The Potential Role of a Surface-Modified Additive-Manufactured Healing Abutment on the Expression of Integrins α2, β1, αv, and β6 in the Peri-Implant Mucosa: A Preliminary Human Study
Abstract
:1. Introduction
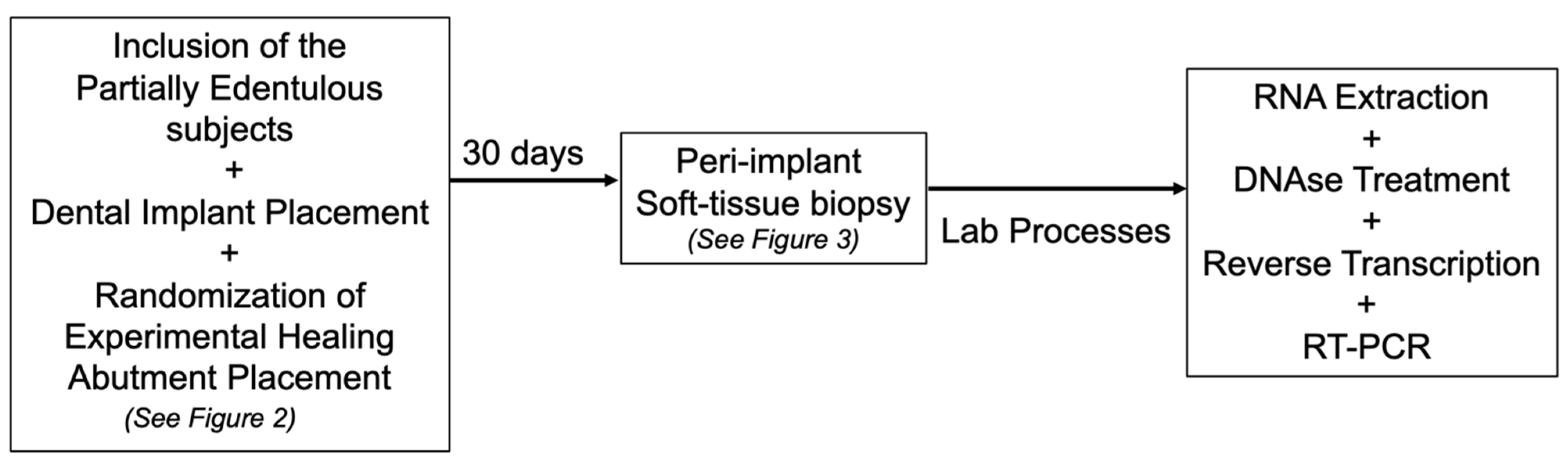
2. Materials and Methods
2.1. Study Population, Inclusion, and Exclusion Criteria
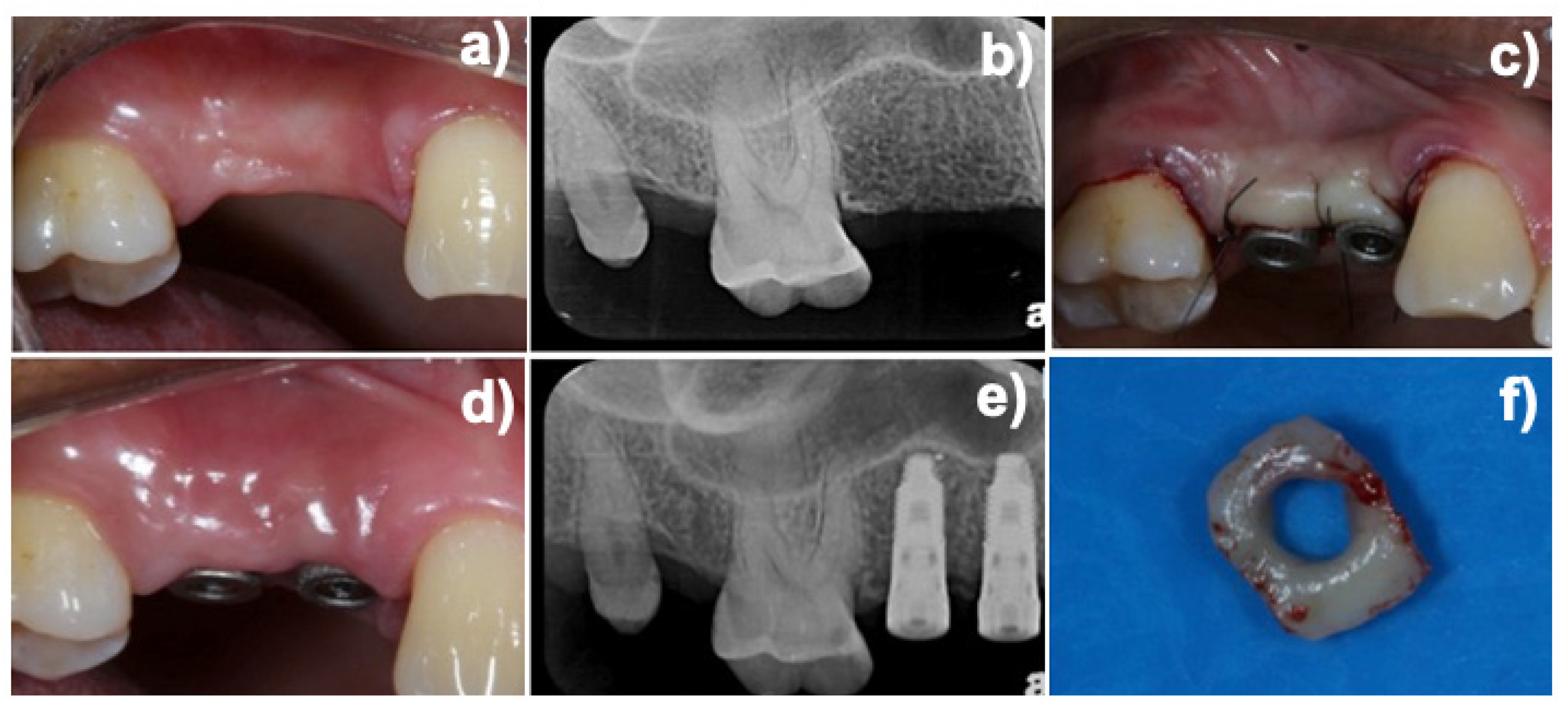
2.2. Experimental Design and Additive Manufactured (DMLS) Healing Abutment
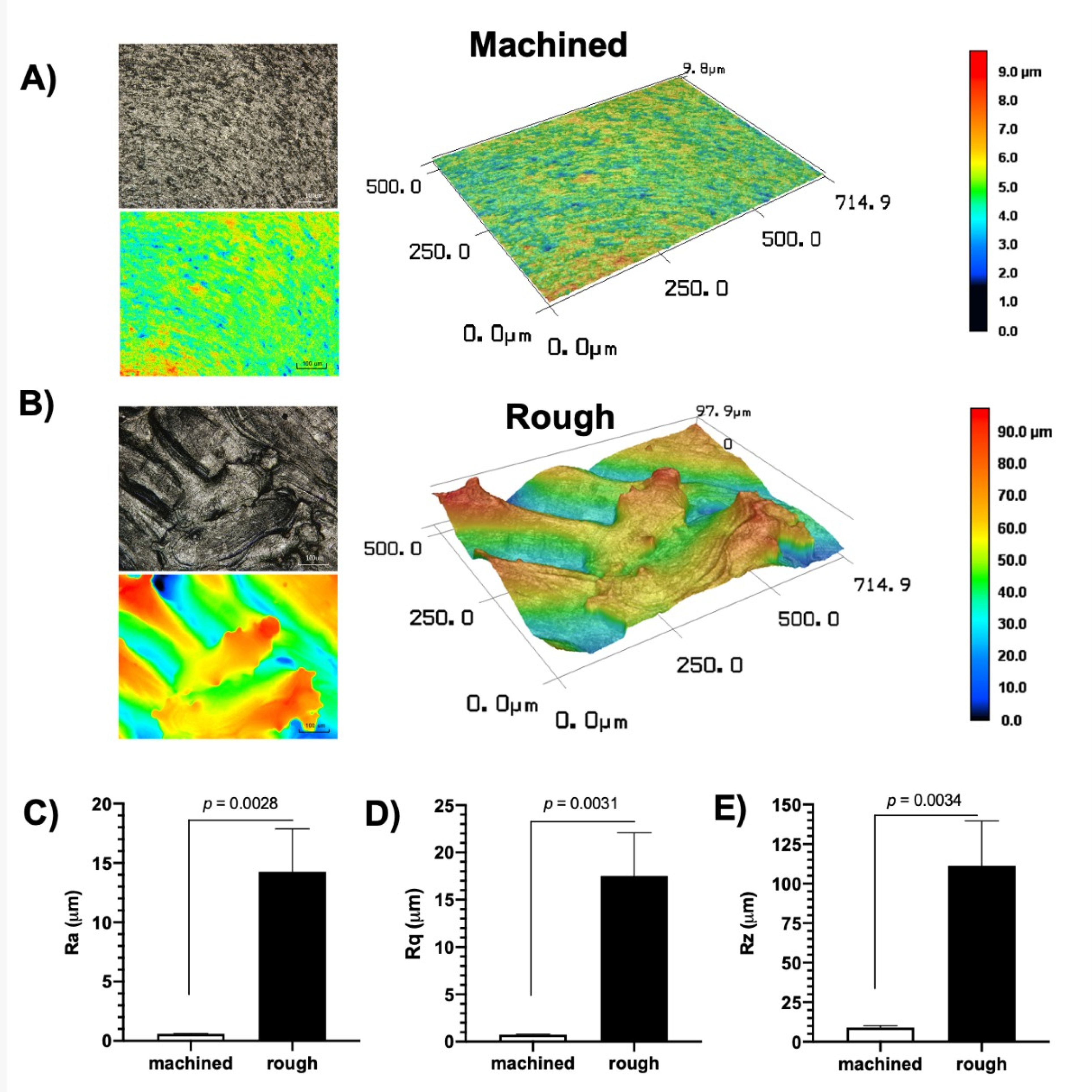
2.3. Abutment Surface Topography Characterization
2.4. Gene Expression Evaluation
2.4.1. RNA Extraction
2.4.2. DNAse Treatment
2.4.3. Reverse Transcription
2.5. Real-Time PCR (RT-PCR) Gene Expression Analysis
2.5.1. Primer Design
2.5.2. Reaction Optimization
2.5.3. RT-PCR Reactions
2.6. Statistical Analysis
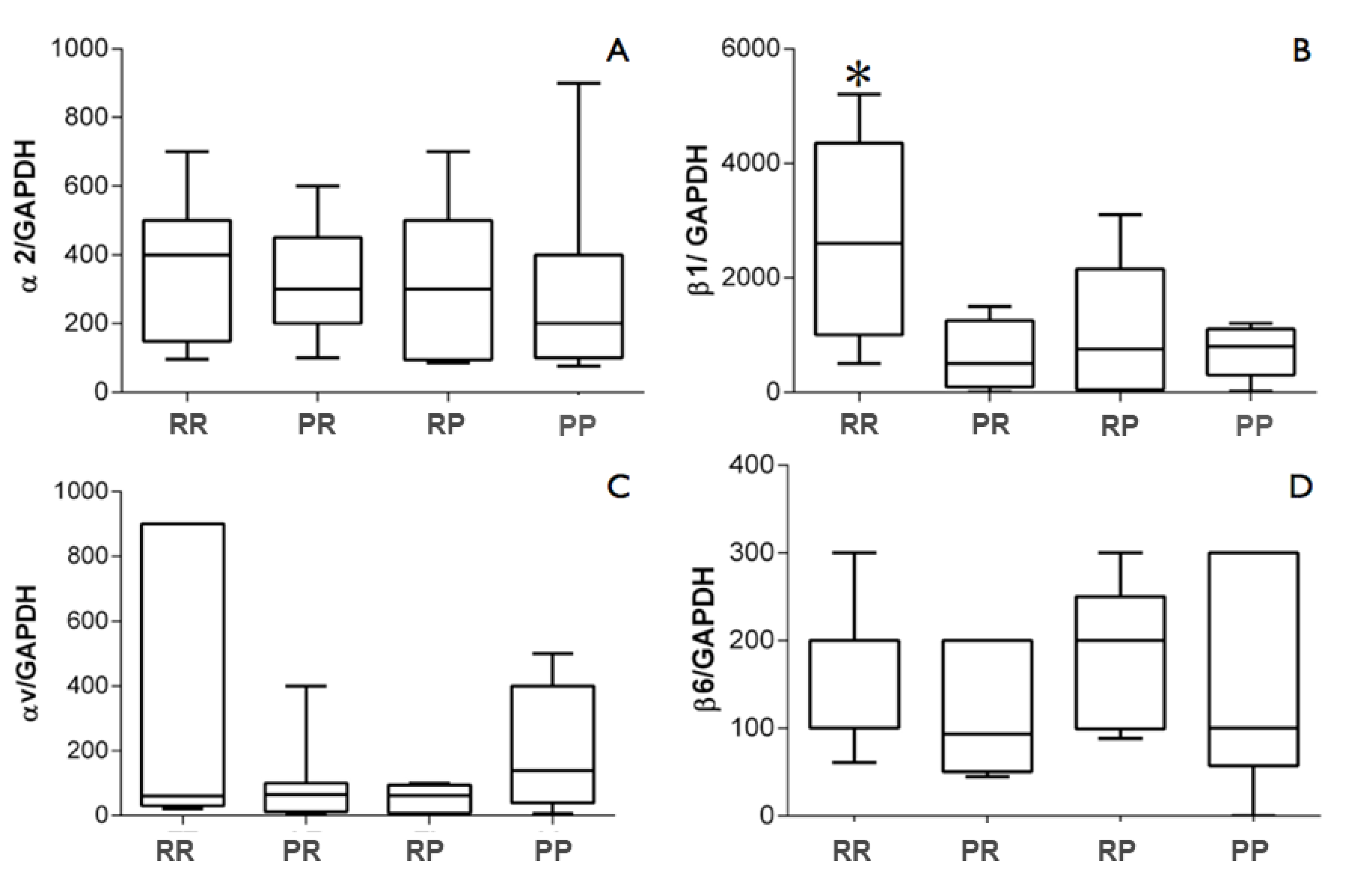
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Canullo, L.; Annunziata, M.; Pesce, P.; Tommasato, G.; Nastri, L.; Guida, L. Influence of Abutment Material and Modifications on Peri-Implant Soft-Tissue Attachment: A Systematic Review and Meta-Analysis of Histological Animal Studies. J. Prosthet. Dent. 2021, 125, 426–436. [Google Scholar] [CrossRef]
- Villar, C.C.; Huynh-Ba, G.; Mills, M.P.; Cochran, D.L. Wound Healing Around Dental Implants. In Oral Wound Healing; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 287–311. ISBN 9781118704509. [Google Scholar]
- Canullo, L.; Menini, M.; Santori, G.; Rakic, M.; Sculean, A.; Pesce, P. Titanium Abutment Surface Modifications and Peri-Implant Tissue Behavior: A Systematic Review and Meta-Analysis. Clin. Oral Investig. 2020, 24, 1113–1124. [Google Scholar] [CrossRef]
- Salvi, G.E.; Bosshardt, D.D.; Lang, N.P.; Abrahamsson, I.; Berglundh, T.; Lindhe, J.; Donos, N. Temporal Sequence of Hard and Soft Tissue Healing around Titanium Dental Implants. Periodontololgy 2000 2015, 68, 135–152. [Google Scholar] [CrossRef] [PubMed]
- Larjava, H.; Koivisto, L.; Häkkinen, L.; Heino, J. Epithelial Integrins with Special Reference to Oral Epithelia. J. Dent. Res. 2011, 90, 1367–1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koivisto, L.; Häkkinen, L.; Larjava, H. Re-Epithelialization of Wounds. Endod. Top. 2011, 24, 59–93. [Google Scholar] [CrossRef]
- Gräber, H.-G.; Conrads, G.; Wilharm, J.; Lampert, F.; Periodontol, J. Role of Interactions Between Integrins and Extracellular Matrix Components in Healthy Epithelial Tissue and Establishment of a Long Junctional Epithelium During Periodontal Wound Healing: A Review State of the Art Review. J. Periodontol. 1999, 70, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, L.; Larjava, K.; Hakkinen, L.; Uitto, V.-J.; Jyrki, H.H.; Larjava, H. Different Integrins Mediate Cell Spreading, Haptotaxis and Lateral Migration of HaCaT Keratinocytes on fibronectin. Cell Adhes. Commun. 1999, 7, 245–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, J.; Koticha, T.; Eubanks, D.; Rudek, I.; Molz, F.; Chiavaccini, L.; Claude, A.; Elder, S.; Wang, H.-L. Influence of Microtextured Implant Surfaces on Peri-Implantitis and Its Treatment: A Preclinical Trial. Int. J. Oral Maxillofac. Implant. 2018, 33, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, F.; Mihatovic, I.; Becker, J.; Bormann, K.H.; Keeve, P.L.; Friedmann, A. Histological Evaluation of Different Abutments in the Posterior Maxilla and Mandible: An Experimental Study in Humans. J. Clin. Periodontol. 2013, 40, 807–815. [Google Scholar] [CrossRef]
- Mangano, C.; Mangano, F.G.; Shibli, J.A.; Roth, L.A.; D’ Addazio, G.; Piattelli, A.; Iezzi, G. Immunohistochemical Evaluation of Peri-Implant Soft Tissues around Machined and Direct Metal Laser Sintered (DMLS) Healing Abutments in Humans. Int. J. Environ. Res. Public Health 2018, 15, 1611. [Google Scholar] [CrossRef] [Green Version]
- Degidi, M.; Artese, L.; Piattelli, A.; Scarano, A.; Shibli, J.A.; Piccirilli, M.; Perrotti, V.; Iezzi, G. Histological and Immunohistochemical Evaluation of the Peri-Implant Soft Tissues around Machined and Acid-Etched Titanium Healing Abutments: A Prospective Randomised Study. Clin. Oral Investig. 2012, 16, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Glauser, R.; Schüpbach, P.; Gottlow, J.; Hämmerle, C.H.F. Periimplant Soft Tissue Barrier at Experimental One-Piece Mini-Implants with Different Surface Topography in Humans: A Light-Microscopic Overview and Histometric Analysis. Clin. Implant Dent. Relat. Res. 2005, 7 (Suppl. S1), S44–S51. [Google Scholar] [CrossRef] [PubMed]
- Shibli, J.A.; Mangano, C.; D’Avila, S.; Piattelli, A.; Pecora, G.E.; Mangano, F.; Onuma, T.; Cardoso, L.A.; Ferrari, D.S.; Aguiar, K.C.; et al. Influence of Direct Laser Fabrication Implant Topography on Type IV Bone: A Histomorphometric Study in Humans. J. Biomed. Mater. Res.—Part A 2010, 93, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Mangano, C.; Piattelli, A.; Scarano, A.; Raspanti, M.; Shibli, J.; Mangano, F.; Perrotti, V.; Iezzi, G. A Light and Scanning Electron Microscopy Study of Human Direct Laser Metal Forming Dental Implants. Int. J. Periodontics Restor. Dent. 2014, 34, e9–e17. [Google Scholar] [CrossRef]
- Wennerberg, A.; Sennerby, L.; Kultje, C.; Lekholm, U. Some Soft Tissue Characteristics at Implant Abutments with Different Surface Topography A Study in Humans. J. Clin. Periodontol. 2003, 30, 88–94. [Google Scholar] [CrossRef]
- Souza, J.G.S.; Costa Oliveira, B.E.; Bertolini, M.; Lima, C.V.; Retamal-Valdes, B.; de Faveri, M.; Feres, M.; Barão, V.A.R. Titanium Particles and Ions Favor Dysbiosis in Oral Biofilms. J. Periodontal Res. 2020, 55, 258–266. [Google Scholar] [CrossRef]
- Schwarz, F.; Ferrari, D.; Herten, M.; Mihatovic, I.; Wieland, M.; Sager, M.; Becker, J. Effects of Surface Hydrophilicity and Microtopography on Early Stages of Soft and Hard Tissue Integration at Non-Submerged Titanium Implants: An Immunohistochemical Study in Dogs. J. Periodontol. 2007, 78, 2171–2184. [Google Scholar] [CrossRef]
- Larjava, H.; Salo, T.; Haapasalmi, K.; Kramer, R.H.; Heinoll, J. Expression of Integrins and Basement Membrane Components by Wound Keratinocytes. J. Clin. Investig. 1993, 92, 1425–1435. [Google Scholar] [CrossRef] [Green Version]
- Häkkinen, L.; Koivisto, L.; Gardner, H.; Saarialho-Kere, U.; Carroll, J.M.; Lakso, M.; Rauvala, H.; Laato, M.; Heino, J.; Larjava, H. Increased Expression of Β6-Integrin in Skin Leads to Spontaneous Development of Chronic Wounds. Am. J. Pathol. 2004, 164, 229–242. [Google Scholar] [CrossRef]
- Atsuta, I.; Yamaza, T.; Yoshinari, M.; Goto, T.; Kido, M.A.; Kagiya, T.; Mino, S.; Shimono, M.; Tanaka, T. Ultrastructural Localization of Laminin-5 (Γ2 Chain) in the Rat Peri-Implant Oral Mucosa around a Titanium-Dental Implant by Immuno-Electron Microscopy. Biomaterials 2005, 26, 6280–6287. [Google Scholar] [CrossRef]
- Shimono, M.; Ishikawa, T.; Enokiya, Y.; Muramatsu, T.; Matsuzaka, K.-I.; Inoue, T.; Abiko, Y.; Yamaza, T.; Kido, M.A.; Tanaka, T.; et al. Biological Characteristics of the Junctional Epithelium. J. Electron. Microsc. 2003, 52, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Bosshardt, D.D.; Lang, N.P. The Junctional Epithelium: From Health to Disease. J. Dent. Res. 2005, 84, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Hormia, M.; Ylanne1, J.; Virtanen’, I. Expression of Integrins in Human Gingiva. J. Dent. Res. 1990, 69, 1817–1823. [Google Scholar] [CrossRef]
- Larjava, H.; Peltonen, J.; Akiyama, S.K.; Yamada, S.S.; Gralnick, H.R.; Uitto, J.; Yamada, K.M. Novel Function for Β1 Integrins in Keratinocyte Cell-Cell Interactions. J. Cell Biol. 1990, 110, 803–815. [Google Scholar] [CrossRef] [Green Version]
- Haapasalmi, K.; Mdkeli, M.; Oksala, O.; Heino, J.; Yamada, K.M.; Uitto, V.-J.; Larjavat, H. Expression of Epithelial Adhesion Proteins and Integrins in Chronic Inflammation. Am. J. Pathol. 1995, 147, 193–206. [Google Scholar] [PubMed]
- del Castillo, L.F.; Schlegel Gómez, R.; Pelka, M.; Hornstein, O.P.; Johannessen, A.C.; von Den Driesch, P. Immunohistochemical Localization of Very Late Activation Integrins in Healthy and Diseased Human Gingiva. J. Periodontal Res. 1996, 31, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Larjava, H. Expression of β1 Integrins in Normal Human Keratinocytes. Am. J. Med. Sci. 1991, 301, 63–68. [Google Scholar] [CrossRef]
- Abrahamsson, I.; Zitzmann, N.U.; Berglundh, T.; Linder, E.; Wennerberg, A.; Lindhe, J. The mucosal attachment to titanium implants with different surface characteristics: An experimental study in dogs. J. Clin. Periodontol. 2002, 29, 448–455. [Google Scholar] [CrossRef]
- Hermann, J.S.; Jones, A.A.; Bakaeen, L.G.; Buser, D.; Schoolfield, J.D.; Cochran, D.L. Influence of a Machined Collar on Crestal Bone Changes Around Titanium Implants: A Histometric Study in the Canine Mandible. J. Periodontol. 2011, 82, 1329–1338. [Google Scholar] [CrossRef]
- Ghannad, F.; Nica, D.; Garcia Fulle, M.I.; Grenier, D.; Putnins, E.E.; Johnston, S.; Eslami, A.; Koivisto, L.; Jiang, G.; McKee, M.D.; et al. Absence of Avβ6 Integrin Is Linked to Initiation and Progression of Periodontal Disease. Am. J. Pathol. 2008, 172, 1271–1286. [Google Scholar] [CrossRef] [Green Version]
- An, N.; Rausch-Fan, X.; Wieland, M.; Matejka, M.; Andrukhov, O.; Schedle, A. Initial Attachment, Subsequent Cell Proliferation/Viability and Gene Expression of Epithelial Cells Related to Attachment and Wound Healing in Response to Different Titanium Surfaces. Dent. Mater. 2012, 28, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Atsuta, I.; Ayukawa, Y.; Furuhashi, A.; Ogino, Y.; Moriyama, Y.; Tsukiyama, Y.; Koyano, K. In Vivo and in Vitro Studies of Epithelial Cell Behavior around Titanium Implants with Machined and Rough Surfaces. Clin. Implant. Dent. Relat. Res. 2014, 16, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Laird, N.Z.; Malkawi, W.I.; Chakka, J.L.; Acri, T.M.; Elangovan, S.; Salem, A.K. A Proof of Concept Gene-Activated Titanium Surface for Oral Implantology Applications. J. Tissue Eng. Regen. Med. 2020, 14, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.C.; Nagay, B.E.; Bertolini, M.; Costa-Oliveira, B.E.; Sampaio, A.A.; Retamal-Valdes, B.; Shibli, J.A.; Feres, M.; Barão, V.A.R.; Souza, J.G.S. Fitting Pieces into the Puzzle: The Impact of Titanium-Based Dental Implant Surface Modifications on Bacterial Accumulation and Polymicrobial Infections. Adv. Colloid Interface Sci. 2021, 298, 102551. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Martín, I.; Sanz-Sánchez, I.; Carrillo de Albornoz, A.; Figuero, E.; Sanz, M. Effects of Modified Abutment Characteristics on Peri-Implant Soft Tissue Health: A Systematic Review and Meta-Analysis. Clin. Oral Implant. Res. 2018, 29, 118–129. [Google Scholar] [CrossRef]
- Quirynen, M.; van der Mei, H.; Bollen, C.; Schotte, A.; Marechal, M.; Doornbusch, G.; Busscher, H.; van Steenberghe, D. An in vivo study of the influence of the surface roughness of implants on the microbiology of supra- and subgingival plaque. J. Dent. Res. 1993, 72, 1304–1309. [Google Scholar] [CrossRef]
- Teughels, W.; van Assche, N.; Sliepen, I.; Quirynen, M. Effect of Material Characteristics and/or Surface Topography on Biofilm Development. Clin. Oral Implant. Res. 2006, 17, 68–81. [Google Scholar] [CrossRef]
- Pingueiro, J.; Piattelli, A.; Paiva, J.; de Figueiredo, L.C.; Feres, M.; Shibli, J.; Bueno-Silva, B. Additive Manufacturing of Titanium Alloy Could Modify the Pathogenic Microbial Profile: An in Vitro Study. Braz. Oral Res. 2019, 33. [Google Scholar] [CrossRef] [Green Version]
- Quirynen, M.; de Soete, M.; van Steenberghe, D. Infectious Risks for Oral Implants: A Review of the Literature. Clin. Oral Impl. Res. 2002, 13, 1–19. [Google Scholar] [CrossRef]

| Gene | Sequence (5′–3′) | Amplification Profile [Temperature (°C)/Time (s)] | Amplicon Size (bp) |
|---|---|---|---|
| F: AGCCTATTCGAGCTGCC | 95/10; 56/5; 72/10 | 290 | |
| α2 | R: CAGTGTTGTATGCACTTTCCC | ||
| β1 | F: GTAACAATGGAGAGTGCGTC | 95/10; 54/10; 72/10 | 300 |
| R: GCTCTGCACTGAACACATTC | |||
| αv | F: CACCAACTCCACATTGGTTAC | 95/10; 56/7; 72/10 | 289 |
| R: CTGCAGTTAAGTTTCTGAGTTTCC | |||
| β6 | F: GTACTGCAACTGCACCAC | 95/10; 56/7; 72/10 | 295 |
| R: GCAGCTCCGTTTAGAGTTAC | |||
| F: CTGAGTACGTCGTGGAGTC | 95/10; 56/5; 72/7 | 250 | |
| GAPDH | |||
| R: TGATGATCTTGAGGCTGTTGTC |
| Patients | Age (Years) | Healing Abutments per Patient | Groups | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| 1 | 41 | 3 | - | 1 | 1 | 1 |
| 2 | 33 | 3 | 1 * | 1 | 1 | - |
| 3 | 33 | 6 | 1 | 1 + 1 * | 2 | 1 |
| 4 | 41 | 3 | 1 | 1 | - | 1 |
| 5 | 32 | 3 | 1 | - | 1 * | 1 |
| 6 | 31 | 1 | 1 | - | - | - |
| 7 | 35 | 4 | 1 | 2 | 1 | - |
| 8 | 46 | 3 | - | 1 | 1 | 1 |
| 9 | 45 | 4 | 1 | 1 | 1 | 1 |
| 10 | 45 | 1 | - | - | - | 1 |
| 11 | 50 | 4 | 1 | 1 | 1 | 1 * |
| 12 | 26 | 4 | 1 | - | 1 | 2 |
| 13 | 50 | 1 | 1 | - | - | - |
| Total | 39.07 | 40 | 10 | 10 | 10 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roth, L.A.; Bastos, M.F.; Melo, M.A.; Barão, V.A.R.; Costa, R.C.; Giro, G.; Souza, J.G.S.; Grzech-Leśniak, K.; Shibli, J.A. The Potential Role of a Surface-Modified Additive-Manufactured Healing Abutment on the Expression of Integrins α2, β1, αv, and β6 in the Peri-Implant Mucosa: A Preliminary Human Study. Life 2022, 12, 937. https://doi.org/10.3390/life12070937
Roth LA, Bastos MF, Melo MA, Barão VAR, Costa RC, Giro G, Souza JGS, Grzech-Leśniak K, Shibli JA. The Potential Role of a Surface-Modified Additive-Manufactured Healing Abutment on the Expression of Integrins α2, β1, αv, and β6 in the Peri-Implant Mucosa: A Preliminary Human Study. Life. 2022; 12(7):937. https://doi.org/10.3390/life12070937
Chicago/Turabian StyleRoth, Leandro Amadeu, Marta Ferreira Bastos, Marcelo A. Melo, Valentim A. R. Barão, Raphael C. Costa, Gabriela Giro, João Gabriel Silva Souza, Kinga Grzech-Leśniak, and Jamil Awad Shibli. 2022. "The Potential Role of a Surface-Modified Additive-Manufactured Healing Abutment on the Expression of Integrins α2, β1, αv, and β6 in the Peri-Implant Mucosa: A Preliminary Human Study" Life 12, no. 7: 937. https://doi.org/10.3390/life12070937







