Mineral Element Insiders and Outliers Play Crucial Roles in Biological Evolution
Abstract
:1. Introduction
2. Materials and Methods
Code and Data Availability
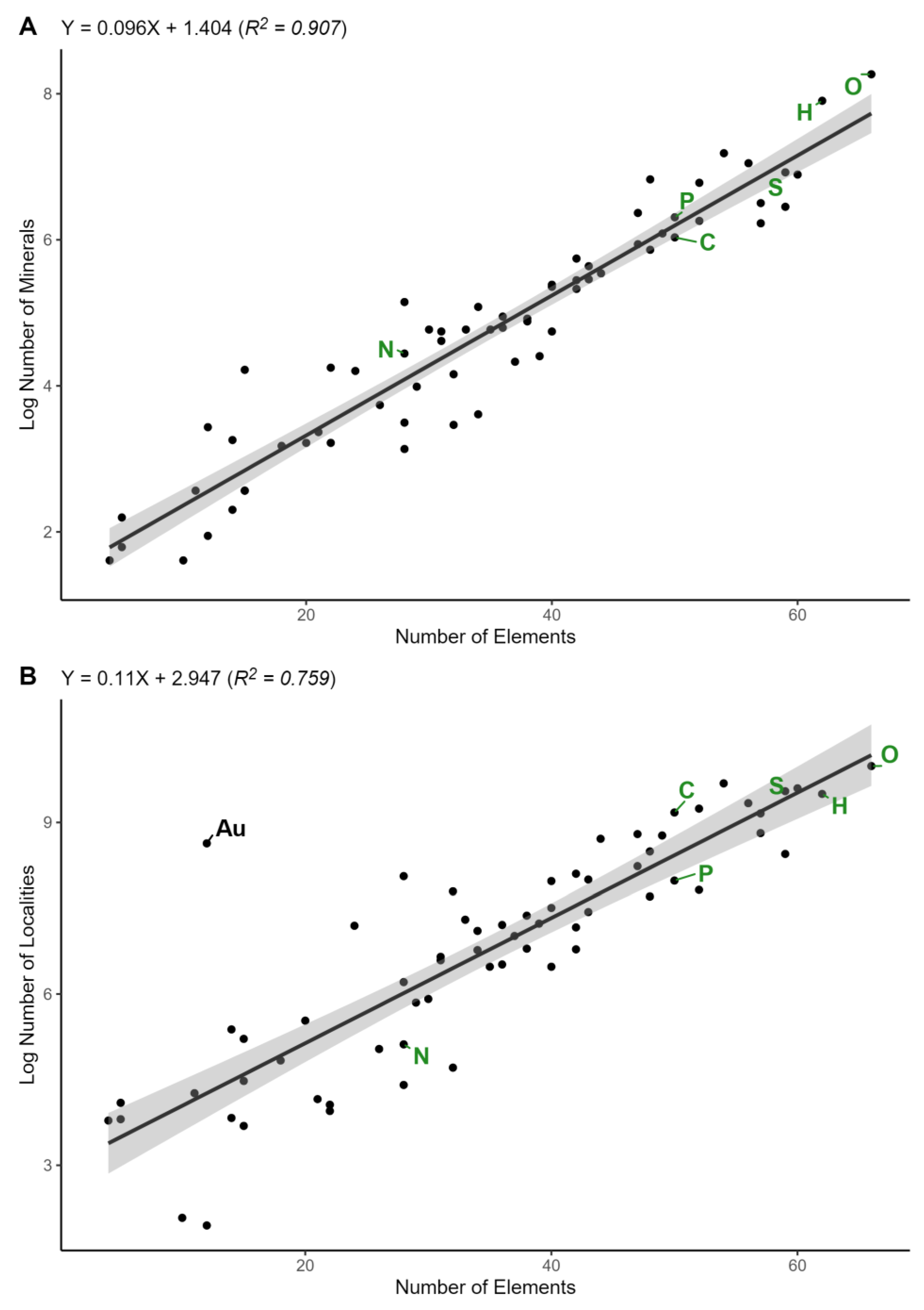
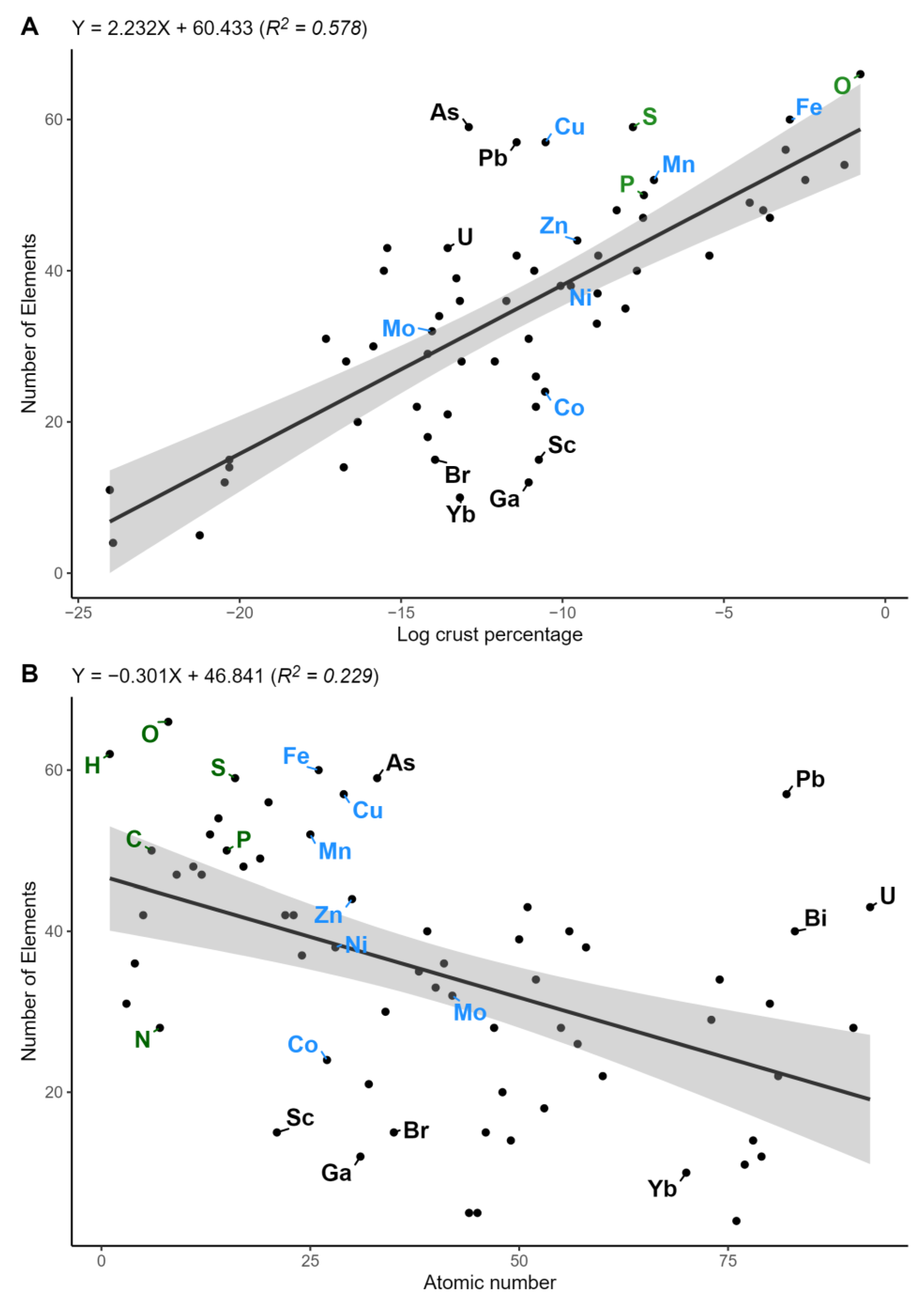
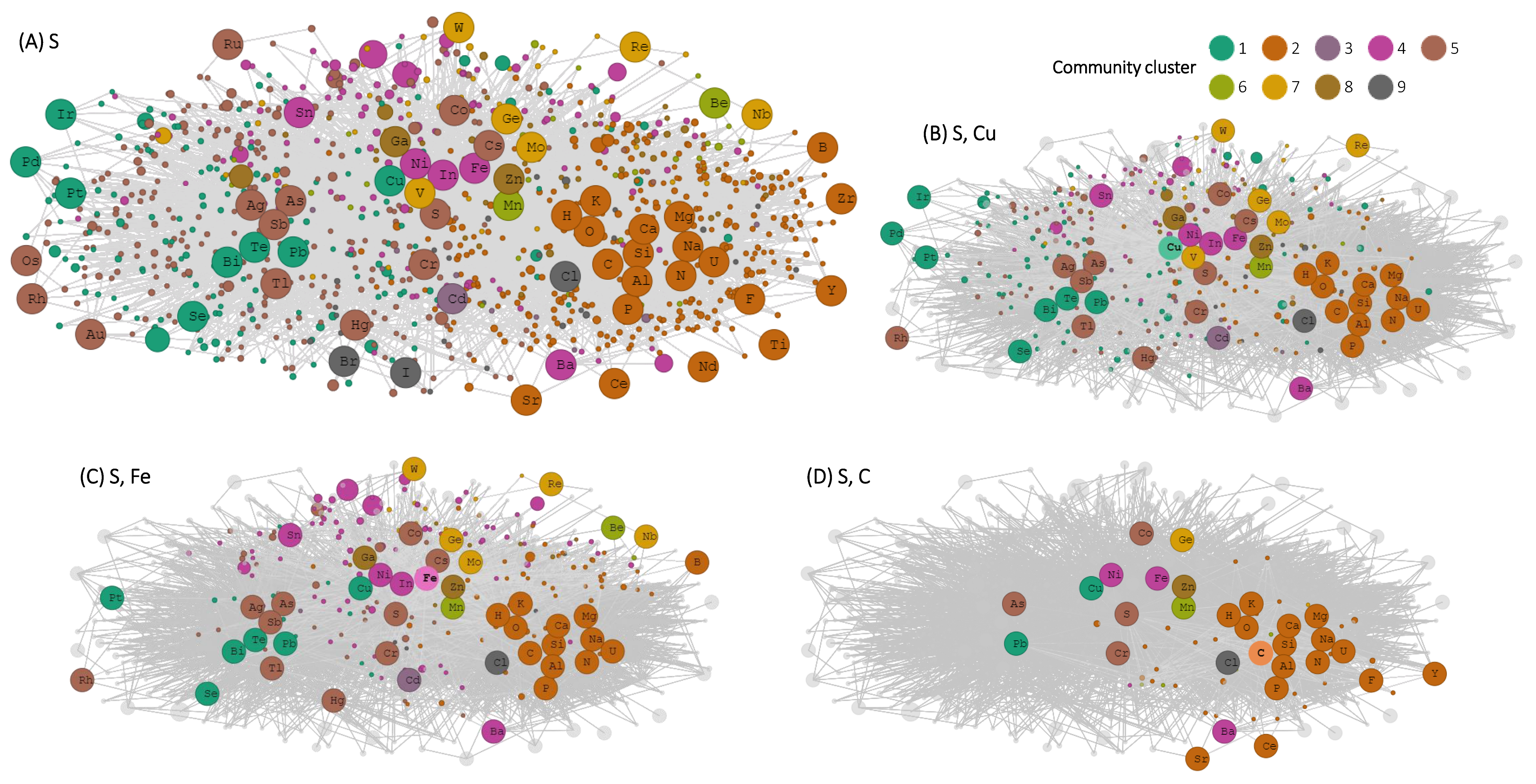
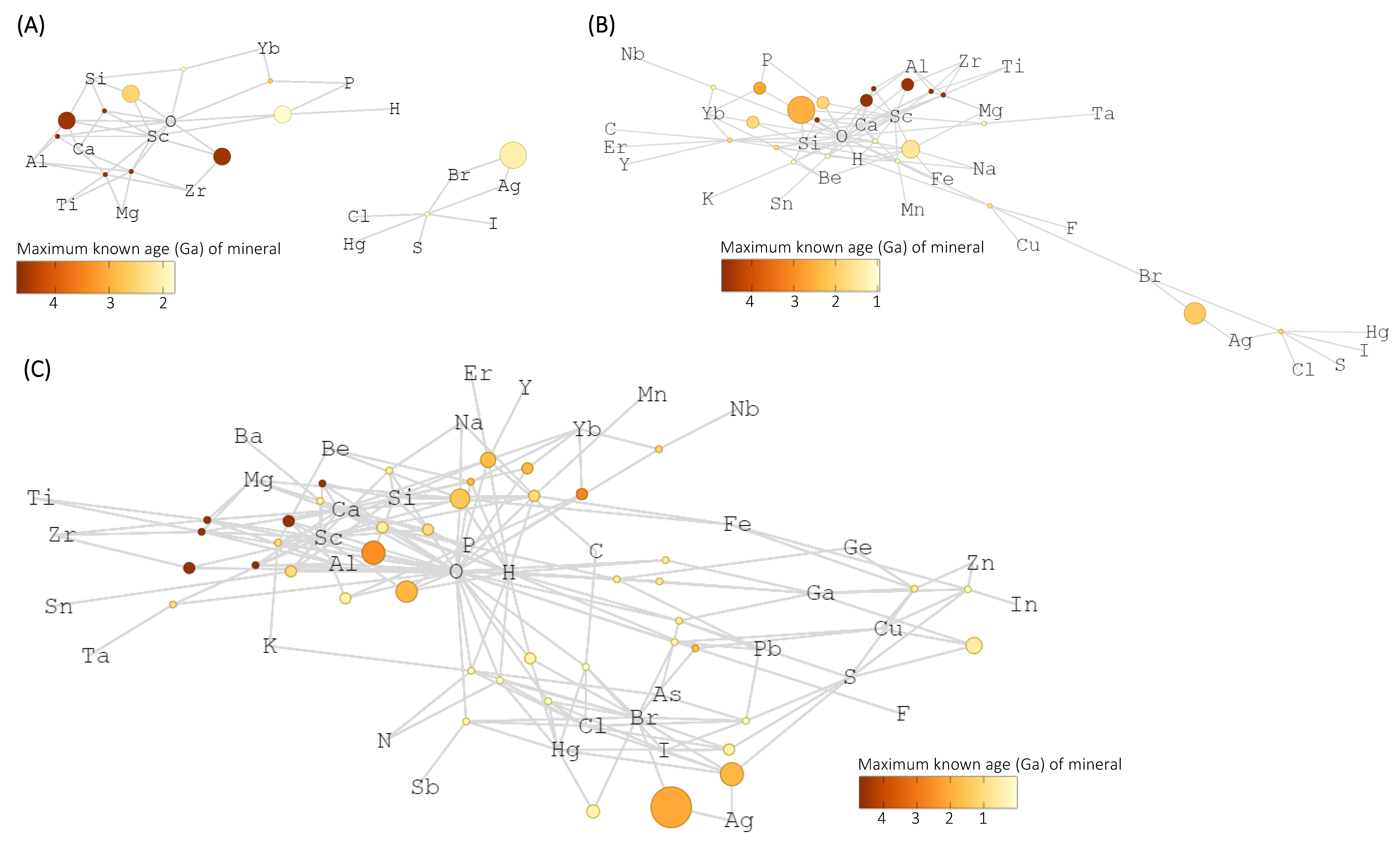
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| #-Mineral- | Crust Percent | ||||
|---|---|---|---|---|---|
| Element | Element | Minerals | Localities | HSAB | Weight |
| Ag | 28 | 172 | 3166 | Soft acid | 5.58 × 10−8 |
| Al | 52 | 881 | 10,322 | Hard acid | 0.083816762 |
| As | 59 | 634 | 4664 | Hard acid | 2.49 × 10−6 |
| Au | 12 | 31 | 5616 | Soft acid | 1.29 × 10−9 |
| B | 42 | 232 | 1295 | Soft acid | 1.10 × 10−5 |
| Ba | 40 | 218 | 2904 | Hard acid | 4.54 × 10−4 |
| Be | 36 | 121 | 1352 | Hard acid | 1.89 × 10−6 |
| Bi | 40 | 212 | 1821 | Int. acid | 1.79 × 10−7 |
| Br | 15 | 13 | 88 | Soft base | 8.76 × 10−7 |
| C | 50 | 417 | 9650 | Soft base | NA |
| Ca | 56 | 1152 | 11,362 | Hard acid | 0.045629216 |
| Cd | 20 | 25 | 253 | Soft acid | 7.97× 10−8 |
| Ce | 38 | 132 | 893 | Hard acid | 4.28 × 10−5 |
| Cl | 48 | 352 | 2219 | Int. base | 2.43 × 10−4 |
| Co | 24 | 67 | 1334 | Int. acid | 2.65 × 10−5 |
| Cr | 37 | 76 | 1115 | Hard acid | 1.34 × 10−4 |
| Cs | 28 | 23 | 82 | Hard acid | 1.99 × 10−6 |
| Cu | 57 | 667 | 9489 | Int. acid | 2.69 × 10−5 |
| F | 47 | 380 | 3775 | Hard base | 5.51 × 10−4 |
| Fe | 60 | 986 | 14,730 | Int. acid | 0.051949338 |
| Ga | 12 | 7 | 7 | Hard acid | 1.59 × 10−5 |
| Ge | 21 | 29 | 64 | Hard acid | 1.29 × 10−6 |
| H | 62 | 2708 | 13,356 | Hard acid | NA |
| Hg | 31 | 101 | 773 | Soft acid | 2.99 × 10−8 |
| I | 18 | 24 | 126 | Soft base | 6.97 × 10−7 |
| In | 14 | 10 | 46 | Int. acid | 5.18 × 10−8 |
| Ir | 11 | 13 | 71 | Soft acid | 3.69 × 10−11 |
| K | 49 | 440 | 6449 | Hard acid | 0.014965767 |
| La | 26 | 42 | 154 | Hard acid | 1.99 × 10−5 |
| Li | 31 | 115 | 728 | Hard acid | 1.59 × 10−5 |
| Mg | 47 | 583 | 6611 | Hard acid | 0.027989626 |
| Mn | 52 | 523 | 2492 | Int. acid | 7.71 × 10−4 |
| Mo | 32 | 64 | 2427 | Int. acid | 7.97 × 10−7 |
| N | 28 | 85 | 167 | Int. base | NA |
| Na | 48 | 923 | 4877 | Hard acid | 0.022740134 |
| Nb | 36 | 141 | 677 | Hard acid | 7.97 × 10−6 |
| Nd | 22 | 25 | 58 | Hard acid | 1.99 × 10−5 |
| Ni | 38 | 137 | 1590 | Int. acid | 5.88 × 10−5 |
| O | 66 | 3892 | 21,754 | Hard base | 0.462294236 |
| Os | 4 | 5 | 44 | Soft acid | 4.08 × 10−11 |
| P | 50 | 549 | 2931 | Soft base | 5.65 × 10−4 |
| Pb | 57 | 506 | 6728 | Int. acid | 1.10 × 10−5 |
| Pd | 15 | 68 | 184 | Soft acid | 1.49 × 10−9 |
| Pt | 14 | 26 | 217 | Soft acid | 1.49 × 10−9 |
| RE | 32 | 32 | 111 | NA | NA |
| Rh | 5 | 9 | 45 | Soft acid | NA |
| Ru | 5 | 6 | 60 | Soft acid | 5.98 × 10−10 |
| S | 59 | 1016 | 14,012 | Int. base | 4.02 × 10−4 |
| Sb | 43 | 235 | 2984 | Hard acid | 1.99 × 10−7 |
| Sc | 15 | 13 | 40 | Hard acid | 2.18 × 10−5 |
| Se | 30 | 118 | 370 | Soft base | 1.29 × 10−7 |
| Si | 54 | 1321 | 16,057 | Hard acid | 0.28214177 |
| Sn | 39 | 82 | 1384 | Hard acid | 1.69 × 10−6 |
| Sr | 35 | 118 | 651 | Hard acid | 3.19 × 10−4 |
| Ta | 29 | 54 | 347 | Int. acid | 6.97 × 10−7 |
| Te | 34 | 161 | 870 | Soft base | NA |
| Th | 28 | 33 | 497 | Hard acid | 5.58 × 10−6 |
| Ti | 42 | 312 | 3300 | Hard acid | 0.004298094 |
| Tl | 22 | 70 | 52 | Soft acid | 4.98 × 10−7 |
| U | 43 | 281 | 1694 | Hard acid | 1.29 × 10−6 |
| V | 42 | 206 | 882 | Hard acid | 1.37 × 10−4 |
| W | 34 | 37 | 1218 | Int. acid | 9.96 × 10−7 |
| Y | 40 | 115 | 650 | Hard acid | 1.89 × 10−5 |
| Yb | 10 | 5 | 8 | Hard acid | 1.89 × 10−6 |
| Zn | 44 | 255 | 6089 | Int. acid | 7.17 × 10−5 |
| Zr | 33 | 118 | 1482 | Hard acid | 1.31 × 10−4 |
References
- Goldschmidt, V.M. The Principles of Distribution of Chemical Elements in Minerals and Rocks. The Seventh Hugo Müller Lecture, Delivered before the Chemical Society on March 17th, 1937. J. Chem. Soc. 1937, 655–673. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Gao, S. 3.01-Composition of the Continental Crust. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Pergamon: Oxford, UK, 2003; Volume 3, pp. 1–64. ISBN 978-0-08-043751-4. [Google Scholar]
- McDonough, W.F.; Sun, S.-S. The Composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Williams, R.J.P. The Bakerian Lecture, 1981 Natural Selection of the Chemical Elements. Proc. R. Soc. Lond. Ser. B Boil. Sci. 1981, 213, 361–397. [Google Scholar] [CrossRef]
- Da Silva, J.J.R.F.; Williams, R.J.P. The Uptake of Elements by Biological Systems. Struct. Bond. 1976, 29, 69–121. [Google Scholar]
- Martin, W.; Baross, J.; Kelley, D.; Russell, M.J. Hydrothermal Vents and the Origin of Life. Nat. Rev. Microbiol. 2008, 6, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.K.; Jelen, B.I.; Giovannelli, D.; Raanan, H.; Falkowski, P.G. Metal Availability and the Expanding Network of Microbial Metabolisms in the Archaean Eon. Nat. Geosci. 2017, 10, 629–636. [Google Scholar] [CrossRef]
- Muchowska, K.B.; Varma, S.J.; Moran, J. Synthesis and Breakdown of Universal Metabolic Precursors Promoted by Iron. Nature 2019, 569, 104. [Google Scholar] [CrossRef]
- Morrison, S.M.; Runyon, S.E.; Hazen, R.M. The Paleomineralogy of the Hadean Eon Revisited. Life 2018, 8, 64. [Google Scholar] [CrossRef] [Green Version]
- Berner, R.A.; Lasaga, A.C.; Garrels, R.M. The Carbonate-Silicate Geochemical Cycle and Its Effect on Atmospheric Carbon Dioxide over the Past 100 Million Years. Am. J. Sci. 1983, 283, 641–683. [Google Scholar] [CrossRef]
- Lipp, A.G.; Shorttle, O.; Sperling, E.A. The Composition and Weathering of the Continents over Geologic Time. Geochem. Perspect. Lett. 2021, 17, 21–26. [Google Scholar] [CrossRef]
- Hazen, R.M.; Papineau, D.; Bleeker, W.; Downs, R.T.; Ferry, J.M.; McCoy, T.J.; Sverjensky, D.A.; Yang, H. Mineral Evolution. Am. Mineral. 2008, 93, 1693–1720. [Google Scholar] [CrossRef]
- Anbar, A.D.; Knoll, A.H. Proterozoic Ocean Chemistry and Evolution: A Bioinorganic Bridge? Science 2002, 297, 1137–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fike, D. Earth’s Redox Evolution. Nat. Geosci. 2010, 3, 453–454. [Google Scholar] [CrossRef]
- Jelen, B.I.; Giovannelli, D.; Falkowski, P.G. The Role of Microbial Electron Transfer in the Coevolution of the Biosphere and Geosphere. Annu. Rev. Microbiol. 2016, 70, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Sidborn, M.; Neretnieks, I. Long Term Redox Evolution in Granitic Rocks: Modelling the Redox Front Propagation in the Rock Matrix. Appl. Geochem. 2007, 22, 2381–2396. [Google Scholar] [CrossRef]
- Hazen, R.M.; Ferry, J.M. Mineral Evolution: Mineralogy in the Fourth Dimension. Elements 2010, 6, 9–12. [Google Scholar] [CrossRef]
- Tosca, N.J.; Johnston, D.T.; Mushegian, A.; Rothman, D.H.; Summons, R.E.; Knoll, A.H. Clay Mineralogy, Organic Carbon Burial, and Redox Evolution in Proterozoic Oceans. Geochim. Cosmochim. Acta 2010, 74, 1579–1592. [Google Scholar] [CrossRef] [Green Version]
- Spielman, S.J.; Moore, E.K. Dragon: A New Tool for Exploring Redox Evolution Preserved in the Mineral Record. Front. Earth Sci. 2020, 8, 585087. [Google Scholar] [CrossRef]
- Hazen, R.M.; Downs, R.T.; Eleish, A.; Fox, P.; Gagné, O.C.; Golden, J.J.; Grew, E.S.; Hummer, D.R.; Hystad, G.; Krivovichev, S.V.; et al. Data-Driven Discovery in Mineralogy: Recent Advances in Data Resources, Analysis, and Visualization. Engineering 2019, 5, 397–405. [Google Scholar] [CrossRef]
- Hummer, D.R. Fractal Distribution of Mineral Species among the Crystallographic Point Groups. Am. Mineral. 2021, 106, 1574–1579. [Google Scholar] [CrossRef]
- Moore, E.K.; Hao, J.; Prabhu, A.; Zhong, H.; Jelen, B.I.; Meyer, M.; Hazen, R.M.; Falkowski, P.G. Geological and Chemical Factors that Impacted the Biological Utilization of Cobalt in the Archean Eon. J. Geophys. Res. Biogeosci. 2018, 123, 743–759. [Google Scholar] [CrossRef]
- Moore, E.K.; Hao, J.; Spielman, S.J.; Yee, N. The Evolving Redox Chemistry and Bioavailability of Vanadium in Deep Time. Geobiology 2020, 18, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Spielman, S.J.; Morrison, S.M.; Moore, E.K. Geological Factors Impacted Cadmium Availability and Use as an Alternative Cofactor for Zinc in the Carbon Fixation Pathways of Marine Diatoms. J. Geophys. Res. Biogeosci. 2021, 126, e2020JG005966. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.M. The Geochemical Evolution of the Continental Crust. Rev. Geophys. 1995, 33, 241–265. [Google Scholar] [CrossRef]
- Ingrin, J.; Blanchard, M. Diffusion of Hydrogen in Minerals. Rev. Mineral. Geochem. 2006, 62, 291–320. [Google Scholar] [CrossRef]
- Li, Y.; Wiedenbeck, M.; Shcheka, S.; Keppler, H. Nitrogen Solubility in Upper Mantle Minerals. Earth Planet. Sci. Lett. 2013, 377–378, 311–323. [Google Scholar] [CrossRef]
- Morrison, S.M.; Buongiorno, J.; Downs, R.T.; Eleish, A.; Fox, P.; Giovannelli, D.; Golden, J.J.; Hummer, D.R.; Hystad, G.; Kellogg, L.H.; et al. Exploring Carbon Mineral Systems: Recent Advances in C Mineral Evolution, Mineral Ecology, and Network Analysis. Front. Earth Sci. 2020, 8, 208. [Google Scholar] [CrossRef]
- Golden, J.J. Mineral Evolution Database: Data Model for Mineral Age Associations. Master’s Thesis, University of Arizona, Tucson, AZ, USA, 2019. [Google Scholar]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. The Power of Databases: The RRUFF Project. In Highlights in Mineralogical Crystallography; Armbruster, T., Danisi, R.M., Eds.; W. De Gruyter: Berlin, Germany, 2015; pp. 1–30. [Google Scholar]
- Krivovichev, V.G.; Charykova, M.V. Number of Minerals of Various Chemical Elements: Statistics 2012 (a New Approach to an Old Problem). Geol. Ore Depos. 2014, 56, 553–559. [Google Scholar] [CrossRef]
- Christy, A.G. Causes of Anomalous Mineralogical Diversity in the Periodic Table. Mineral. Mag. 2015, 79, 33–49. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Krivovichev, V.G.; Hazen, R.M. Structural and Chemical Complexity of Minerals: Correlations and Time Evolution. Eur. J. Mineral. 2018, 30, 231–236. [Google Scholar] [CrossRef] [Green Version]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Lüdecke, D.; Ben-Shachar, M.S.; Patil, I.; Waggoner, P.; Makowski, D. Performance: An R Package for Assessment, Comparison and Testing of Statistical Models. J. Open Source Softw. 2021, 6, 3139. [Google Scholar] [CrossRef]
- Blondel, V.D.; Guillaume, J.-L.; Lambiotte, R.; Lefebvre, E. Fast Unfolding of Communities in Large Networks. J. Stat. Mech. 2008, 2008, P10008. [Google Scholar] [CrossRef] [Green Version]
- Hazen, R.M. An Evolutionary System of Mineralogy: Proposal for a Classification of Planetary Materials Based on Natural Kind Clustering. Am. Mineral. 2019, 104, 810–816. [Google Scholar] [CrossRef]
- Hazen, R.M.; Grew, E.S.; Downs, R.T.; Golden, J.; Hystad, G. Mineral Ecology: Chance and Necessity in The Mineral Diversity of Terrestrial Planetsthe Canadian Mineralogist. Can. Mineral. 2015, 53, 295–324. [Google Scholar] [CrossRef]
- Moorbath, S. Palaeobiology: Dating Earliest Life. Nature 2005, 434, 155. [Google Scholar] [CrossRef]
- Hazen, R.M. Genesis: Rocks, Minerals, and the Geochemical Origin of Life. Elements 2005, 1, 135–137. [Google Scholar] [CrossRef]
- Pearce, J.A. Geochemical Fingerprinting of the Earth’s Oldest Rocks. Geology 2014, 42, 175–176. [Google Scholar] [CrossRef] [Green Version]
- Baadsgaard, H.; Nutman, A.P.; Bridgwater, D.; Rosing, M.; McGregor, V.R.; Allaart, J.H. The Zircon Geochronology of the Akilia Association and Isua Supracrustal Belt, West Greenland. Earth Planet. Sci. Lett. 1984, 68, 221–228. [Google Scholar] [CrossRef]
- Dymek, R.F.; Klein, C. Chemistry, Petrology and Origin of Banded Iron-Formation Lithologies from the 3800 MA Isua Supracrustal Belt, West Greenland. Precambr. Res. 1988, 39, 247–302. [Google Scholar] [CrossRef]
- Nutman, A.P.; Bennett, V.C.; Friend, C.R.L.; Norman, M.D. Meta-Igneous (Non-Gneissic) Tonalites and Quartz-Diorites from an Extensive ca. 3800 Ma Terrain South of the Isua Supracrustal Belt, Southern West Greenland: Constraints on Early Crust Formation. Contrib. Miner. Pet. 1999, 137, 364–388. [Google Scholar] [CrossRef]
- Appel, P.W.U.; Moorbath, S.; Touret, J.L.R. Earlly Archean processes and the Isua Greenstone Belt, West Greenland. Introduction to the Isua special issue. Precambr. Res. 2003, 126, 173–394. [Google Scholar] [CrossRef]
- Schidlowski, M. A 3800-Million-Year Isotopic Record of Life from Carbon in Sedimentary Rocks. Nature 1988, 333, 313–318. [Google Scholar] [CrossRef]
- Rosing, M.T. 13C-Depleted Carbon Microparticles in >3700-Ma Sea-Floor Sedimentary Rocks from West Greenland. Science 1999, 283, 674–676. [Google Scholar] [CrossRef]
- Clark, B.C.; Kolb, V.M.; Steele, A.; House, C.H.; Lanza, N.L.; Gasda, P.J.; VanBommel, S.J.; Newsom, H.E.; Martínez-Frías, J. Origin of Life on Mars: Suitability and Opportunities. Life 2021, 11, 539. [Google Scholar] [CrossRef]
- Frey, P.A.; Reed, G.H. The Ubiquity of Iron. ACS Chem. Biol. 2012, 7, 1477–1481. [Google Scholar] [CrossRef]
- Lindley, P.F. Iron in Biology: A Structural Viewpoint. Rep. Prog. Phys. 1996, 59, 867–933. [Google Scholar] [CrossRef]
- Canfield, D.E.; Glazer, A.N.; Falkowski, P.G. The Evolution and Future of Earth’s Nitrogen Cycle. Science 2010, 330, 192–196. [Google Scholar] [CrossRef] [Green Version]
- Zerkle, A.L. Biogeodynamics: Bridging the Gap between Surface and Deep Earth Processes|Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. Philos. Trans. R. Soc. A 2018, 376, 20170401. [Google Scholar] [CrossRef] [Green Version]
- Mikhail, S.; Sverjensky, D.A. Nitrogen Speciation in Upper Mantle Fluids and the Origin of Earth’s Nitrogen-Rich Atmosphere. Nat. Geosci. 2014, 7, 816–819. [Google Scholar] [CrossRef]
- Zerkle, A.L.; Poulton, S.W.; Newton, R.J.; Mettam, C.; Claire, M.W.; Bekker, A.; Junium, C.K. Onset of the Aerobic Nitrogen Cycle during the Great Oxidation Event. Nature 2017, 542, 465–467. [Google Scholar] [CrossRef] [Green Version]
- Watenphul, A.; Wunder, B.; Wirth, R.; Heinrich, W. Ammonium-Bearing Clinopyroxene: A Potential Nitrogen Reservoir in the Earth’s Mantle. Chem. Geol. 2010, 270, 240–248. [Google Scholar] [CrossRef] [Green Version]
- Huppertz, H.; Schnick, W. Ba2Nd7Si11N23—A Nitridosilicate with a Zeolite-Analogous Si–N Structure. Angew. Chem. Int. Ed. Engl. 1997, 36, 2651–2652. [Google Scholar] [CrossRef]
- Zhang, Y.; Zindler, A. Distribution and Evolution of Carbon and Nitrogen in Earth. Earth Planet. Sci. Lett. 1993, 117, 331–345. [Google Scholar] [CrossRef]
- Tolstikhin, I.N.; Marty, B. The Evolution of Terrestrial Volatiles: A View from Helium, Neon, Argon and Nitrogen Isotope Modelling. Chem. Geol. 1998, 147, 27–52. [Google Scholar] [CrossRef]
- Deer, W.A.; Howie, R.A.; Zussman, J. An Introduction to the Rock-Forming Minerals, 3rd ed.; The Minerological Society: London, UK, 1966; ISBN 978-0-903056-33-5. [Google Scholar]
- Middlemost, E.A.K. Naming Materials in the Magma/Igneous Rock System. Earth-Sci. Rev. 1994, 37, 215–224. [Google Scholar] [CrossRef]
- Canfield, D.E.; Raiswell, R. The Evolution of the Sulfur Cycle. Am. J. Sci. 1999, 299, 697–723. [Google Scholar] [CrossRef]
- Jacob, C.; Giles, G.I.; Giles, N.M.; Sies, H. Sulfur and Selenium: The Role of Oxidation State in Protein Structure and Function. Angew. Chem. Int. Ed. 2003, 42, 4742–4758. [Google Scholar] [CrossRef]
- Bentley, R. Role of Sulfur Chirality in the Chemical Processes of Biology. Chem. Soc. Rev. 2005, 34, 609–624. [Google Scholar] [CrossRef]
- Neurath, H. The Proteins Composition, Structure, and Function V1; Academic Press Inc.: New York, NY, USA, 1963; ISBN 978-0-323-16138-1. [Google Scholar]
- Lippard, S.J. Iron Sulfur Coordination Compounds and Proteins. Acc. Chem. Res. 1973, 6, 282–288. [Google Scholar] [CrossRef]
- Vahrenkamp, H. Sulfur Atoms as Ligands in Metal Complexes. Angew. Chem. Int. Ed. Engl. 1975, 14, 322–329. [Google Scholar] [CrossRef]
- Stiefel, E.I. Transition Metal Sulfur Chemistry: Biological and Industrial Significance and Key Trends. In Transition Metal Sulfur Chemistry; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1996; Volume 653, pp. 2–38. ISBN 978-0-8412-3476-5. [Google Scholar]
- Rees, D.C.; Howard, J.B. The Interface Between the Biological and Inorganic Worlds: Iron-Sulfur Metalloclusters. Science 2003, 300, 929–931. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J. Iron–Sulfur Protein Folds, Iron–Sulfur Chemistry, and Evolution. JBIC J. Biol. Inorg. Chem. 2008, 13, 157–170. [Google Scholar] [CrossRef]
- Mutter, A.C.; Tyryshkin, A.M.; Campbell, I.J.; Poudel, S.; Bennett, G.N.; Silberg, J.J.; Nanda, V.; Falkowski, P.G. De Novo Design of Symmetric Ferredoxins that Shuttle Electrons in vivo. Proc. Natl. Acad. Sci. USA 2019, 116, 14557–14562. [Google Scholar] [CrossRef] [Green Version]
- Beinert, H.; Holm, R.H.; Münck, E. Iron-Sulfur Clusters: Nature’s Modular, Multipurpose Structures. Science 1997, 277, 653–659. [Google Scholar] [CrossRef]
- Beinert, H. A Tribute to Sulfur. Eur. J. Biochem. 2000, 267, 5657–5664. [Google Scholar] [CrossRef] [Green Version]
- Linder, M.C.; Hazegh-Azam, M. Copper Biochemistry and Molecular Biology. Am. J. Clin. Nutr. 1996, 63, 797S–811S. [Google Scholar] [CrossRef]
- Solomon, E.I.; Heppner, D.E.; Johnston, E.M.; Ginsbach, J.W.; Cirera, J.; Qayyum, M.; Kieber-Emmons, M.T.; Kjaergaard, C.H.; Hadt, R.G.; Tian, L. Copper Active Sites in Biology. Chem. Rev. 2014, 114, 3659–3853. [Google Scholar] [CrossRef] [Green Version]
- Goyer, R.A. Lead Toxicity: Current Concerns. Environ. Health Perspect. 1993, 100, 177–187. [Google Scholar] [CrossRef]
- Hughes, M.F. Arsenic Toxicity and Potential Mechanisms of Action. Toxicol. Lett. 2002, 133, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Dubey, R. Lead Toxicity in Plants. Braz. J. Plant Physiol. 2005, 17, 35–52. [Google Scholar] [CrossRef] [Green Version]
- Finnegan, P.; Chen, W. Arsenic Toxicity: The Effects on Plant Metabolism. Front. Physiol. 2012, 3, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farquhar, J.; Bao, H.; Thiemens, M. Atmospheric Influence of Earth’s Earliest Sulfur Cycle. Science 2000, 289, 756–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fru, E.C.; Somogyi, A.; Albani, A.E.; Medjoubi, K.; Aubineau, J.; Robbins, L.J.; Lalonde, S.V.; Konhauser, K.O. The Rise of Oxygen-Driven Arsenic Cycling at ca. 2.48 Ga. Geology 2019, 47, 243–246. [Google Scholar] [CrossRef] [Green Version]
- Nyström, J.O.; Wickman, F.E. The Ordovician Chondrite from Brunflo, Central Sweden, II. Secondary Minerals. Lithos 1991, 27, 167–185. [Google Scholar] [CrossRef]
- Karwowski, Ł.; Muszyński, A. Multimineral Inclusions in the Morasko Coarse Octahedrite. Meteorit. Planet. Sci. 2008, 43, 5232. [Google Scholar]
- Hazen, R.M.; Morrison, S.M. An Evolutionary System of Mineralogy, Part V: Aqueous and Thermal Alteration of Planetesimals (~4565 to 4550 Ma). Am. Mineral. 2021, 106, 1388–1419. [Google Scholar] [CrossRef]
- Burton, J.D.; Culkin, F.; Riley, J.P. The Abundances of Gallium and Germanium in Terrestrial Materials. Geochim. Cosmochim. Acta 1959, 16, 151–180. [Google Scholar] [CrossRef]
- Eby, G.N. Scandium Geochemistry of the Oka Carbonatite Complex, Oka, Quebec. Am. Mineral. 1973, 58, 819–825. [Google Scholar]
- Combs, D.W. Mineralogy, Petrology and Bromine Geochemistry of Selected Samples of the Salado Salt, Lea and Eddy Counties, New Mexico: A Potential Horizon for the Disposal of Radioactive Waste; United States Department of Energy Office of Scientific and Technical Information: Oak Ridge, TN, USA, 1975; p. 87. [Google Scholar]
- Steeman, E.; Temmerman, E.; Verbeek, F. Pulse Polarographic Determination of Ytterbium in Minerals. Bull. Sociétés Chim. Belg. 1977, 86, 499–502. [Google Scholar] [CrossRef]
- Hazen, R.M.; Golden, J.; Downs, R.T.; Hystad, G.; Grew, E.S.; Azzolini, D.; Sverjensky, D.A. Mercury (Hg) Mineral Evolution: A Mineralogical Record of Supercontinent Assembly, Changing Ocean Geochemistry, and the Emerging Terrestrial Biosphere. Am. Mineral. 2012, 97, 1013–1042. [Google Scholar] [CrossRef]
- Falkowski, P.G.; Fenchel, T.; Delong, E.F. The Microbial Engines that Drive Earth’s Biogeochemical Cycles. Science 2008, 320, 1034–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hazen, R.M.; Ausubel, J.H. On the Nature and Significance of Rarity in Mineralogy. Am. Mineral. 2016, 101, 1245–1251. [Google Scholar] [CrossRef] [Green Version]
- Hazen, R.M.; Morrison, S.M. On the Paragenetic Modes of Minerals: A Mineral Evolution Perspective. Am. Mineral. 2022; in press. [Google Scholar]
- Kim, J.D.; Senn, S.; Harel, A.; Jelen, B.I.; Falkowski, P.G. Discovering the Electronic Circuit Diagram of Life: Structural Relationships among Transition Metal Binding Sites in Oxidoreductases. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120257. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moore, E.K.; Martinez, D.L.; Srivastava, N.; Morrison, S.M.; Spielman, S.J. Mineral Element Insiders and Outliers Play Crucial Roles in Biological Evolution. Life 2022, 12, 951. https://doi.org/10.3390/life12070951
Moore EK, Martinez DL, Srivastava N, Morrison SM, Spielman SJ. Mineral Element Insiders and Outliers Play Crucial Roles in Biological Evolution. Life. 2022; 12(7):951. https://doi.org/10.3390/life12070951
Chicago/Turabian StyleMoore, Eli K., Daniella L. Martinez, Naman Srivastava, Shaunna M. Morrison, and Stephanie J. Spielman. 2022. "Mineral Element Insiders and Outliers Play Crucial Roles in Biological Evolution" Life 12, no. 7: 951. https://doi.org/10.3390/life12070951







