Improves Diastolic Relaxation in Hyperglycemic In Vitro in Rats. Evid.-Based Complement. Altern. Med. 2022, 2022, 4158762. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, R.; Shi, H.; Li, X.; Li, Y.; Taha, A.; Xu, C. Protective effect of curcumin against ultraviolet A irradiation-induced photoaging in human dermal fibroblasts. Mol. Med. Rep. 2018, 17, 7227–7237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowley, C.; Wong, J.M.; Fisher, D.M.; Khan, W.S. A systematic review on preclinical and clinical studies on the use of scaffolds for bone repair in skeletal defects. Curr. Stem Cell Res. Ther. 2013, 8, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Chen, H.; Zhang, H.; Guo, C.; Yang, K.; Chen, K.; Cheng, R.; Qian, N.; Sandler, N.; Zhang, Y.S.; et al. Vascularized 3D printed scaffolds for promoting bone regeneration. Biomaterials 2019, 190–191, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Deng, M. Novel Biocompatible Polymeric Blends for Bone Regeneration: Material and Matrix Design and Development; University of Virginia ProQuest Dissertations Publishing: Charlottesville, VA, USA, 2010; p. 3437483. [Google Scholar]
- Holzapfel, B.M.; Rudert, M.; Hutmacher, D.W. Gerüstträgerbasiertes Knochen-Tissue-Engineering [Scaffold-based Bone Tissue Engineering]. Orthopade 2017, 46, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Mourino, V.; Boccaccini, A.R. Bone tissue engineering therapeutics: Controlled drug delivery in three-dimensional scaffolds. J. R. Soc. Interface 2010, 7, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seitz, H.; Rieder, W.; Irsen, S.; Leukers, B.; Tille, C. Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part B 2005, 74, 782. [Google Scholar] [CrossRef]
- Jones, A.C.; Arns, C.H.; Sheppard, A.P.; Hutmacher, D.W.; Milthorpe, B.K.; Knackstedt, M.A. Assessment of bone ingrowth into porous biomaterials using MICRO-CT. Biomaterials 2007, 28, 2491. [Google Scholar] [CrossRef]
- Siddiqui, N.; Asawa, S.; Birru, B.; Baadhe, R.; Rao, S. PCL-Based Composite Scaffold Matrices for Tissue Engineering Applications. Mol. Biotechnol. 2018, 60, 506–532. [Google Scholar] [CrossRef]
- Chen, X.; Gleeson, S.E.; Yu, T.; Khan, N.; Yucha, R.W.; Marcolongo, M.; Li, C.Y. Hierarchically ordered polymer nanofiber shish kebabs as a bone scaffold material. J. Biomed. Mater. Res. Part A 2017, 105, 1786–1798. [Google Scholar] [CrossRef]
- Barba, A.; Maazouz, Y.; Diez-Escudero, A.; Rappe, K.; Espanol, M.; Montufar, E.B.; Öhman-Mägi, C.; Persson, C.; Fontecha, P.; Manzanares, M.C.; et al. Osteogenesis by foamed and 3D-printed nanostructured calcium phosphate scaffolds: Effect of pore architecture. Acta Biomater. 2018, 79, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Ouyang, L.; Xu, R.; Yang, Y.; Sun, W. Responsive biomaterials for 3D bioprinting: A review. Mater. Today 2022, 52, 112–132. [Google Scholar] [CrossRef]
- Jordana, F.; Le Visage, C.; Weiss, P. Substituts osseux [Bone substitutes]. Med. Sci. 2017, 33, 60–65. [Google Scholar]
- Khalaf, A.T.; Sun, Y.; Wang, F.; Sheng, M.; Li, Y.; Liu, X. Photodynamic Therapy Using HMME for Port-Wine Stains: Clinical Effectiveness and Sonographic Appearance. BioMed Res. Int. 2020, 2020, 6030581. [Google Scholar] [CrossRef]
- Cheng, L.; Lin, T.; Khalaf, A.T.; Zhang, Y.; He, H.; Yang, L.; Yan, S.; Zhu, J.; Shi, Z. The preparation and application of calcium phosphate biomedical composites in filling of weight-bearing bone defects. Sci. Rep. 2021, 11, 4283. [Google Scholar] [CrossRef]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as biomaterial for bone scaffolds: Review of literature. J. Oral Biol. Craniofac. Res. 2020, 10, 381–388. [Google Scholar] [CrossRef]
- Khalaf, A.T.; Wei, Y.; Alneamah, S.J.A.; Al-Shawi, S.G.; Kadir, S.Y.A.; Zainol, J.; Liu, X. What Is New in the Preventive and Therapeutic Role of Dairy Products as Nutraceuticals and Functional Foods? BioMed Res. Int. 2021, 2021, 8823222. [Google Scholar] [CrossRef]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110698. [Google Scholar] [CrossRef]
- Wang, L.; Khalaf, A.T.; Lei, D.; Gale, M.; Li, J.; Jiang, P.; Du, J.; Yinayeti, X.; Abudureheman, M.; Wei, Y. Structured oral examination as an effective assessment tool in lab-based physiology learning sessions. Adv. Physiol. Educ. 2020, 44, 453–458. [Google Scholar] [CrossRef]
- Christen, M.O.; Vercesi, F. Polycaprolactone: How a Well-Known and Futuristic Polymer Has Become an Innovative Collagen-Stimulator in Esthetics. Clin. Cosmet Investig. Dermatol. 2020, 13, 31–48. [Google Scholar] [CrossRef] [Green Version]
- Nii, T.; Katayama, Y. Biomaterial-Assisted Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 8657. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Khalaf, A.T.; Lin, T.; Ran, L.; Shi, Z.; Wan, J.; Zhou, X.; Zou, L. Exercise Promotes the Osteoinduction of HA/β-TCP Biomaterials via the Wnt Signaling Pathway. Metabolites 2020, 10, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalaf, A.T.; Wan, J. The Influence of Polysaccharide Nucleic Acid of BCG on Serum sIL-2R, IL-10 and TNF-α in Patients with Vitiligo. Basic Clin. Pharmacol. Toxicol. 2020, 126, 64–65. [Google Scholar]
- Zhao, S.; Xie, K.; Guo, Y.; Tan, J.; Wu, J.; Yang, Y.; Fu, P.; Wang, L.; Jiang, W.; Hao, Y. Fabrication and Biological Activity of 3D-Printed Polycaprolactone/Magnesium Porous Scaffolds for Critical Size Bone Defect Repair. ACS Biomater. Sci. Eng. 2020, 6, 5120–5131. [Google Scholar] [CrossRef]
- Keller, L.; Regiel-Futyra, A.; Gimeno, M.; Eap, S.; Mendoza, G.; Andreu, V.; Wagner, Q.; Kyzioł, A.; Sebastian, V.; Stochel, G.; et al. Chitosan-based nanocomposites for the repair of bone defects. Nanomedicine 2017, 13, 2231–2240. [Google Scholar] [CrossRef]
- Kozusko, S.D.; Riccio, C.; Goulart, M.; Bumgardner, J.; Jing, X.L.; Konofaos, P. Chitosan as a Bone Scaffold Biomaterial. J. Craniofac. Surg. 2018, 29, 1788–1793. [Google Scholar] [CrossRef]
- Lee, Y.H.; Lee, B.W.; Jung, Y.C.; Yoon, B.I.; Woo, H.M.; Kang, B.J. Application of alginate microbeads as a carrier of bone morphogenetic protein-2 for bone regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 286–294. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Li, R.; Bai, H.; Zhu, Z.; Zhu, L.; Zhu, C.; Che, Z.; Liu, H.; Wang, J.; et al. Collagen-based biomaterials for bone tissue engineering. Mater. Des. 2021, 210, 110049. [Google Scholar] [CrossRef]
- Yu, L.; Rowe, D.W.; Perera, I.P.; Zhang, J.; Suib, S.L.; Xin, X.; Wei, M. Intrafibrillar Mineralized Collagen-Hydroxyapatite-Based Scaffolds for Bone Regeneration. ACS Appl. Mater. Interfaces 2020, 12, 18235–18249. [Google Scholar] [CrossRef]
- Hwang, C.; Park, S.; Kang, I.G.; Kim, H.E.; Han, C.M. Tantalum-coated polylactic acid fibrous membranes for guided bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 115, 111112. [Google Scholar] [CrossRef]
- Shirzaei Sani, I.; Rezaei, M.; Baradar Khoshfetrat, A.; Razzaghi, D. Preparation and characterization of polycaprolactone/chitosan-g-polycaprolactone/hydroxyapatite electrospun nanocomposite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2021, 182, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Khalaf, A.T.; Zhang, R.; Xu, C.; Liu, X. Evolving Understanding of Palisaded Encapsulated Neuroma: An Unusual Presentation of Multiple Lesions on the Lips. Basic Clin. Pharmacol. Toxicol. 2019, 125 (Suppl. 6), 4–5. [Google Scholar]
- Khalaf, A.T.; Wan, J.; Hu, W.; Wang, J.; Liu, X.; Tang, S.; Zhang, M.; Shen, F.; Hai, T. Gene Anti-Tumor Therapy Applications to Lung Carcinoma: Adenovirus TOA2 Shows Low Toxicity and Inhibition Effects on Tumor Growth in Nude Mice. Basic Clin. Pharmacol. Toxicol. 2019, 125, 215. [Google Scholar]
- Wen, Y.; Xun, S.; Haoye, M.; Baichuan, S.; Peng, C.; Xuejian, L.; Kaihong, Z.; Xuan, Y.; Jiang, P.; Shibi, L. 3D printed porous ceramic scaffolds for bone tissue engineering: A review. Biomater. Sci. 2017, 5, 1690–1698. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Liu, A.; Shao, H.; Yang, X.; Ma, C.; Yan, S.; Liu, Y.; He, Y.; Gou, Z. Systematical Evaluation of Mechanically Strong 3D Printed Diluted Magnesium Doping Wollastonite Scaffolds on Osteogenic Capacity in Rabbit Calvarial Defects. Sci Rep. 2016, 6, 34029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauria, I.; Kramer, M.; Schröder, T.; Kant, S.; Hausmann, A.; Böke, F.; Leube, R.; Telle, R.; Fischer, H. Inkjet printed periodical micropatterns made of inert alumina ceramics induce contact guidance and stimulate osteogenic differentiation of mesenchymal stromal cells. Acta Biomater. 2016, 44, 85–96. [Google Scholar] [CrossRef]
- Mussano, F.; Genova, T.; Serra, F.G.; Carossa, M.; Munaron, L.; Carossa, S. Nano-Pore Size of Alumina Affects Osteoblastic Response. Int. J. Mol. Sci. 2018, 19, 528. [Google Scholar] [CrossRef] [Green Version]
- Hanawa, T. Zirconia versus titanium in dentistry: A review. Dent. Mater. J. 2020, 39, 24–36. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Lawn, B.R. Novel Zirconia Materials in Dentistry. J. Dent. Res. 2018, 97, 140–147. [Google Scholar] [CrossRef]
- Nommeots-Nomm, A.; Lee Peter, D.; Jones Julian, R. Direct ink writing of highly bioactive glasses. J. Eur. Ceram. Soc. 2018, 38, 837–844. [Google Scholar] [CrossRef] [Green Version]
- Qi, X.; Wang, H.; Zhang, Y.; Pang, L.; Xiao, W.; Jia, W.; Zhao, S.; Wang, D.; Huang, W.; Wang, Q. Mesoporous bioactive glass-coated 3D printed borosilicate bioactive glass scaffolds for improving repair of bone defects. Int. J. Biol. Sci. 2018, 14, 471–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, L.; Engqvist, H.; Xia, W. Glass-Ceramics in Dentistry: A Review. Materials 2020, 13, 1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, G.; Kumar, V.; Baino, F.; Mauro, J.C.; Pickrell, G.; Evans, I.; Bretcanu, O. Mechanical properties of bioactive glasses, ceramics, glass-ceramics, and composites: State-of-the-art review and future challenges. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109895. [Google Scholar] [CrossRef]
- Maciel, P.P.; Pessôa, J.A.M.; de Medeiros, E.L.G.; Batista, A.U.D.; Fernandes Figueiredo, L.R.; de Medeiros, E.S.; de Oliveira Duarte, D.F.; Alves, A.F. Use of strontium doping glass-ceramic material for bone regeneration in critical defect: In vitro and in vivo analyses. Ceram. Int. 2020, 46, 24940–24954. [Google Scholar] [CrossRef]
- Buss, D.J.; Kröger, R.; McKee, M.D.; Reznikov, N. Hierarchical organization of bone in three dimensions: A twist of twists. J. Struct. Biol. X 2021, 6, 100057. [Google Scholar] [CrossRef]
- Mahdy, E.A.; Sahbal, K.M.; Mabrouk, M.; Beherei, H.H.; Abdel-Monem, Y.K. Enhancement of glass-ceramic performance by TiO2 doping: In vitro cell viability, proliferation, and differentiation. Ceram. Int. 2021, 47, 6251–6261. [Google Scholar] [CrossRef]
- Beig, B.; Liaqat, U.; Niazi, M.F.K.; Douna, I.; Zahoor, M.; Niazi, M.B.K. Current Challenges and Innovative Developments in Hydroxyapatite-Based Coatings on Metallic Materials for Bone Implantation: A Review. Coatings 2020, 10, 1249. [Google Scholar] [CrossRef]
- Arcos, D.; Vallet-Regí, M. Substituted hydroxyapatite coatings of bone implants. J. Mater. Chem. B 2020, 8, 1781–1800. [Google Scholar] [CrossRef]
- Yeo, T.; Ko Y-Gwang Kim, E.J.; Kwon, O.K.; Chung, H.Y.; Kwon, O.H. Promoting bone regeneration by 3D-printed poly (glycolic acid)/hydroxyapatite composite scaffolds. J. Ind. Eng. Chem. 2020, 94, 343–351. [Google Scholar] [CrossRef]
- Parent, M.; Baradari, H.; Champion, E.; Damia, C.; Viana-Trecant, M. Design of calcium phosphate ceramics for drug delivery applications in bone diseases: A review of the parameters affecting the loading and release of the therapeutic substance. J. Control. Release 2017, 252, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Montelongo, S.A.; Chiou, G.; Ong, J.L.; Bizios, R.; Guda, T. Development of bioinks for 3D printing microporous, sintered calcium phosphate scaffolds. J. Mater. Sci. Mater. Med. 2021, 32, 94. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, A.T.; Wan, J.; Al-Jashamy, K.; Kadir, S.Y.A.; Zainol, J.; Doustjalali, S.R.; Sabet, N.S.; Aung, M.K.; Arafeh, Y.H.; Shin, J.L.X.; et al. Nicotine Replacement Therapy and Electronic Cigarettes: Awareness among Medical Students. J. Pharm. Res. Int. 2019, 31, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Woodbine, L.; Carr, A.M.; Pillai, A.R.; Nokhodchi, A.; Maniruzzaman, M. 3D Printed Calcium Phosphate Cement (CPC) Scaffolds for Anti-Cancer Drug Delivery. Pharmaceutics 2020, 12, 1077. [Google Scholar] [CrossRef] [PubMed]
- Baiguera, S.; del Gaudio, C.; di Nardo, P.; Manzari, V.; Carotenuto, F.; Teodori, L. 3D Printing Decellularized Extracellular Matrix to Design Biomimetic Scaffolds for Skeletal Muscle Tissue Engineering. BioMed Res. Int. 2020, 2020, 2689701. [Google Scholar] [CrossRef]
- Khalaf, A.T.; Liu, J.; Wang, Y.; Samiah, A.K.; Zainol, J.; Doustjalali, S.R.; Sabet, N.S.; Appalanaidu, V.; Al-Jashamy, K.; Bhuiyan, M.; et al. Emotional and Social Wellbeing in Middle and Primary Schools: Hubei, China. Res. J. Med. Sci. 2017, 11, 138–142. [Google Scholar]
- Khalaf, A.T.; Feng, L.L.; Samiah, A.K.; Zainol, J.; Doustjalali, S.R.; Sabet, N.S.; Appalanaidu, V.; Al-Jashamy, K.; Muftah, A.E. Integrative and Holistic Approach for Immunological Disorders using Electroacupuncture. Int. J. Appl. Bus. Econ. Res. 2017, 15, 21. [Google Scholar]
- Khalaf, T.; Song, J.Q.; Gao, T.T.; Yu, X.P.; Lei, T.C. CTLA-4 gene polymorphism and the risk of systemic lupus erythematosus in the Chinese population. J. Biomed. Biotechnol. 2011, 2011, 167395. [Google Scholar] [CrossRef] [Green Version]
- Asa’ad, F.; Pagni, G.; Pilipchuk, S.P.; Giannì, A.B.; Giannobile, W.V.; Rasperini, G. 3D-Printed Scaffolds and Biomaterials: Review of Alveolar Bone Augmentation and Periodontal Regeneration Applications. Int. J. Dent. 2016, 2016, 1239842. [Google Scholar] [CrossRef] [Green Version]
- Romanazzo, S.; Molley, T.G.; Nemec, S.; Lin, K.; Sheikh, R.; Gooding, J.J.; Wan, B.; Li, Q.; Kilian, K.A.; Roohani, I. Synthetic bone-like structures through omnidirectional ceramic bioprinting in cell suspensions. Adv. Funct. Mater. 2021, 31, 2008216. [Google Scholar] [CrossRef]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Adv. Mater. Sci. Eng. 2019, 2019, 3429527. [Google Scholar] [CrossRef] [Green Version]
- Khalaf, A.T.; Liu, X.M.; Sheng, W.X.; Tan, J.Q.; Abdalla, A.N. Efficacy and safety of desloratadine combined with dipyridamole in the treatment of chronic urticarial. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, A.T.; Li, W.; Jinquan, T. Current advances in the management of urticarial. Arch. Immunol. Ther. Exp. 2008, 56, 103. [Google Scholar] [CrossRef]
- Yousefi, A.; James, P.F.; Akbarzadeh, R.; Subramanian, A.; Flavin, C.; Oudadesse, H. Prospect of Stem Cells in Bone Tissue Engineering: A Review. Stem Cells 2016, 2016, 6180487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drummer, D.; Rietzel, D.; Kühnlein, F. Development of a characterization approach for the sintering behavior of new thermoplastics for selective laser sintering. Phys. Procedia 2010, 5, 533–542. [Google Scholar] [CrossRef] [Green Version]
- Shahzad, K.; Deckers, J.; Zhang, Z.; Kruth, J.-P.; Vleugels, J. Additive manufacturing of zirconia parts by indirect selective laser sintering. J. Eur. Ceram. Soc. 2014, 34, 81–89. [Google Scholar] [CrossRef]
- Hwa, L.C.; Rajoo, S.; Noor, A.M.; Ahmad, N.; Uday, M.B. Recent advances in 3D printing of porous ceramics: A review. Curr. Opin. Solid State Mater. Sci. 2017, 21, 323–347. [Google Scholar] [CrossRef]
- Gogolewski, D.; Kozior, T.; Zmarzły, P.; Mathia, T.G. Morphology of Models Manufactured by SLM Technology and the Ti6Al4V Titanium Alloy Designed for Medical Applications. Materials 2021, 14, 6249. [Google Scholar] [CrossRef]
- Yang, J.W.; Liu, Q.; Yue, Z.G.; Hou, J.X.; Afrashtehfar, K.I. Digital Workflow for Full-Arch Immediate Implant Placement Using a Stackable Surgical Guide Fabricated Using SLM Technology. J. Prosthodont. 2021, 30, 645–650. [Google Scholar] [CrossRef]
- Lewis, J.A. Direct Ink writing of 3D functional materials. Adv. Funct. Mater. 2006, 16, 2193–2204. [Google Scholar] [CrossRef]
- Lewis, J.A. Direct-write assembly of ceramics from colloidal inks. Curr. Opin. Solid State Mater. Sci. 2002, 6, 245–250. [Google Scholar] [CrossRef]
- Feilden, E.; Blanca, E.G.-T.; Giuliani, F.; Saiz, E.; Vandeperre, L. Robocasting of structural ceramic parts with hydrogel inks. J. Eur. Ceram. Soc. 2016, 36, 2525–2533. [Google Scholar] [CrossRef]
- Pfaffinger, M.; Mitteramskogler, G.; Gmeiner, R.; Stampfl, J. Thermal debinding of ceramic-filled photopolymers. Mater. Sci. Forum 2015, 825–826, 75–81. [Google Scholar] [CrossRef]
- Griffith, M.L.; Halloran, J.W. Freeform fabrication of ceramics via stereolithography. J. Am. Ceram. Soc. 1996, 79, 2601–2608. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.H.; Padzil, F.N.B.M.; Lee, S.H.; Ainun, Z.M.A.; Abdullah, L.C. Potential for Natural Fiber Reinforcement in PLA Polymer Filaments for Fused Deposition Modeling (FDM) Additive Manufacturing: A Review. Polymers 2021, 13, 1407. [Google Scholar] [CrossRef]
- Pervaiz, S.; Qureshi, T.A.; Kashwani, G.; Kannan, S. 3D Printing of Fiber-Reinforced Plastic Composites Using Fused Deposition Modeling: A Status Review. Materials 2021, 14, 4520. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, C.; Chang, J. Bioceramics to regulate stem cells and their microenvironment for tissue regeneration. Mater. Today 2019, 24, 41–56. [Google Scholar] [CrossRef]
- Katagiri, T.; Watabe, T. Bone morphogenetic proteins. Cold Spring Harb. Perspect. Biol. 2016, 8, a021899. [Google Scholar] [CrossRef] [Green Version]
- Ishack, S.; Mediero, A.; Wilder, T.; Ricci, J.L.; Cronstein, B.N. Bone regeneration in critical bone defects using three–dimensionally printed beta-tricalcium phosphate/hydroxyapatite scaffolds is enhanced by coating scaffolds with either dipyridamole or BMP-2. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 366–375. [Google Scholar] [CrossRef] [Green Version]
- Lai, Y.; Cao, H.; Wang, X.; Chen, S.; Zhang, M.; Wang, N.; Yao, Z.; Dai, Y.; Xie, X.; Zhang, P.; et al. Porous composite scaffold incorporating osteogenic phytomolecule icariin for promoting skeletal regeneration in challenging osteonecrotic bone in rabbits. Biomaterials 2018, 153, 1–13. [Google Scholar] [CrossRef]
- Sun, H.; Hu, C.; Zhou, C.; Wu, L.; Sun, J.; Zhou, X.; Xing, F.; Long, C.; Kong, Q.; Liang, J.; et al. 3D printing of calcium phosphate scaffolds with controlled release of antibacterial functions for jaw bone repair. Mater. Des. 2020, 189, 108540. [Google Scholar] [CrossRef]
- García-Alvarez, R.; Izquierdo-Barba, I.; Vallet-Regí, M. 3D scaffold with effective multidrug sequential release against bacteria biofilm. Acta Biomater. 2017, 49, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The essential metals for humans: A brief overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.S.B.; Harwood, A.J. Lithium therapy and signal transduction. Trends Pharmacol. Sci. 2000, 21, 61–64. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, S.; Wu, C.; Lu, P.; Hu, C.; Xiong, S.; Chang, J.; Heng, B.C.; Xiao, Y.; Ouyang, H.W. A BilLineage conducive scaffold for osteochondral defect regeneration. Adv. Funct. Mater. 2014, 24, 4473–4483. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y.; Hao, J.; Ma, B.; Di, T.; Dong, H. Evaluation of the degradation, biocompatibility and osteogenesis behavior of lithium-doped calcium polyphosphate for bone tissue engineering. Biomed. Mater. Eng. 2019, 30, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Zhai, D.; Chen, L.; Chen, Y.; Zhu, Y.; Xiao, Y.; Wu, C. Lithium silicate-based bioceramics promoting chondrocyte maturation by immunomodulating M2 macrophage polarization. Biomater. Sci. 2020, 8, 4521–4534. [Google Scholar] [CrossRef]
- Kargozar, S.; Montazerian, M.; Fiume, E.; Baino, F. Multiple and promising applications of strontium (Sr)-containing bioactive glasses in bone tissue engineering. Front. Bioeng. Biotechnol. 2019, 7, 161. [Google Scholar] [CrossRef] [Green Version]
- Fuleihan, G.E.-H. Strontium ranelate—A novel therapy for osteoporosis or a permutation of the same? N. Engl. J. Med. 2004, 350, 504–506. [Google Scholar] [CrossRef]
- Zeng, J.; Guo, J.; Sun, Z.; Deng, F.; Ning, C.; Xie, Y. Osteoblastic and anti-osteoclastic activities of strontium-substituted silicocarnotite ceramics: In vitro and in vivo studies. Bioact. Mater. 2020, 5, 435–446. [Google Scholar] [CrossRef]
- Lin, K.; Xia, L.; Li, H.; Jiang, X.; Pan, H.; Xu, Y.; Lu, W.W.; Zhang, Z.; Chang, J. Enhanced osteoporotic bone regeneration by strontium-substituted calcium silicate bioactive ceramics. Biomaterials 2013, 34, 10028–10042. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, H.; Lin, C.; Ning, C.; Lin, K. Synergetic topography and chemistry cues guiding osteogenic differentiation in bone marrow stromal cells through ERK1/2 and p38 MAPK signaling pathway. Biomater. Sci. 2018, 6, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Pierantozzi, D.; Scalzone, A.; Jindal, S.; Stīpniece, L.; Šalma-Ancāne, K.; Dalgarno, K.; Gentile, P.; Mancuso, E. 3D printed Sr-containing composite scaffolds: Effect of structural design and material formulation towards new strategies for bone tissue engineering. Compos. Sci. Technol. 2020, 191, 108069. [Google Scholar] [CrossRef]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. Trace elements in human physiology and pathology. Copper. Biomed. Pharmacother. 2003, 57, 386–398. [Google Scholar] [CrossRef]
- Ai, F.; Chen, L.; Yan, J.; Yang, K.; Li, S.; Duan, H.; Cao, C.; Li, W.; Zhou, K. Hydroxyapatite scaffolds containing copper for bone tissue engineering. J. Sol-Gel Sci. Technol. 2020, 95, 168–179. [Google Scholar] [CrossRef]
- Baino, F. Copper-doped Ordered Mesoporous Bioactive Glass: A Promising Multifunctional Platform for Bone Tissue Engineering. Bioengineering 2020, 7, 45. [Google Scholar] [CrossRef]
- Lin, R.; Deng, C.; Li, X.; Liu, Y.; Zhang, M.; Qin, C.; Yao, Q.; Wang, L.; Wu, C. Copper-incorporated bioactive glass-ceramics inducing anti-inflammatory phenotype and regeneration of cartilage/bone interface. Theranostics 2019, 9, 6300–6313. [Google Scholar] [CrossRef]
- Liu, Y.; Li, T.; Ma, H.; Zhai, D.; Deng, C.; Wang, J.; Zhuo, S.; Chang, J.; Wu, C. 3D-printed scaffolds with bioactive elements-induced photothermal effect for bone tumor therapy. Acta Biomater. 2018, 73, 531–546. [Google Scholar] [CrossRef]
- Abu-Amer, Y.; Darwech, I.; Clohisy, J.C. Aseptic loosening of total joint replacements: Mechanisms underlying osteolysis and potential therapies. Arthritis Res. Ther. 2007, 9, S6. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Yi, D.; Zheng, X.; Chang, J.; Wu, C.; Xiao, Y. Nutrient element–based bioceramic coatings on titanium alloy stimulating osteogenesis by inducing beneficial osteoimmmunomodulation. J. Mater. Chem. B 2014, 2, 6030–6043. [Google Scholar] [CrossRef] [Green Version]
- Yuehua, Y.; Khalaf, A.T.; Xiaoxang, Z.; Xinggang, W. Narrow-band ultraviolet and convention UVB phototherapy in psoriasis: A randomized controlled trial. Am. J. Appl. Sci. 2008, 5, 905–908. [Google Scholar]
- Su, J.; Du, Z.; Xiao, L.; Wei, F.; Yang, Y.; Li, M.; Qiu, Y.; Liu, J.; Chen, J.; Xiao, Y. Graphene oxide coated titanium surfaces with osteoimmunomodulatory role to enhance osteogenesis. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 113, 110983. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, S.; Shafieyan, Y.; Mirzadeh, H.; Bagheri-Khoulenjani, S.; Rabiee, S.M.; Imani, M.; Atai, M.; Shokrgozar, M.A.; Hatampoor, A. Hydroxyapatite scaffolds infiltrated with thermally crosslinked polycaprolactone fumarate and polycaprolactone itaconate. J. Biomed. Mater. Res. A 2011, 98, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xia, L.; Zhai, D.; Shi, M.; Luo, Y.; Feng, C.; Fang, B.; Yin, J.; Chang, J.; Wu, C. Mesoporous bioactive glass nanolayer-functionalized 3D-printed scaffolds for accelerating osteogenesis and angiogenesis. Nanoscale 2015, 7, 19207–19221. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhai, D.; Xu, M.; Yao, Q.; Zhu, H.; Chang, J.; Wu, C. 3D-printed bioceramic scaffolds with antibacterial and osteogenic activity. Biofabrication 2017, 9, 025037. [Google Scholar] [CrossRef]
- Ma, H.; Jiang, C.; Zhai, D.; Luo, Y.; Chen, Y.; Lv, F.; Yi, Z.; Deng, Y.; Wang, J.; Chang, J.; et al. A Bifunctional biomaterial with photothermal effect for tumor therapy and bone regeneration. Adv. Funct. Mater. 2016, 26, 1197–1208. [Google Scholar] [CrossRef]
- Ma, H.; Luo, J.; Sun, Z.; Xia, L.; Shi, M.; Liu, M.; Chang, J.; Wu, C. 3D printing of biomaterials with mussel-inspired nanostructures for tumor therapy and tissue regeneration. Biomaterials 2016, 111, 138–148. [Google Scholar] [CrossRef]
- Berman, B. 3-D printing: The new industrial revolution. Bus Horiz. 2012, 55, 155–162. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Med. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef] [Green Version]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Bobbert, F.S.L.; Zadpoor, A.A. Effects of bone substitute architecture and surface properties on cell response, angiogenesis, and structure of new bone. J. Mater. Chem. B 2017, 5, 6175–6192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Jinkang, Z.; Zhen, W.; Jianxi, L.; Jiang, C.; Jian, L.; Guolin, M.; Xin, D. The effect of pore size on tissue ingrowth and neovascularization in porous bioceramics of controlled architecture in vivo. Biomed. Mater. 2011, 6, 015007. [Google Scholar] [CrossRef] [PubMed]
- Tsuruga, E.; Takita, H.; Itoh, H.; Wakisaka, Y.; Kuboki, Y. Pore size of porous hydroxyapatite as the cell-substratum controls BMP-induced osteogenesis. J. Biochem. 1997, 121, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Entezari, A.; Roohani, I.; Li, G.; Dunstan, C.R.; Rognon, P.; Li, Q.; Jiang, X.; Zreiqat, H. Architectural design of 3D printed scaffolds controls the volume and functionality of newly formed bone. Adv. Healthc Mater. 2019, 8, e1801353. [Google Scholar] [CrossRef] [Green Version]
- Rumpler, M.; Woesz, A.; Dunlop, J.W.; van Dongen, J.T.; Fratzl, P. The effect of geometry on three-dimensional tissue growth. J. R. Soc. Interface 2008, 5, 1173–1180. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, L.; Fan, Y.; Feng, Q.; Cui, F.Z.; Watari, F. Nanostructured scaffolds for bone tissue engineering. J. Biomed. Mater. Res. A 2013, 101, 2424–2435. [Google Scholar] [CrossRef]
- Pan, H.; Xie, Y.; Zhang, Z.; Li, K.; Hu, D.; Zheng, X.; Tang, T. Hierarchical macropore/nano surface regulates stem cell fate through a ROCK-related signaling pathway. RSC Adv. 2017, 7, 8521–8532. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.J.; Khalaf, A.T.; Liu, X.M.; Xu, C.X.; Zhao, W.; Cheng, S.; Zhang, R.Z. Zinc finger A20 and NF-κB correlate with high-risk human papillomavirus of squamous cell carcinoma patients. Tumour Biol. 2014, 35, 11855–11860. [Google Scholar] [CrossRef]
- Pan, H.; Xie, Y.; Zhang, Z.; Li, K.; Hu, D.; Zheng, X.; Fan, Q.; Tang, T. YAP-mediated mechanotransduction regulates osteogenic and adipogenic differentiation of BMSCs onhierarchical structure. Colloids Surf. B Biointerfaces 2017, 152, 344–353. [Google Scholar] [CrossRef]
- Jiquan, S.; Khalaf, A.T.; Jinquan, T.; Xiaoming, L. Necrobiosis lipoidica: A case with histopathological findings revealed asteroid bodies and was successfully treated with dipyridamole plus intralesional triamcinolone. J. Dermatolog. Treat. 2008, 19, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Guo, T.; Yang, F.; Feng, G.; Shi, F.; Li, J.; Wang, D.; Duan, K.; Weng, J. In situ formation of nanostructured calcium phosphate coatings on porous hydroxyapatite scaffolds using a hydrothermal method and the effect on mesenchymal stem cell behavior. Ceram. Int. 2017, 43, 1588–1596. [Google Scholar] [CrossRef]
- Elrayah, A.; Zhi, W.; Feng, S.; Al-Ezzi, S.; Lei, H.; Weng, J. Preparation of micro/nano-structure copper-substituted hydroxyapatite scaffolds with improved angiogenesis capacity for bone regeneration. Materials 2018, 11, 1516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, L.; Lin, K.; Jiang, X.; Xu, Y.; Zhang, M.; Chang, J.; Zhang, Z. Enhanced osteogenesis through nano-structured surface design of macroporous hydroxyapatite bioceramic scaffolds via activation of ERK and p38 MAPK signaling pathways. J. Mater. Chem. B 2013, 1, 5403–5416. [Google Scholar] [CrossRef]
- Deng, C.; Lin, R.; Zhang, M.; Qin, C.; Yao, Q.; Wang, L.; Chang, J.; Wu, C. Micro/nanometer–structured scaffolds for regeneration of both cartilage and subchondral bone. Adv. Func. Mater. 2019, 29, 1806068. [Google Scholar] [CrossRef]
- Bar-Cohen, Y. Biomimetics: Biologically Inspired Technologies; Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2006. [Google Scholar]
- Cohen, Y.H.; Reich, Y. Biomimetic Design Method for Innovation and Sustainability; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Li, H.; Wang, H.; Pan, J.; Li, J.; Zhang, K.; Duan, W.; Liang, H.; Chen, K.; Geng, D.; Shi, Q.; et al. Nanoscaled Bionic Periosteum Orchestrating the Osteogenic Microenvironment for Sequential Bone Regeneration. ACS Appl. Mater. Interfaces 2020, 12, 36823–36836. [Google Scholar] [CrossRef]
- Barthlott, C.N.W. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Zheng, X.; Chen, Z.; Zhou, Q.; Chen, Y. 3D-printed biomimetic super-hydrophobic structure for microdroplet manipulation and oil/water separation. Adv. Mater. 2018, 30, 1704912. [Google Scholar] [CrossRef]
- Munch, E.; Launey, M.E.; Alsem, D.H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Tough, bio-inspired hybrid materials. Science 2008, 322, 1516–1520. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Li, X.; Chu, M.; Sun, H.; Jin, J.; Yu, K.; Wang, Q.; Zhou, Q.; Chen, Y. Electrically assisted 3D printing of nacre-inspired structures with self-sensing capability. Sci. Adv. 2019, 5, eaau9490. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Lin, R.; Wang, X.; Xue, J.; Deng, C.; Feng, C.; Zhuang, H.; Ma, J.; Qin, C.; Wan, L.; et al. 3D printing of Haversianbone-mimicking scaffolds for multicellular delivery in bone regeneration. Sci. Adv. 2020, 6, eaaz6725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, C.; Zhang, W.; Deng, C.; Li, G.; Chang, J.; Zhang, Z.; Jiang, X.; Wu, C. 3D Printing of lotus root-like biomimetic materials for cell delivery and tissue regeneration. Adv. Sci. 2017, 4, 1700401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Feng, C.; Yang, G.; Li, G.; Ding, X.; Wang, S.; Dou, Y.; Zhang, Z.; Chang, J.; Wu, C.; et al. 3D-printed scaffolds with synergistic effect of hollow-pipe structure and bioactive ions for vascularized bone regeneration. Biomaterials 2017, 135, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhai, D.; Ma, B.; Xue, J.; Zhao, P.; Chang, J.; Gelinsky, M.; Wu, C. 3D Printing of hot dog-like biomaterials with hierarchical architecture and distinct bioactivity. Adv. Sci. 2019, 6, 1901146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef]
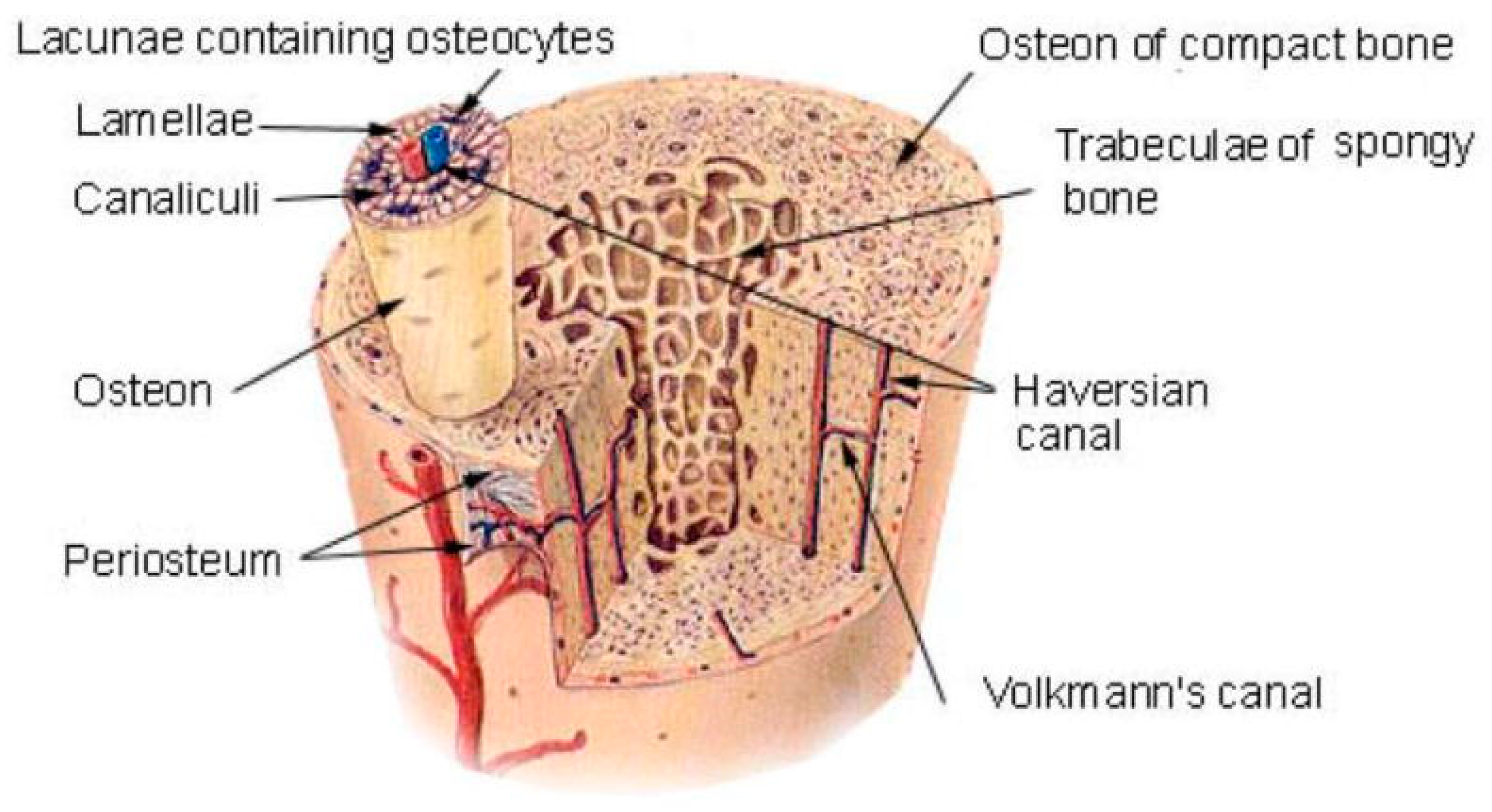

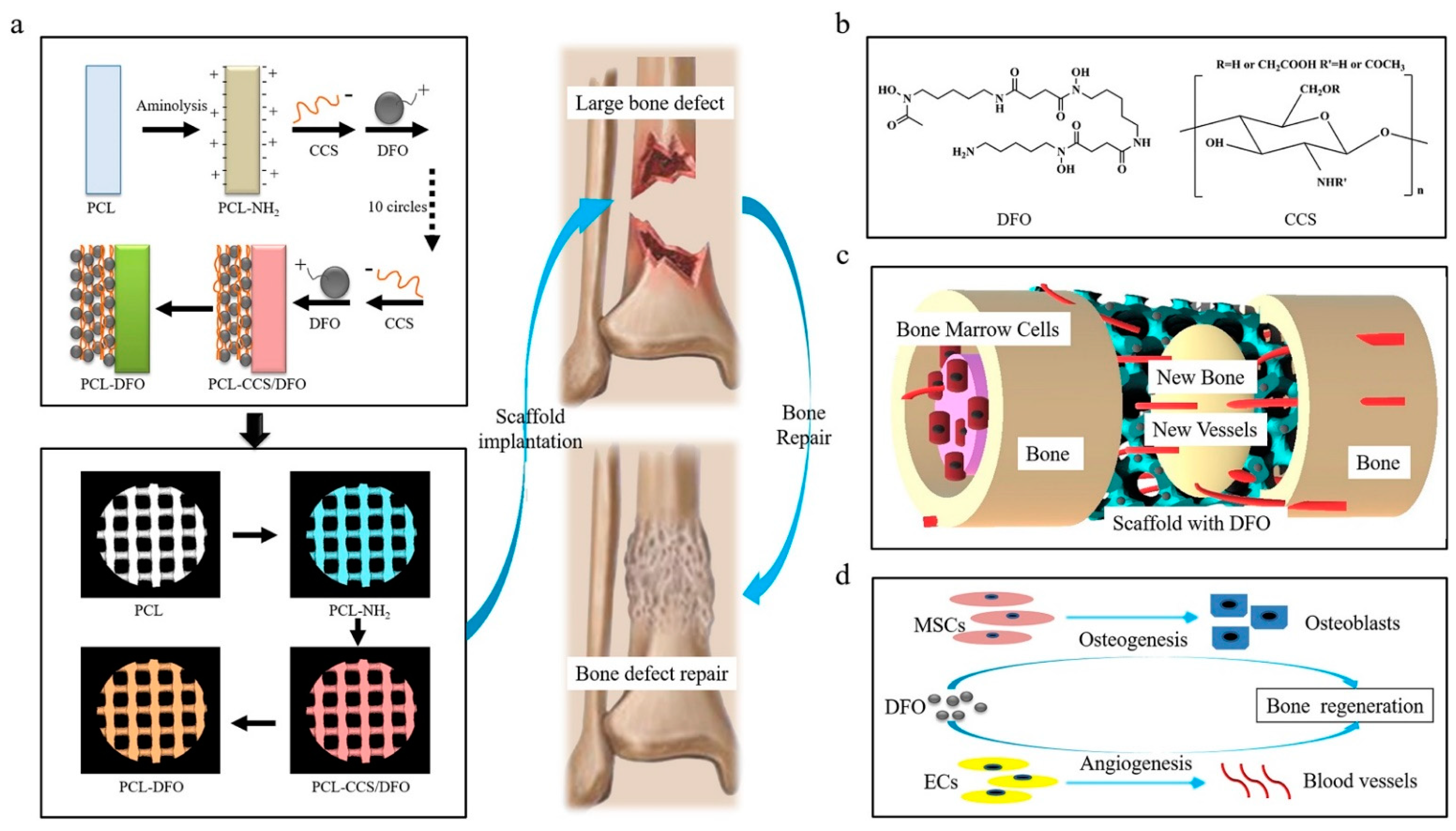
| Types | Biomaterials | Advantages | Disadvantages | Composite Materials | In Vitro Study | In Vivo Study | Reference |
|---|---|---|---|---|---|---|---|
| Natural polymer materials | Chitosan | Excellent biocompatibility, osteogenic potential, compatibility, cytocompatibility. | Strong biodegradability, fast degradation speed, easy to deform | Chitosan-based SiO2 nanocomposites | Human osteoblasts (HOBs)s were used to detect cell adhesion and proliferation of scaffolds. | Scaffolds were implanted in nude mice to verify osteogenesis and vascularization. | [36,37] |
| Alginate | Excellent biocompatibility, biodegradability, hydrophilicity, and low cost can be shaped. | Poor bioactivity, antioxidant, mechanical strength, and bone conductivity. | Alginate microbeads (AM) loaded with BMP-2. | Active expression of ALP in mesenchymal stem cells was used to examine the release of alginate microbeads carrier BMP-2. | Skull defect model rats and mice were injected subcutaneously to verify the higher osteogenic efficiency of alginate microbeads carrier BMP-2. | [38] | |
| Collagen | Excellent biocompatibility and biodegradability; easily degrades and strong plasticity and low immunogenicity. | Fast degradation rate and poor mechanical properties | Mineralized collagen-hydroxyapatite-based scaffolds | Mouse calvarial 3T3 (MC3T3) cells were used to examine the in vitro cytocompatibility of various scaffolds. Osteogenic differentiation with fluorescent multi reporter mice BMSCs. | A mouse skull defect model was used to observe the bone regeneration ability of different scaffolds in vivo. | [39,40] | |
| Artificial synthetic materials | Polylactic acid | Good biodegradability, biocompatibility, and processability; high mechanical strength. | Slow degradation rate, poor osteoconductivity. | Tantalum-coated polylactic acid fibrous membranes. | Preosteoblast cell lines (MC3T3-E1) were used to verify the biocompatibility of Ta-PLA electrospun membranes. | Rabbits with cylindrical skull defects were used to examine the osteogenic effect of Ta-PLA electrospun membranes. | [41] |
| Polycaprolactone (PCL) | Good biocompatibility, biodegradability, and processability. | Poor bioactivity, Slow degradation rate, and long degradation cycle. | Polycaprolactone/ chitosan-g-polycaprolactone/hydroxyapatite electrospun nanocomposite scaffolds. | NIH 3T3 fibroblast cells and MG-63 cells were used to study the in vitro cytocompatibility of nanocomposite scaffolds. | PCL implantation in bone defect mice can promote bone defect repair with good cellular compatibility. | [42,35] |
| Bioceramic Materials | Characteristic | Advantages | Disadvantages | Products | Reference |
|---|---|---|---|---|---|
| Alumina | Alumina is an inert ceramic material with good chemical stability and high mechanical strength. Abundant raw materials, low price, wide use, high mechanical strength, pressure resistance, high-temperature resistance, corrosion resistance, high-temperature insulation, and excellent dielectric properties. | Stability, biocompatibility, and excellent wear resistance, non-cytotoxic. | Limited strength, low mechanical properties. | Inert alumina ceramics, nanoporous alumina. | [47,48,49] |
| Zirconia | Similar to inkjet 3D printing, a liquid binder is used to bind the powder together and then the support layer is printed layer by layer, finally, the powder printing stand is melted directly. High mechanical strength, high strength, high toughness, high hardness, excellent chemical corrosion and wear resistance, low thermal conductivity, good insulation, and self-lubrication. | Fracture resistance and flexural strength characteristics. | Micro-cracks or inducing a phase transformation (grind or sandblasting dental treatment), Chemical aging, and wear. | Yttria-stabilized tetragonal zirconia polycrystalline (Y-TZP), zirconias versus silica-based ceramics. | [50,51] |
| Bioactive glass | Bioactive glass exhibits uniform interconnected macro-pores, high porosity, and high compressive strength. It can promote the expression of osteogenic genes in human bone marrow stromal cells. High biological activity, osteogenesis, osteoinduction, good combination with bone and soft tissue, and many functions. | Good bioactivity, biocompatibility, and no cytotoxicity promote bone and soft tissue regeneration. | Poor mechanical strength and intrinsic brittleness. | Bioactive glass ink; bioactive borosilicate glass (BG) scaffolds. | [52,53] |
| Glass-ceramics | Glass-ceramics are mainly composed of ~70 vol % of interlocked rod-like lithium disilicate crystals with high compressive strength. High mechanical strength, adjustable thermal expansion, chemical corrosion resistance, and wide application. | It has sufficient strength and chemical stability, with outstanding aesthetics, transparency, as well as low thermal conductivity with adequate strength. In addition to biocompatibility, corrosion resistance, and chemical durability. | The production process is complicated and high cost. | Strontium doping glass-ceramic material, TiO2-containing glass-ceramics. | [54,55] |
| Hydroxyapatite | Principal inorganic component of human or animal bones and teeth. | Good biocompatibility, bioactivity, and bone conductivity. | The degradation rate is slow, has a poor bone induction effect, and has high brittleness. | Hydroxyapatite coatings, poly (glycolic acid)/hydroxyapatite composite scaffolds. | [56,57,58] |
| Calcium phosphates | Similar in composition to bone minerals, the most widely used synthetic bone substitutes. | Excellent biocompatibility, bioactivity, bone conductivity, and absorbability. | Low compressive strength, no toughness, slow degradation. | Beta-tricalcium phosphate (β-TCP)-based bioinks, 3D printed calcium phosphate cement (CPC). | [59,60,61] |
| 3D Printing Technologies | Principle | Advantages | Disadvantages | Reference |
|---|---|---|---|---|
| Inkjet 3D printing technology | The print head sprays an adhesive over a specific area to bind the powder material together, then accumulates layer by layer to form the final scaffold frame. | Low cost, a wide range of applications, printing does not require additional support. | The mechanical properties of the scaffold are low, the surface is very rough, and poor printing accuracy. | [9,10,11,12] |
| Selective laser sintering technology | Similar to inkjet 3D printing, a liquid binder is used to bind the powder together and then the support layer is printed layer by layer, finally, the powder printing stand is melted directly. | No additional support is required, printed metal material. | High cost, low efficiency, the rough surface of the scaffold, low resolution, and long printing time. | [13,14,15] |
| Ink direct writing 3D printing technology | The mobile print head directly extrudes the printing ink layer by layer to build a three-dimensional scaffold. | Fast printing speed, easy operation, low cost, good printing accuracy, widely used. | Low printing accuracy, additional support is needed to assist with printing, sag and deformation may occur. | [16,17,18] |
| SLA printing technology | The 3D scaffold is printed layer by layer through photoinduced polymerization of photosensitive resin. | High accuracy allows printing of scaffolds with complex porous structures and very high resolution. | Need additional support, post-cleaning takes a lot of time and energy and affects roughness. | [19,20] |
| Challenges | Solutions |
|---|---|
| Existing bioceramic scaffolds have insufficient toughness and are easy to fracture, so they cannot be used for bearing bones. | 3D printing technology and bionic technology to prepare composite multi-materials, with excellent mechanical properties of 3D-printed bioceramic scaffold. |
| Clinical practice often requires the simultaneous treatment of the patient’s disease and repair of bone defects. | 3D printing technology combined with drug-carrying materials and bone growth-promoting factors has developed a 3D-printed multifunctional bioceramic scaffold that can be used for both disease treatment and tissue regeneration. The scaffolds can both treat disease and promote bone tissue regeneration. |
| Existing 3D-printed bioceramics scaffolds are difficult to accurately mimic the highly complex and ordered microstructure of natural bone tissue. | Other micro-nano manufacturing technologies—such as hydrothermal processing, laser engraving, and electrospinning—are being combined with existing 3D printing technologies to produce scaffolds with finer structures. |
| Existing 3D-printed bioceramic scaffolds cannot restore the full function of bone tissue. | Through the multi-channel 3D printing technology, a variety of materials and cells are combined to simulate the real situation of bone tissue in the body as much as possible. |
| Existing 3D printing technology is difficult to be accurate to the nanometer scale, and can only be made into a scaffold and change its shape through physical and chemical methods. | The development of nano-scale 3D printing technology can prepare multi-tissue scaffolds with spatial and functional regulation. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalaf, A.T.; Wei, Y.; Wan, J.; Zhu, J.; Peng, Y.; Abdul Kadir, S.Y.; Zainol, J.; Oglah, Z.; Cheng, L.; Shi, Z. Bone Tissue Engineering through 3D Bioprinting of Bioceramic Scaffolds: A Review and Update. Life 2022, 12, 903. https://doi.org/10.3390/life12060903
Khalaf AT, Wei Y, Wan J, Zhu J, Peng Y, Abdul Kadir SY, Zainol J, Oglah Z, Cheng L, Shi Z. Bone Tissue Engineering through 3D Bioprinting of Bioceramic Scaffolds: A Review and Update. Life. 2022; 12(6):903. https://doi.org/10.3390/life12060903
Chicago/Turabian StyleKhalaf, Ahmad Taha, Yuanyuan Wei, Jun Wan, Jiang Zhu, Yu Peng, Samiah Yasmin Abdul Kadir, Jamaludin Zainol, Zahraa Oglah, Lijia Cheng, and Zheng Shi. 2022. "Bone Tissue Engineering through 3D Bioprinting of Bioceramic Scaffolds: A Review and Update" Life 12, no. 6: 903. https://doi.org/10.3390/life12060903
APA StyleKhalaf, A. T., Wei, Y., Wan, J., Zhu, J., Peng, Y., Abdul Kadir, S. Y., Zainol, J., Oglah, Z., Cheng, L., & Shi, Z. (2022). Bone Tissue Engineering through 3D Bioprinting of Bioceramic Scaffolds: A Review and Update. Life, 12(6), 903. https://doi.org/10.3390/life12060903








