Abundance and Niche Differentiation of Comammox in the Sludges of Wastewater Treatment Plants That Use the Anaerobic–Anoxic–Aerobic Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Physicochemical Analysis
2.2. DNA Extraction and Quantitative PCR (qPCR)
2.3. High-Throughput Sequencing of the 16S rRNA Gene and Comammox amoA Gene
2.4. Data Processing and Statistical Analyses
2.5. Sequence Accession Numbers
3. Results
3.1. Physicochemical Parameters of the WWTPs
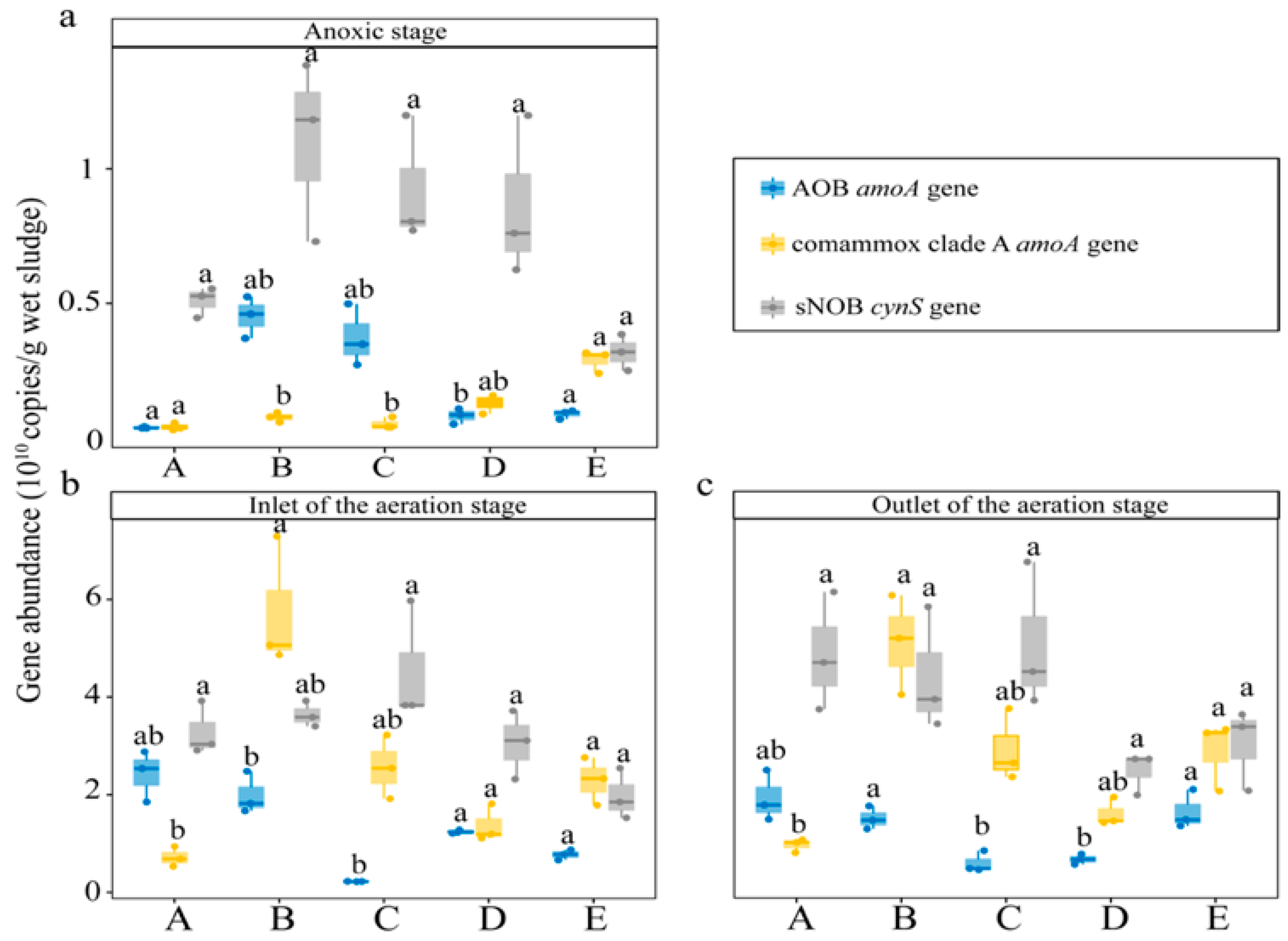
3.2. Abundance of Comammox and Other Nitrifiers in WWTPs
3.3. Composition and Distribution of Nitrospira and Nitrosomonas in WWTPs
3.4. Correlation of Comammox and Other Nitrifiers with Environmental Parameters
3.5. Network Analysis of Nitrospira and Nitrosomonas Communities
3.6. Phylogenetic Analysis and Relationships of Comammox OTUs with Environmental Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuypers MM, M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Arp, D.J.; Sayavedra-Soto, L.A.; Hommes, N.G. Molecular biology and biochemistry of ammonia oxidation by Nitrosomonas europaea. Arch. Microbiol. 2002, 178, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Konneke, M.; Bernhard, A.E.; de la Torre, J.R.; Walker, C.B.; Waterbury, J.B.; Stahl, D.A. Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 2005, 437, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Teske, A.; Alm, E.; Regan, J.M.; Toze, S.; Rittmann, B.E.; Stahl, D.A. Evolutionary relationships among ammonia-oxidizing and nitrite-oxidizing bacteria. J. Bacteriol. 1994, 176, 6623–6630. [Google Scholar] [CrossRef] [Green Version]
- Winogradsky, S. Recherches sur les organismes de la nitrification. Ann. Inst. Pasteur. 1890, 4, 213–231. [Google Scholar]
- Costa, E.; Perez, J.; Kreft, J.U. Why is metabolic labour divided in nitrification? Trends Microbiol. 2006, 14, 213–219. [Google Scholar] [CrossRef]
- Daims, H.; Lebedeva, E.V.; Pjevac, P.; Han, P.; Herbold, C.; Albertsen, M.; Jehmlich, N.; Palatinszky, M.; Vierheilig, J.; Bulaev, A.; et al. Complete nitrification by Nitrospira bacteria. Nature 2015, 528, 504–509. [Google Scholar] [CrossRef]
- van Kessel, M.A.; Speth, D.R.; Albertsen, M.; Nielsen, P.H.; Op den Camp, H.J.; Kartal, B.; Jetten, M.S.; Lucker, S. Complete nitrification by a single microorganism. Nature 2015, 528, 555–559. [Google Scholar] [CrossRef] [Green Version]
- Sakoula, D.; Koch, H.; Frank, J.; Jetten, M.S.M.; van Kessel, M.A.; Lucker, S. Enrichment and physiological characterization of a novel comammox Nitrospira indicates ammonium inhibition of complete nitrification. ISME J. 2021, 15, 1010–1024. [Google Scholar] [CrossRef]
- Kits, K.D.; Sedlacek, C.J.; Lebedeva, E.V.; Han, P.; Bulaev, A.; Pjevac, P.; Daebeler, A.; Romano, S.; Albertsen, M.; Stein, L.Y. Kinetic analysis of a complete nitrifier reveals an oligotrophic lifestyle. Nature 2017, 549, 269–272. [Google Scholar] [CrossRef] [Green Version]
- Pjevac, P.; Schauberger, C.; Poghosyan, L.; Herbold, C.W.; van Kessel, M.; Daebeler, A.; Steinberger, M.; Jetten, M.S.M.; Lucker, S.; Wagner, M.; et al. AmoA-targeted polymerase chain reaction primers for the specific detection and quantification of comammox Nitrospira in the environment. Front. Microbiol. 2017, 8, 1508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, F.; Wang, J.G.; Zhu, T.; Zou, B.; Rhee, S.K.; Quan, Z.X. Ubiquity and diversity of complete ammonia oxidizers (comammox). Appl. Environ. Microbiol. 2018, 84, e01390-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.Q.; Zhou, C.H.; Nie, M.; Gu, J.D.; Quan, Z.X. Abundance and niche specificity of different types of complete ammonia oxidizers (comammox) in salt marshes covered by different plants. Sci. Total Environ. 2021, 768, 144993. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.J.; Marcus, D.N.; Ijaz, U.Z.; Bautista-de Lose Santos, Q.M.; Dick, G.J.; Raskin, L. Metagenomic evidence for the presence of comammox Nitrospira-like bacteria in a drinking water system. mSphere 2016, 1, e00054-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Jiang, Y.; Wang, S.; Wang, X.; Zhu, G. Biogeographic distribution of comammox bacteria in diverse terrestrial habitats. Sci. Total Environ. 2020, 717, 137257. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.K.; Zhou, L.; He, B.P.; Qian, L.; Wan, S.; Qu, L.P. Nitrogen and phosphorus removal using fluidized-carriers in a full-scale A(2)O biofilm system. Biochem. Eng. J. 2016, 115, 47–55. [Google Scholar] [CrossRef]
- Massara, T.M.; Solis, B.; Guisasola, A.; Katsou, E.; Baeza, J.A. Development of an ASM2d-N2O model to describe nitrous oxide emissions in municipal WWTPs under dynamic conditions. Chem. Eng. J. 2018, 335, 185–196. [Google Scholar] [CrossRef] [Green Version]
- Oehmen, A.; Lopez-Vazquez, C.M.; Carvalho, G.; Reis, M.A.M.; van Loosdrecht, M.C.M. Modelling the population dynamics and metabolic diversity of organisms relevant in anaerobic/anoxic/aerobic enhanced biological phosphorus removal processes. Water Res. 2010, 44, 4473–4486. [Google Scholar] [CrossRef]
- Wiesmann, U. Biological nitrogen removal from wastewater. Adv. Biochem. Eng. Biotechnol. 1994, 51, 113–154. [Google Scholar]
- Otawa, K.; Asano, R.; Ohba, Y.; Sasaki, T.; Kawamura, E.; Koyama, F.; Nakamura, S.; Nakai, Y. Molecular analysis of ammonia-oxidizing bacteria community in intermittent aeration sequencing batch reactors used for animal wastewater treatment. Environ. Microbiol. 2006, 8, 1985–1996. [Google Scholar] [CrossRef]
- Ge, S.; Wang, S.; Yang, X.; Qiu, S.; Li, B.; Peng, Y. Detection of nitrifiers and evaluation of partial nitrification for wastewater treatment: A review. Chemosphere 2015, 140, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Burrell, P.C.; Phalen, C.M.; Hovanec, T.A. Identification of bacteria responsible for ammonia oxidation in freshwater aquaria. Appl. Environ. Microbiol. 2001, 67, 5791–5800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, L.; Zhang, T. Ammonia-oxidizing bacteria dominates over ammonia-oxidizing archaea in a saline nitrification reactor under low DO and high nitrogen loading. Biotechnol. Bioeng. 2011, 108, 2544–2552. [Google Scholar] [CrossRef] [PubMed]
- Pan, K.L.; Gao, J.F.; Fan, X.Y.; Li, D.C.; Dai, H.H. The more important role of archaea than bacteria in nitrification of wastewater treatment plants in cold season despite their numerical relationships. Water Res. 2018, 145, 552–561. [Google Scholar] [CrossRef]
- Whang, L.M.; Chien, I.C.; Yuan, S.L.; Wu, Y.J. Nitrifying community structures and nitrification performance of full-scale municipal and swine wastewater treatment plants. Chemosphere 2009, 75, 234–242. [Google Scholar] [CrossRef]
- Kim, Y.M.; Park, H.; Chandran, K. Nitrification inhibition by hexavalent chromium Cr(VI)—Microbial ecology, gene expression and off-gas emissions. Water Res. 2016, 92, 254–261. [Google Scholar] [CrossRef] [Green Version]
- Zheng, M.S.; Wang, M.Y.; Zhao, Z.R.; Zhou, N.; He, S.S.; Liu, S.F.; Wang, J.W.; Wang, X.K. Transcriptional activity and diversity of comammox bacteria as a previously overlooked ammonia oxidizing prokaryote in full-scale wastewater treatment plants. Sci. Total Environ. 2019, 656, 717–722. [Google Scholar] [CrossRef]
- Cotto, I.; Dai, Z.; Huo, L.; Anderson, C.L.; Vilardi, K.J.; Ijaz, U.; Khunjar, W.; Wilson, C.; De Clippeleir, H.; Gilmore, K.; et al. Long solids retention times and attached growth phase favor prevalence of comammox bacteria in nitrogen removal systems. Water Res. 2020, 169, 115268. [Google Scholar] [CrossRef]
- Palomo, A.; Pedersen, A.G.; Fowler, S.J.; Dechesne, A.; Sicheritz-Ponten, T.; Smets, B.F. Comparative genomics sheds light on niche differentiation and the evolutionary history of comammox Nitrospira. ISME J. 2018, 12, 1779–1793. [Google Scholar] [CrossRef] [Green Version]
- Roots, P.; Wang, Y.; Rosenthal, A.F.; Griffin, J.S.; Sabba, F.; Petrovich, M.; Yang, F.; Kozak, J.A.; Zhang, H.; Wells, G.F. Comammox Nitrospira are the dominant ammonia oxidizers in a mainstream low dissolved oxygen nitrification reactor. Water Res. 2019, 157, 396–405. [Google Scholar] [CrossRef]
- He, S.; Zhao, Z.; Tian, Z.; Xu, C.; Liu, Y.; He, D.; Zhang, Y.; Zheng, M. Comammox bacteria predominate among ammonia-oxidizing microorganisms in municipal but not in refinery wastewater treatment plants. J. Environ. Manag. 2022, 316, 115271. [Google Scholar] [CrossRef] [PubMed]
- Holman, J.B.; Wareham, D.G. COD, ammonia and dissolved oxygen time profiles in the simultaneous nitrification/denitrification process. Biochem. Eng. J. 2005, 22, 125–133. [Google Scholar] [CrossRef]
- Insel, G. Effects of design and aeration control parameters on simultaneous nitrification and denitrification (SNdN) performance for activated sludge process. Environ. Eng. Sci. 2007, 24, 675–686. [Google Scholar] [CrossRef]
- Wang, M.Y.; Huang, G.H.; Zhao, Z.R.; Dang, C.Y.; Liu, W.; Zheng, M.S. Newly designed primer pair revealed dominant and diverse comammox amoA gene in full-scale wastewater treatment plants. Bioresour. Technol. 2018, 270, 580–587. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fert. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Hood-Nowotny, R.; Hinko-Najera Umana, N.; Inselbacher, E.; Oswald-Lachouani, P.; Wanek, W. Alternative methods for measuring inorganic, organic, and total dissolved nitrogen in soil. Soil Sci. Soc. Am. J. 2010, 74, 1018–1027. [Google Scholar] [CrossRef]
- Jiang, R.; Wang, J.G.; Zhu, T.; Zou, B.; Wang, D.Q.; Rhee, S.K.; An, D.; Ji, Z.Y.; Quan, Z.X. Use of newly designed primers for quantification of complete ammonia-oxidizing (comammox) bacterial clades and strict nitrite oxidizers in the genus Nitrospira. Appl. Environ. Microbiol. 2020, 86, e01775-20. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glockner, F.O. The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res. 2014, 42, D643–D648. [Google Scholar] [CrossRef] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.G.; Xia, F.; Zeleke, J.; Zou, B.; Rhee, S.K.; Quan, Z.X. An improved protocol with a highly degenerate primer targeting copper-containing membrane-bound monooxygenase genes for community analysis of methane- and ammonia-oxidizing bacteria. FEMS Microbiol. Ecol. 2017, 93, fiw244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stecher, G.; Tamura, K.; Kumar, S. Molecular evolutionary genetics analysis (MEGA) for macOS. Mol. Biol. Evol. 2020, 37, 1237–1239. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Yang, Y.C.; Herbold, C.W.; Jung, M.Y.; Qin, W.; Cai, M.W.; Du, H.; Lin, J.G.; Li, X.Y.; Li, M.; Gu, J.D. Survival strategies of ammonia-oxidizing archaea (AOA) in a full-scale WWTP treating mixed landfill leachate containing copper ions and operating at low-intensity of aeration. Water Res. 2021, 191, 116798. [Google Scholar] [CrossRef]
- Zhou, X.; Li, B.; Wei, J.; Ye, Y.; Xu, J.; Chen, L.; Lu, C. Temperature influenced the comammox community composition in drinking water and wastewater treatment plants. Microb. Ecol. 2021, 82, 870–884. [Google Scholar] [CrossRef]
- Daims, H.; Nielsen, J.L.; Nielsen, P.H.; Schleifer, K.H.; Wagner, M. In situ characterization of Nitrospira-like nitrite oxidizing bacteria active in wastewater treatment plants. Appl. Environ. Microb. 2001, 67, 5273–5284. [Google Scholar] [CrossRef] [Green Version]
- Gruber-Dorninger, C.; Pester, M.; Kitzinger, K.; Savio, D.F.; Loy, A.; Rattei, T.; Wagner, M.; Daims, H. Functionally relevant diversity of closely related Nitrospira in activated sludge. ISME J. 2015, 9, 643–655. [Google Scholar] [CrossRef]
- Juretschko, S.; Timmermann, G.; Schmid, M.; Schleifer, K.H.; Pommerening-Roser, A.; Koops, H.P.; Wagner, M. Combined molecular and conventional analyses of nitrifying bacterium diversity in activated sludge: Nitrosococcus mobilis and Nitrospira-like bacteria as dominant populations. Appl. Environ. Microb. 1998, 64, 3042–3051. [Google Scholar] [CrossRef] [Green Version]
- Kruse, M.; Zumbragel, S.; Bakker, E.; Spieck, E.; Eggers, T.; Lipski, A. The nitrite-oxidizing community in activated sludge from a municipal wastewater treatment plant determined by fatty acid methyl ester-stable isotope probing. Syst. Appl. Microbiol. 2013, 36, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Maixner, F.; Noguera, D.R.; Anneser, B.; Maixner, F.; Noguera, D.R.; Anneser, B.; Stoecker, K.; Wegl, G.; Wagner, M.; Daims, H. Nitrite concentration influences the population structure of Nitrospira-like bacteria. Environ. Microbiol. 2006, 8, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Daims, H.; Lucker, S.; Wagner, M. A new perspective on microbes formerly known as nitrite-oxidizing bacteria. Trends Microbiol. 2016, 24, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Poly, F.; Wertz, S.; Brothier, E.; Degrange, V. First exploration of Nitrobacter diversity in soils by a PCR cloning-sequencing approach targeting functional gene nxrA. FEMS Microbiol. Ecol. 2008, 63, 132–140. [Google Scholar] [CrossRef] [Green Version]
- Alawi, M.; Off, S.; Kaya, M.; Spieck, E. Temperature influences the population structure of nitrite-oxidizing bacteria in activated sludge. Environ. Microbiol. Rep. 2009, 1, 184–190. [Google Scholar] [CrossRef]
- Rotthauwe, J.H.; Witzel, K.P.; Liesack, W. The ammonia monooxygenase structural gene amoA as a functional marker: Molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl. Environ. Microbiol. 1997, 63, 4704–4712. [Google Scholar] [CrossRef] [Green Version]
- Orschler, L.; Agrawal, S.; Lackner, S. Lost in translation: The quest for Nitrosomonas cluster 7-specific amoA primers and TaqMan probes. Microb. Biotechnol. 2020, 13, 2069–2076. [Google Scholar] [CrossRef]
- Camejo, P.Y.; Santo Domingo, J.; McMahon, K.D.; Noguera, D.R. Genome-enabled insights into the ecophysiology of the comammox bacterium “Candidatus Nitrospira nitrosa”. mSystems 2017, 2, e00059-17. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Fang, F.; Liu, G. Efficient nitrification and low-level N2O emission in a weakly acidic bioreactor at low dissolved-oxygen levels are due to comammox. Appl. Environ. Microbiol. 2021, 87, e00154-21. [Google Scholar] [CrossRef]
- Jiang, Q.; Xia, F.; Zhu, T.; Wang, D.; Quan, Z. Distribution of comammox and canonical ammonia-oxidizing bacteria in tidal flat sediments of the Yangtze River estuary at different depths over four seasons. J. Appl. Microbiol. 2019, 127, 533–543. [Google Scholar] [CrossRef]
- Sun, D.; Tang, X.; Zhao, M.; Zhang, Z.; Hou, L.; Liu, M.; Wang, B.; Klumper, U.; Han, P. Distribution and diversity of comammox Nitrospira in coastal wetlands of China. Front. Microbiol. 2020, 11, 589268. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Fujitani, H.; Hirono, Y.; Tago, K.; Wang, Y.; Hayatsu, M.; Tsuneda, S. Enrichment of comammox and nitrite-oxidizing Nitrospira from acidic soils. Front. Microbiol. 2020, 11, 1737. [Google Scholar] [CrossRef]
- Beach, N.K.; Noguera, D.R. Design and assessment of species-level qPCR primers targeting comammox. Front. Microbiol. 2019, 10, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belevich, I.; Borisov, V.B.; Konstantinov, A.A.; Verkhovsky, M.I. Oxygenated complex of cytochrome bd from Escherichia coli: Stability and photolability. FEBS Lett. 2005, 579, 4567–4570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Laz, S.; Kpebe, A.; Bauzan, M.; Lignon, S.; Rousset, M.; Brugna, M. A biochemical approach to study the role of the terminal oxidases in aerobic respiration in Shewanella oneidensis MR-1. PLoS ONE 2014, 9, e86343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borisov, V.B.; Gennis, R.B.; Hemp, J.; Verkhovsky, M.I. The cytochrome bd respiratory oxygen reductases. Biochim. Biophys. Acta 2011, 1807, 1398–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Straka, L.L.; Meinhardt, K.A.; Bollmann, A.; Stahl, D.A.; Winkler, M.H. Affinity informs environmental cooperation between ammonia-oxidizing archaea (AOA) and anaerobic ammonia-oxidizing (Anammox) bacteria. ISME J. 2019, 13, 1997–2004. [Google Scholar] [CrossRef]
- Ehrich, S.; Behrens, D.; Lebedeva, E.; Ludwig, W.; Bock, E. A new obligately chemolithoautotrophic, nitrite-oxidizing bacterium, Nitrospira moscoviensis sp. nov. and its phylogenetic relationship. Arch. Microbiol. 1995, 164, 16–23. [Google Scholar] [CrossRef]
- Koch, H.; Lucker, S.; Albertsen, M.; Kitzinger, K.; Herbold, C.; Spieck, E.; Nielsen, P.H.; Wagner, M.; Daims, H. Expanded metabolic versatility of ubiquitous nitrite-oxidizing bacteria from the genus Nitrospira. Proc. Natl. Acad. Sci. USA 2015, 112, 11371–11376. [Google Scholar] [CrossRef] [Green Version]
- Lucker, S.; Wagner, M.; Maixner, F.; Pelletier, E.; Koch, H.; Vacherie, B.; Rattei, T.; Damste, J.S.; Spieck, E.; Le Paslier, D.; et al. A Nitrospira metagenome illuminates the physiology and evolution of globally important nitrite-oxidizing bacteria. Proc. Natl. Acad. Sci. USA 2010, 107, 13479–13484. [Google Scholar] [CrossRef] [Green Version]
- Hosler, J.P.; Fetter, J.; Tecklenburg, M.M.; Espe, M.; Lerma, C.; Ferguson-Miller, S. Cytochrome aa3 of Rhodobacter sphaeroides as a model for mitochondrial cytochrome c oxidase. Purification, kinetics, proton pumping, and spectral analysis. J. Biol. Chem. 1992, 267, 24264–24272. [Google Scholar] [CrossRef]
- Muyzer, G.; de Waal, E.C.; Uitterlinden, A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakravorty, S.; Connell, N.; Alland, D. A detailed analysis of 16S ribosomal RNA gene segments for the diagnosis of pathogenic bacteria. J. Microbiol. Methods 2007, 69, 330–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, C.A.; Santoro, A.E.; Oakley, B.B. Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc. Natl. Acad. Sci. USA 2005, 102, 14683–14688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frank, J.A.; Reich, C.I.; Sharma, S.; Weisbaum, J.S.; Wilson, B.A.; Olsen, G.J. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl. Environ. Microbiol. 2008, 74, 2461–2470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amann, R.I.; Binder, B.J.; Olson, R.J.; Chisholm, S.W.; Devereux, R.; Stahl, D.A. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl. Environ. Microbiol. 1990, 56, 1919–1925. [Google Scholar] [CrossRef] [Green Version]




| Nitrate (mg/L) | Ammonium (mg/L) | Temperature (°C) | DO (mg/L) | pH | |
|---|---|---|---|---|---|
| A-A2 1 | 35.34 | 3.42 | 21.00 | 0.20 | 6.96 |
| A-O1 | 68.20 | 1.44 | 20.80 | 6.00 | 7.30 |
| A-O2 | 69.44 | - 2 | 21.10 | 7.50 | 7.35 |
| B-A2 | 89.90 | 14.04 | 19.90 | 0.40 | 6.82 |
| B-O1 | 47.12 | 3.06 | 20.20 | 2.30 | 7.35 |
| B-O2 | 83.70 | - | 19.90 | 1.70 | 7.27 |
| C-A2 | 83.70 | 5.04 | 19.90 | 0.30 | 6.82 |
| C-O1 | 106.02 | 1.80 | 19.70 | 6.70 | 7.20 |
| C-O2 | 114.08 | 0.72 | 20.00 | 12.70 3 | 7.33 |
| D-A2 | 44.64 | 20.70 | 19.30 | 0.50 | 6.80 |
| D-O1 | 73.78 | 8.10 | 19.20 | 8.00 | 7.70 |
| D-O2 | 109.12 | 0.36 | 19.80 | 7.10 | 7.70 |
| E-A2 | 88.66 | 4.50 | 19.30 | 0.50 | 6.77 |
| E-O1 | 107.26 | 3.96 | 19.10 | 7.00 | 7.50 |
| E-O2 | 80.60 | 0.18 | 19.10 | 5.70 | 7.52 |
| Ammonium Inf 1 | Ammonium Eff 2 | DO | Temperature | pH | Finding | Reference |
|---|---|---|---|---|---|---|
| (mg/L) | ||||||
| 18.3–38.3 | 0.4–1.7 | 2–5.2 | 18.8–27.1 | 7.1–7.8 | Comammox actively participate in ammonia oxidation in WWTPs. | [27] |
| 118 ± 15 | 6 ± 2 | 0.07 | 21.2 | 6.7 | AOA outnumber AOB or comammox and contribute the most to ammonia oxidation in WWTPs. | [46] |
| 26.9–49.0 | - | 0.1–3.2 | 15–34 | 7.01–8.07 | Comammox are the dominant ammonia oxidizers in WWTPs in different seasons. | [47] |
| - | 0–10.3 | 0–0.4 | - | 7.0–7.5 | Comammox are the dominant ammonia oxidizers in a sequencing batch reactor under low DO levels. | [58] |
| - | 18.4 ± 4.9 | 0.2–1.0 | 20.3 ± 1.1 | - 3 | Comammox are the dominant ammonia oxidizers in nitrification systems at low DO levels. | [30] |
| - | 0.69 ± 1.08 | 0.5 | - | 6.3–6.8 | Comammox are the dominant ammonia oxidizers under weakly acidic and low DO levels. | [59] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.-N.; Wang, J.-G.; Wang, D.-Q.; Jiang, Q.-Y.; Quan, Z.-X. Abundance and Niche Differentiation of Comammox in the Sludges of Wastewater Treatment Plants That Use the Anaerobic–Anoxic–Aerobic Process. Life 2022, 12, 954. https://doi.org/10.3390/life12070954
Zhang S-N, Wang J-G, Wang D-Q, Jiang Q-Y, Quan Z-X. Abundance and Niche Differentiation of Comammox in the Sludges of Wastewater Treatment Plants That Use the Anaerobic–Anoxic–Aerobic Process. Life. 2022; 12(7):954. https://doi.org/10.3390/life12070954
Chicago/Turabian StyleZhang, Sheng-Nan, Jian-Gong Wang, Dan-Qi Wang, Qiu-Yue Jiang, and Zhe-Xue Quan. 2022. "Abundance and Niche Differentiation of Comammox in the Sludges of Wastewater Treatment Plants That Use the Anaerobic–Anoxic–Aerobic Process" Life 12, no. 7: 954. https://doi.org/10.3390/life12070954






