The Impact of Kefir on Epidermal Water Homeostasis in Healthy Human Skin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Intervention
2.2. Kefir
2.3. Biometrics and Experimental Design
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Elias, P.M. Skin barrier function. Curr. Allergy Asthma Rep. 2008, 8, 299–305. [Google Scholar] [CrossRef]
- Feingold, K.R.; Denda, M. Regulation of permeability barrier homeostasis. Clin. Dermatol. 2012, 30, 263–268. [Google Scholar] [CrossRef]
- Montero-Vilchez, T.; Cuenca-Barrales, C.; Rodriguez-Pozo, J.-A.; Diaz-Calvillo, P.; Tercedor-Sanchez, J.; Martinez-Lopez, A.; Molina-Leyva, A.; Arias-Santiago, S. Epidermal barrier function and skin homeostasis in atopic dermatitis: The impact of age. Life 2022, 12, 132. [Google Scholar] [CrossRef]
- Wieërs, G.; Belkhir, L.; Enaud, R.; Leclercq, S.; Philippart de Foy, J.-M.; Dequenne, I.; de Timary, P.; Cani, P.D. How probiotics affect the microbiota. Front. Cell. Infect. Microbiol. 2020, 9, 454. [Google Scholar] [CrossRef] [Green Version]
- La Fata, G.; Weber, P.; Mohajeri, M.H. Probiotics and the Gut Immune System: Indirect Regulation. Probiotics Antimicrob. Proteins 2018, 10, 11–21. [Google Scholar] [CrossRef]
- Fang, Z.; Lu, W.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; Chen, W. Probiotics modulate the gut microbiota composition and immune responses in patients with atopic dermatitis: A pilot study. Eur. J. Nutr. 2020, 59, 2119–2130. [Google Scholar] [CrossRef]
- Slattery, C.; Cotter, P.D.; O’Toole, P.W. Analysis of health benefits conferred by Lactobacillus species from kefir. Nutrients 2019, 11, 1252. [Google Scholar] [CrossRef] [Green Version]
- Üstün-Aytekin, Ö.; Şeker, A.; Arısoy, S. The effect of in vitro gastrointestinal simulation on bioactivities of kefir. Int. J. Food Sci. Technol. 2020, 55, 283–292. [Google Scholar] [CrossRef]
- Garrote, G.L.; Abraham, A.G.; De Antoni, G.L. Inhibitory power of kefir: The role of organic acids. J. Food Prot. 2000, 63, 364–369. [Google Scholar] [CrossRef]
- Bengoa, A.A.; Iraporda, C.; Garrote, G.L.; Abraham, A.G. Kefir micro-organisms: Their role in grain assembly and health properties of fermented milk. J. Appl. Microbiol. 2019, 126, 686–700. [Google Scholar] [CrossRef] [Green Version]
- Farnworth, E.R. Kefir—A complex probiotic. Food Sci. Technol. Bull. Funct. Foods 2005, 2, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, B.; Gürakan, G.C.; Ünlü, G. Kefir: A multifaceted fermented dairy product. Probiotics Antimicrob. Proteins 2014, 6, 123–135. [Google Scholar] [CrossRef]
- Algiert-Zielińska, B.; Mucha, P.; Rotsztejn, H. Lactic and lactobionic acids as typically moisturizing compounds. Int. J. Dermatol. 2019, 58, 374–379. [Google Scholar] [CrossRef]
- Lew, L.C.; Liong, M.T. Bioactives from probiotics for dermal health: Functions and benefits. J. Appl. Microbiol. 2013, 114, 1241–1253. [Google Scholar] [CrossRef]
- Huseini, H.F.; Rahimzadeh, G.; Fazeli, M.R.; Mehrazma, M.; Salehi, M. Evaluation of wound healing activities of kefir products. Burns 2012, 38, 719–723. [Google Scholar] [CrossRef]
- Rodrigues, K.L.; Caputo, L.R.; Carvalho, J.C.; Evangelista, J.; Schneedorf, J.M. Antimicrobial and healing activity of kefir and kefiran extract. Int. J. Antimicrob. Agents 2005, 25, 404–408. [Google Scholar] [CrossRef]
- Rodrigues, K.L.; Carvalho, J.C.T.; Schneedorf, J.M. Anti-Inflammatory properties of kefir and its polysaccharide extract. Inflammopharmacology 2005, 13, 485–492. [Google Scholar] [CrossRef]
- Poutahidis, T.; Kearney, S.M.; Levkovich, T.; Qi, P.; Varian, B.J.; Lakritz, J.R.; Ibrahim, Y.M.; Chatzigiagkos, A.; Alm, E.J.; Erdman, S.E. Microbial symbionts accelerate wound healing via the neuropeptide hormone oxytocin. PLoS ONE 2013, 8, e78898. [Google Scholar] [CrossRef] [Green Version]
- Alves, E.; Gregório, J.; Baby, A.R.; Rijo, P.; Rodrigues, L.M.; Rosado, C. Homemade kefir consumption improves skin condition —A study conducted in healthy and atopic volunteers. Foods 2021, 10, 2794. [Google Scholar] [CrossRef]
- Alves, E.; Rijo, P.; Rodrigues, L.M.; Rosado, C. Probiotics in the gut-skin axis–the case of kefir. Biomed. Biopharm. Res. 2021, 18, 1–15. [Google Scholar] [CrossRef]
- Association, W.M. World medical association declaration of Helsinki: Ethical Principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [Green Version]
- Ellis, S.R.; Nguyen, M.; Vaughn, A.R.; Notay, M.; Burney, W.A.; Sandhu, S.; Sivamani, R.K. The skin and gut microbiome and its role in common dermatologic conditions. Microorganisms 2019, 7, 550. [Google Scholar] [CrossRef] [Green Version]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of action of probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef] [Green Version]
- Uchida, Y.; Park, K. Ceramides in skin health and disease: An update. Am. J. Clin. Dermatol. 2021, 22, 853–866. [Google Scholar] [CrossRef]
- Akdeniz, M.; Gabriel, S.; Lichterfeld-Kottner, A.; Blume-Peytavi, U.; Kottner, J. Transepidermal water loss in healthy adults: A systematic review and meta-analysis update. Br. J. Dermatol. 2018, 179, 1049–1055. [Google Scholar] [CrossRef]
- Montero-Vilchez, T.; Segura-Fernández-Nogueras, M.-V.; Pérez-Rodríguez, I.; Soler-Gongora, M.; Martinez-Lopez, A.; Fernández-González, A.; Molina-Leyva, A.; Arias-Santiago, S. Skin barrier function in psoriasis and atopic dermatitis: Transepidermal water loss and temperature as useful tools to assess disease severity. J. Clin. Med. 2021, 10, 359. [Google Scholar] [CrossRef]
- Tagami, H.; Yoshikuni, K. Interrelationship between water-barrier and reservoir functions of pathologic stratum corneum. Arch. Dermatol. 1985, 121, 642–645. [Google Scholar] [CrossRef]
- Ye, L.; Wang, Z.; Li, Z.; Lv, C.; Man, M.-Q. Validation of GPSkin Barrier® for assessing epidermal permeability barrier function and stratum corneum hydration in humans. Skin Res. Technol. 2019, 25, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Wouters, J.T.M.; Ayad, E.H.E.; Hugenholtz, J.; Smit, G. Microbes from raw milk for fermented dairy products. Int. Dairy J. 2002, 12, 91–109. [Google Scholar] [CrossRef]
- Bourrie, B.C.T.; Willing, B.P.; Cotter, P.D. The microbiota and health promoting characteristics of the fermented beverage kefir. Front. Microbiol. 2016, 7, 647. [Google Scholar] [CrossRef] [Green Version]
- Dimidi, E.; Cox, S.; Rossi, M.; Whelan, K. Fermented foods: Definitions and characteristics, gastrointestinal health and disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef] [Green Version]
- Iraporda, C.; Abatemarco Júnior, M.; Neumann, E.; Nunes, Á.C.; Nicoli, J.R.; Abraham, A.G.; Garrote, G.L. Biological activity of the non-microbial fraction of kefir: Antagonism against intestinal pathogens. J. Dairy Res. 2017, 84, 339–345. [Google Scholar] [CrossRef] [Green Version]
- Lew, L.C.; Gan, C.Y.; Liong, M.T. Dermal bioactives from Lactobacilli and Bifidobacteria. Ann. Microbiol. 2013, 63, 1047–1055. [Google Scholar] [CrossRef]
- Notay, M.; Foolad, N.; Vaughn, A.R.; Sivamani, R.K. Probiotics, prebiotics, and synbiotics for the treatment and prevention of adult dermatological diseases. Am. J. Clin. Dermatol. 2017, 18, 721–732. [Google Scholar] [CrossRef]
- Rusu, E.; Enache, G.; Cursaru, R.; Alexescu, A.; Radu, R.; Onila, O.; Cavallioti, T.; Rusu, F.; Posea, M.; Jinga, M.; et al. Prebiotics and probiotics in atopic dermatitis (review). Exp. Ther. Med. 2019, 18, 926–931. [Google Scholar] [CrossRef]
- Azizi, N.F.; Kumar, M.R.; Yeap, S.K.; Abdullah, J.O.; Khalid, M.; Omar, A.R.; Osman, M.A.; Mortadza, S.A.S.; Alitheen, N.B. Kefir and its biological activities. Foods 2021, 10, 1210. [Google Scholar] [CrossRef]
- Rather, I.A.; Bajpai, V.K.; Kumar, S.; Lim, J.; Paek, W.K.; Park, Y.H. Probiotics and atopic dermatitis: An overview. Front. Microbiol. 2016, 7, 507. [Google Scholar] [CrossRef] [Green Version]
- Makrgeorgou, A.; Leonardi-Bee, J.; Bath-Hextall, F.J.; Murrell, D.F.; Tang, M.L.K.; Roberts, A.; Boyle, R.J. Probiotics for treating eczema. Cochrane Database Syst. Rev. 2018, 2018, 1–146. [Google Scholar] [CrossRef]
- Bengoa, A.A.; Zavala, L.; Carasi, P.; Trejo, S.A.; Bronsoms, S.; de los Ángeles Serradell, M.; Garrote, G.L.; Abraham, A.G. Simulated Gastrointestinal conditions increase adhesion ability of Lactobacillus paracasei strains isolated from kefir to Caco-2 cells and mucin. Food Res. Int. 2018, 103, 462–467. [Google Scholar] [CrossRef]
- Vinderola, C.G.; Duarte, J.; Thangavel, D.; Perdigón, G.; Farnworth, E.; Matar, C. Immunomodulating capacity of kefir. J. Dairy Res. 2005, 72, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Dallas, D.; Citerne, F.; Tian, T.; Silva, V.; Kalanetra, K.; Frese, S.; Robinson, R.; Mills, D.; Barile, D. Peptidomic analysis reveals proteolytic activity of kefir microorganisms on bovine milk proteins. Food Chem. 2016, 197, 273–284. [Google Scholar] [CrossRef] [Green Version]
- Iraporda, C.; Errea, A.; Romanin, D.E.; Cayet, D.; Pereyra, E.; Pignataro, O.; Sirard, J.C.; Garrote, G.L.; Abraham, A.G.; Rumbo, M. Lactate and short chain fatty acids produced by microbial fermentation downregulate proinflammatory responses in intestinal epithelial cells and myeloid cells. Immunobiology 2015, 220, 1161–1169. [Google Scholar] [CrossRef]
- Saito, Y.; Mihara, T.; Maruyama, K.; Saito, J.; Ikeda, M.; Tomonaga, A.; Kumagai, T. Effects of intake of Lactobacillus casei subsp. casei 327 on skin conditions: A randomized, double-blind, placebo-controlled, parallel-group study in women. Biosci. Microbiota Food Health 2017, 36, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Gueniche, A.; Philippe, D.; Bastien, P.; Reuteler, G.; Blum, S.; Castiel-Higounenc, I.; Breton, L.; Benyacoub, J. Randomised double-blind placebo-controlled study of the effect of Lactobacillus paracasei NCC 2461 on skin reactivity. Benef. Microbes 2014, 5, 137–145. [Google Scholar] [CrossRef]
- Ogawa, M.; Saiki, A.; Matsui, Y.; Tsuchimoto, N.; Nakakita, Y.; Takata, Y.; Nakamura, T. Effects of oral intake of heat-killed Lactobacillus brevis SBC8803 (SBL88TM) on dry skin conditions: A randomized, double-blind, placebo-controlled study. Exp. Ther. Med. 2016, 12, 3863–3872. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.E.; Huh, C.-S.; Ra, J.; Choi, I.-D.; Jeong, J.-W.; Kim, S.-H.; Ryu, J.H.; Seo, Y.K.; Koh, J.S.; Lee, J.-H.; et al. Clinical evidence of effects of Lactobacillus plantarum HY7714 on skin aging: A randomized, double blind, placebo-controlled study. J. Microbiol. Biotechnol. 2015, 25, 2160–2168. [Google Scholar] [CrossRef]
- Kano, M.; Masuoka, N.; Kaga, C.; Sugimoto, S.; Iizuka, R.; Manabe, K.; Sone, T.; Oeda, K.; Nonoka, C.; Miyazaki, K.; et al. Consecutive intake of fermented milk containing bifidobacterium breve strain yakult and galacto-oligosaccharides benefits skin condition in healthy adult women. Biosci. Microbiota Food Health 2013, 21, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Mori, N.; Kano, M.; Masuoka, N.; Konno, T.; Suzuki, Y.; Miyazaki, K.; Ueki, Y. Effect of probiotic and prebiotic fermented milk on skin and intestinal conditions in healthy young female students. Biosci. Microbiota Food Health 2016, 35, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Garrote, G.L.; Abraham, A.G.; De Antoni, G.L. Microbial interactions in kefir: A natural probiotic drink. In Biotechnology of Lactic Acid Bacteria: Novel Applications; Springer: Berlin/Heidelberg, Germany, 2010; pp. 327–340. [Google Scholar] [CrossRef]
- Dai, R.; Hua, W.; Chen, W.; Xiong, L.; Li, L. The effect of milk consumption on acne: A meta-analysis of observational studies. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 2244–2253. [Google Scholar] [CrossRef]
- Rosado, C.; Ferreira, J.; Pinto, P.C.; Rodrigues, L.M. Skin barrier function evaluation by bi-compartmental analisys of TEWL dynamical measurements: Validation of new analytical conditions. Biomed. Biopharm. Res. 2012, 2, 183–189. [Google Scholar] [CrossRef]

|
| Kefir Group (n = 12) | Control Group (n = 15) | p-Value | |
|---|---|---|---|
| Age, mean (SD), years | 29.0 (13.6) | 23.8 (6.39) | 0.461 ¥ |
| Skin Phototype | 0.361 | ||
| Type II, n (%) | 6 (50.0) | 5 (33.3) | |
| Type III, n (%) | 4 (33.3) | 9 (60.0) | |
| Type IV n (%) | 2 (16.7) | 1 (6.70) | |
| BMI, mean (SD), kg/m2 | 22.1 (3.39) | 22.1 (2.91) | 0.661 ¥ |
| Education (highest) | 0.238 | ||
| Graduate, n (%) | 11 (91.7) | 13 (86.7) | |
| Master, n (%) | 0 | 2 (13.3) | |
| Doctorate, n (%) | 1 (8.30) | 0 | |
| Career | 0.255 | ||
| Employed, n (%) | 1 (8.30) | 0 | |
| University student, n (%) | 11 (91.7) | 15 (100) | |
| Residence area | 0.076 | ||
| Urban, n (%) | 8 (66.7) | 14 (93.3) | |
| Rural, n (%) | 4 (33.3) | 1 (6.70) | |
| Smoking habits | 0.053 | ||
| Smoker, n (%) | 3 (25.0) | 0 | |
| Occasional smoker, n (%) | 1 (8.30) | 0 | |
| Non-smoker, n (%) | 8 (66.7) | 15 (100) | |
| Alcohol consumption | 0.522 | ||
| Never, n (%) | 5 (41.7) | 7 (46.7) | |
| 1 to 2 times/week, n (%) | 6 (50.0) | 8 (53.3) | |
| 3 to 6 times/week, n (%) | 1 (8.30) | 0 |
| T0 | T4 | T8 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Kefir (n = 12) | Control (n = 15) | p-Value | Kefir (n = 12) | Control (n = 15) | p-Value | Kefir (n = 12) | Control (n = 15) | p-Value | |
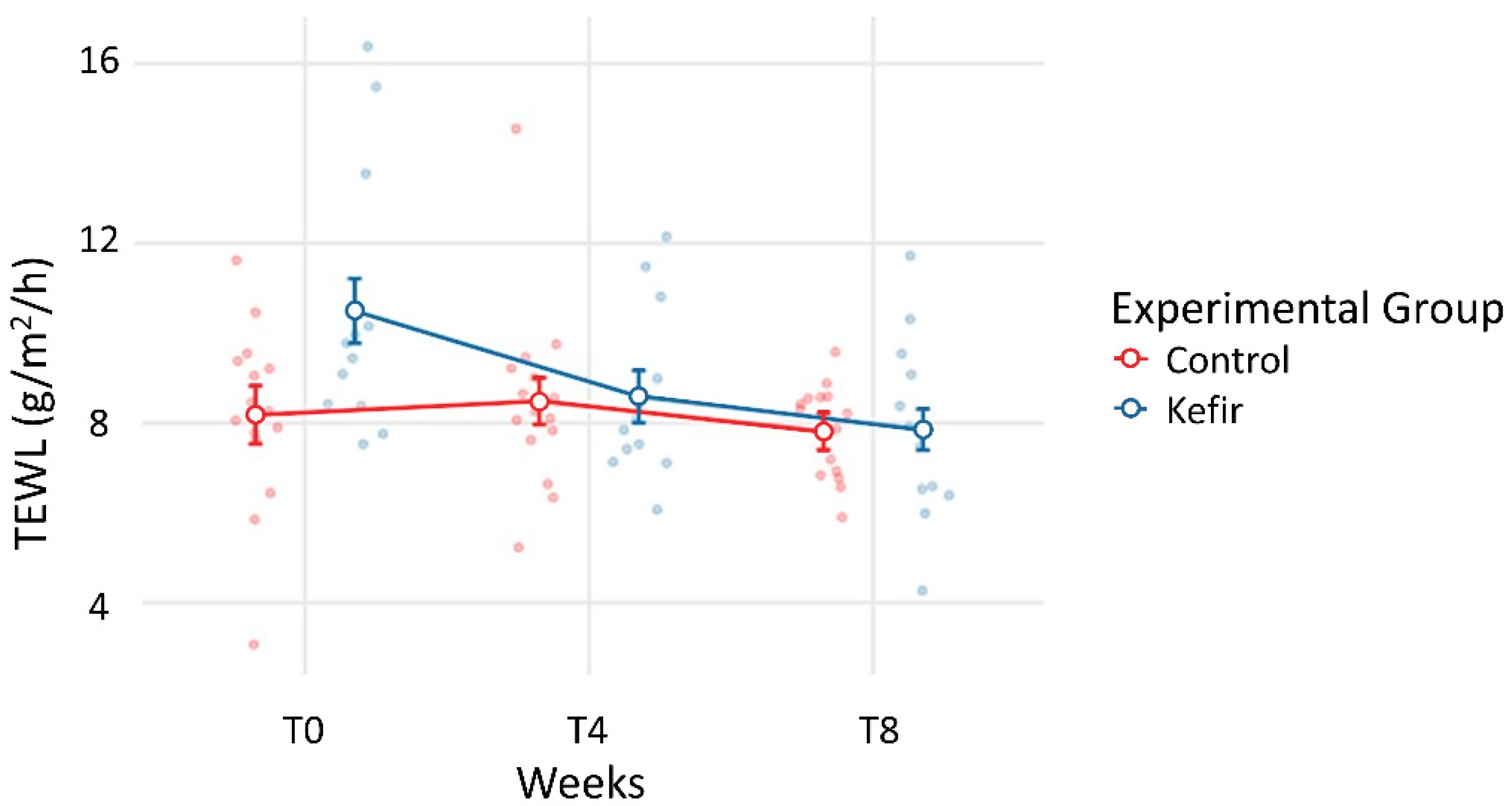
| TEWL (g/m2/h) | 10.49 ± 2.98 | 8.18 ± 2.02 | 0.361 | 8.59 ± 1.91 | 8.49 ± 2.08 | 1.000 | 7.85 ± 2.08 | 7.81 ± 1.04 | 1.000 |
| SC Hydration (a.u.) | 36.25 ± 7.71 | 39.27 ± 7.49 | 0.955 | 38.67 ± 6.97 | 35.80 ± 10.32 | 0.983 | 37.25 ± 6.77 | 31.00 ± 7.32 | 0.417 |
| Kefir Group (n = 12) | Control Group (n = 15) | |||
|---|---|---|---|---|
| T0–T4 (p) | T0–T8 (p) | T0–T4 (p) | T0–T8 (p) | |
| TEWL (g/m2/h) | 1.907 (0.311) | 2.643 (0.043) * | −0.305 (0.999) | 0.369 (0.997) |
| Hydration (a.u.) | −2.417 (0.931) | −1.000 (0.997) | 3.467 (0.650) | 8.526 (0.002) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, E.; Gregório, J.; Rijo, P.; Rosado, C.; Rodrigues, L.M. The Impact of Kefir on Epidermal Water Homeostasis in Healthy Human Skin. Life 2022, 12, 1075. https://doi.org/10.3390/life12071075
Alves E, Gregório J, Rijo P, Rosado C, Rodrigues LM. The Impact of Kefir on Epidermal Water Homeostasis in Healthy Human Skin. Life. 2022; 12(7):1075. https://doi.org/10.3390/life12071075
Chicago/Turabian StyleAlves, Emília, João Gregório, Patrícia Rijo, Catarina Rosado, and Luís Monteiro Rodrigues. 2022. "The Impact of Kefir on Epidermal Water Homeostasis in Healthy Human Skin" Life 12, no. 7: 1075. https://doi.org/10.3390/life12071075
APA StyleAlves, E., Gregório, J., Rijo, P., Rosado, C., & Rodrigues, L. M. (2022). The Impact of Kefir on Epidermal Water Homeostasis in Healthy Human Skin. Life, 12(7), 1075. https://doi.org/10.3390/life12071075










