Characterization and Biomechanical Study of a Novel Magnesium Potassium Phosphate Cement
Abstract
:1. Introduction
2. Materials and Methods
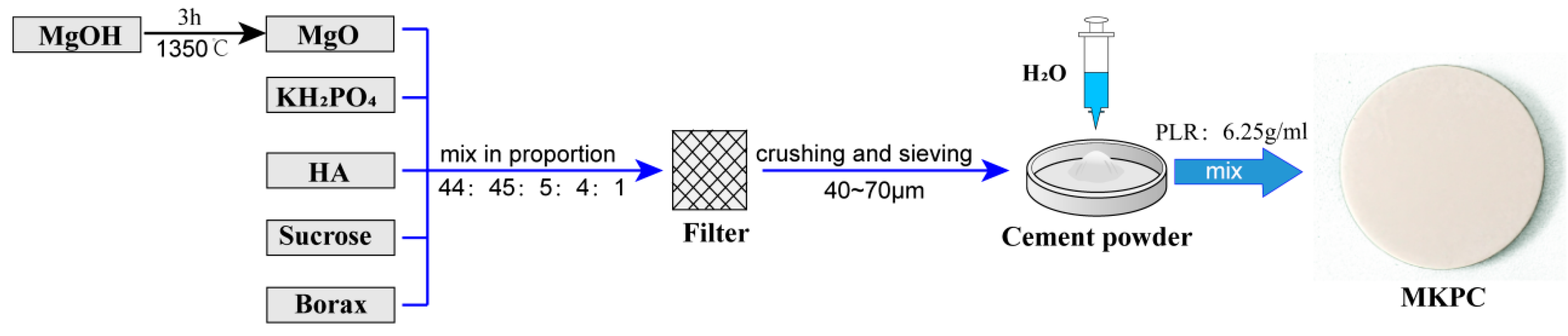
2.1. Cement Preparation
2.2. Compressive Strength, Exothermic Temperature, and Setting Time
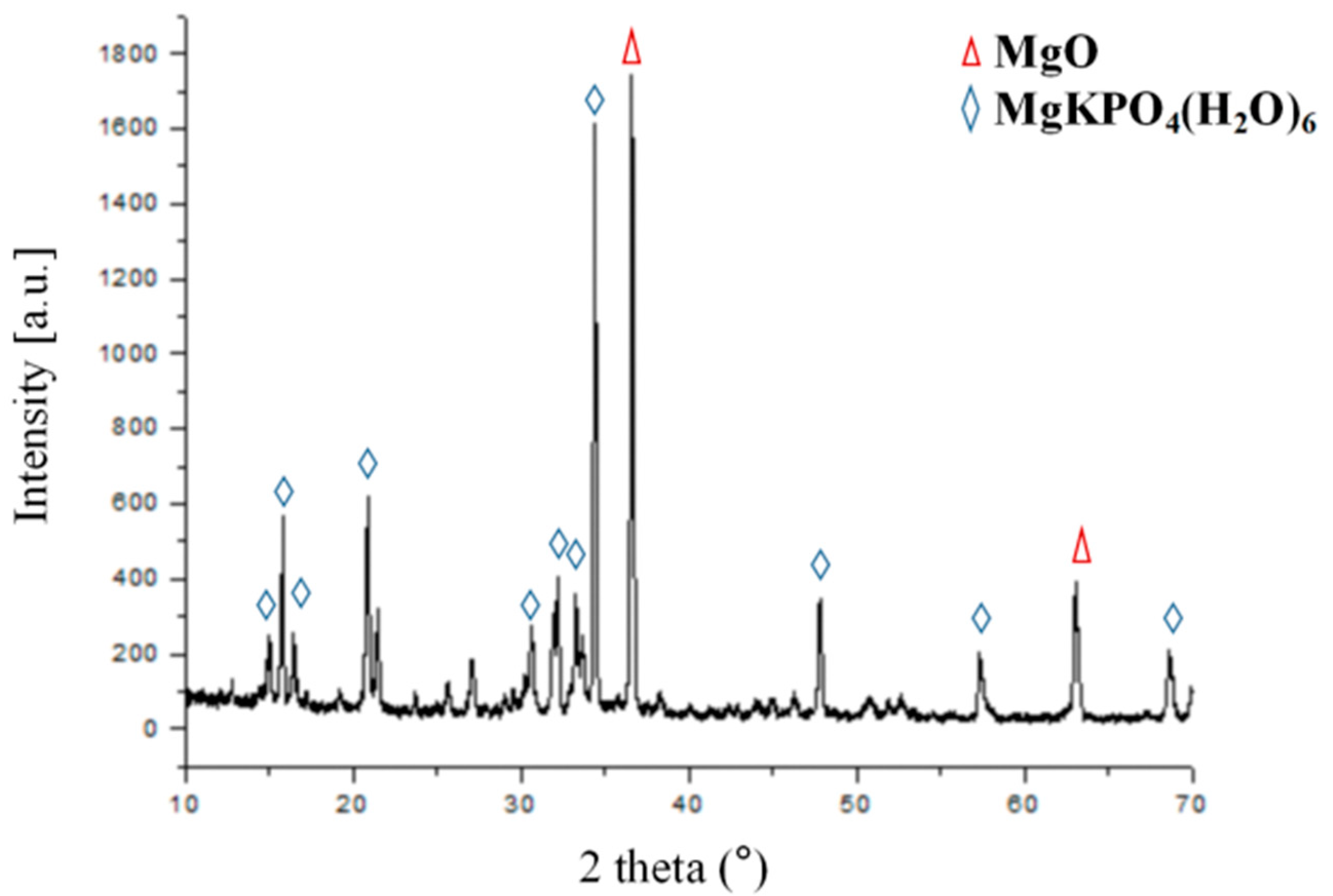
2.3. XRD and SEM
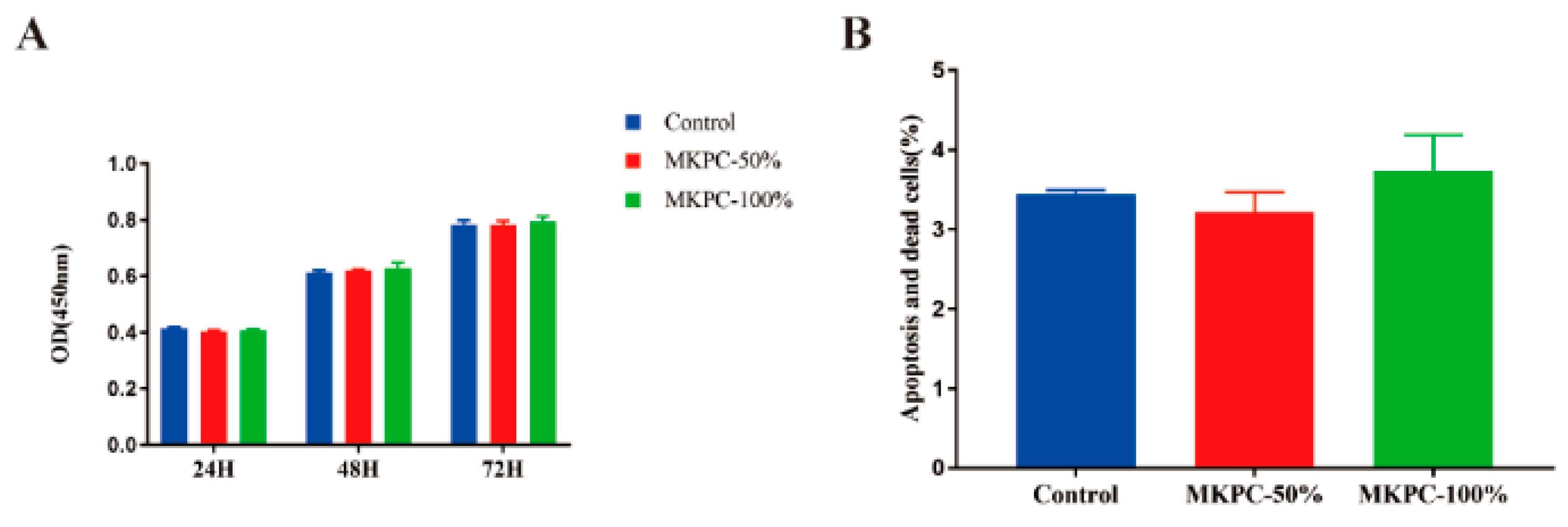
2.4. In Vitro Cytotoxicity Test
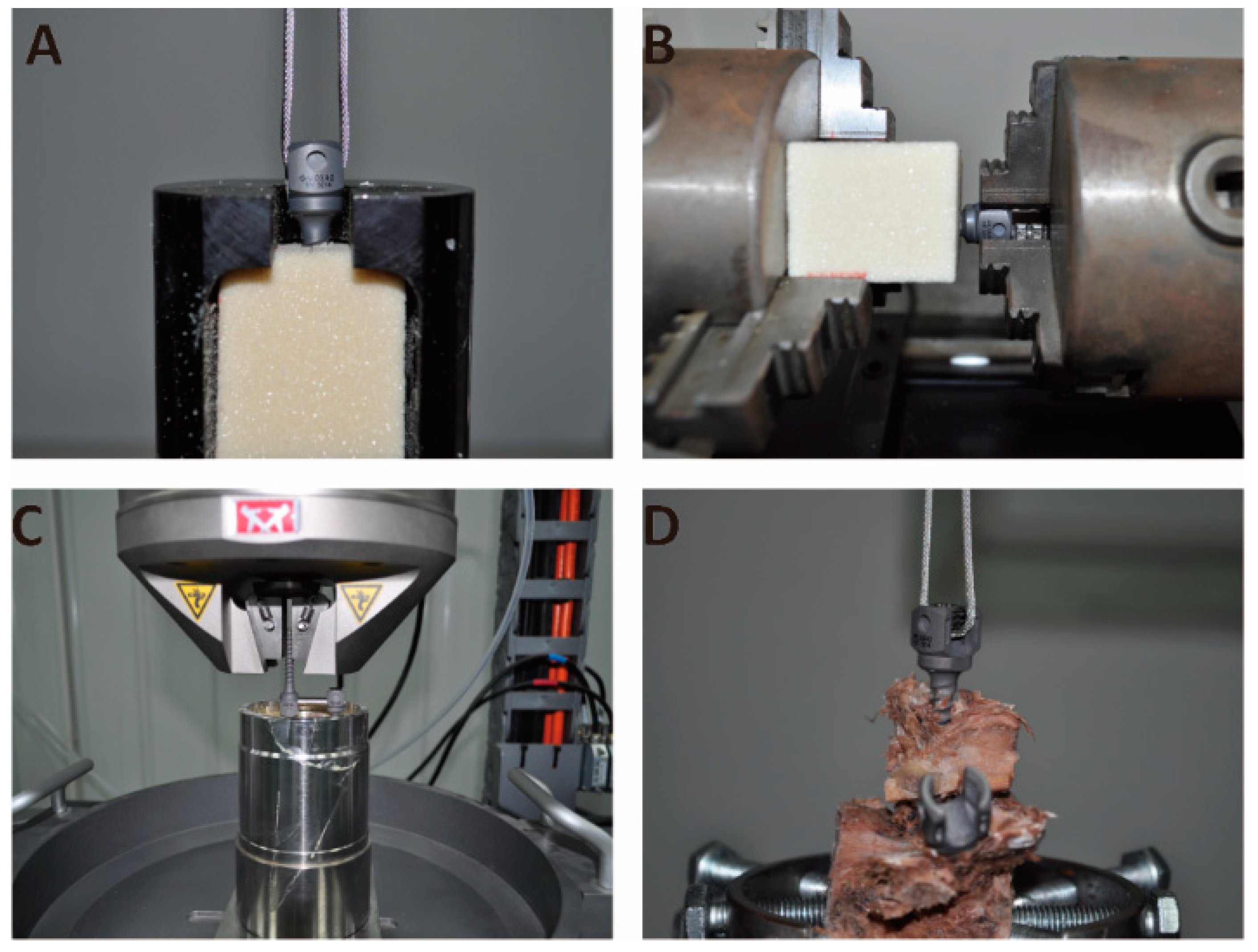
2.5. Biomechanical Tests
2.6. Statistical Analysis
3. Results
3.1. Material Characterization
3.2. Biomechanical Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostrowski, N.; Roy, A.; Kumta, P.N. Magnesium Phosphate Cement Systems for Hard Tissue Applications: A Review. ACS Biomater. Sci. Eng. 2016, 2, 1067–1083. [Google Scholar] [CrossRef] [PubMed]
- Kolk, A.; Handschel, J.; Drescher, W.; Rothamel, D.; Kloss, F.; Blessmann, M.; Heiland, M.; Wolff, K.D.; Smeets, R. Current Trends and Future Perspectives of Bone Substitute Materials—From Space Holders to Innovative Biomaterials. J. Craniomaxillofac. Surg. 2012, 40, 706–718. [Google Scholar] [CrossRef]
- Greenwald, A.S.; Boden, S.D.; Goldberg, V.M.; Khan, Y.; Laurencin, C.T.; Rosier, R.N.; American Academy of Orthopaedic Surgeons; The Committee on Biological Implants. Bone-Graft Substitutes: Facts, Fictions, and Applications. J. Bone Jt. Surg. Am. 2001, 83 (Suppl. 2), S98–S103. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Tang, X.; Gohil, S.V.; Laurencin, C.T. Biomaterials for Bone Regenerative Engineering. Adv. Healthc. Mater. 2015, 4, 1268–1285. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hao, W.; Wu, C.; Li, W.; Tao, J.; Ai, F.; Xin, H.; Wang, X. Injectable and Bioactive Bone Cement With Moderate Setting Time and Temperature Using Borosilicate Bio-Glass-Incorporated Magnesium Phosphate. Biomed. Mater. 2020, 15, 045015. [Google Scholar] [CrossRef]
- Sa, Y.; Yang, F.; Wang, Y.; Wolke, J.G.C.; Jansen, J.A. Modifications of Poly(Methyl Methacrylate) Cement for Application in Orthopedic Surgery. Adv. Exp. Med. Biol. 2018, 1078, 119–134. [Google Scholar] [CrossRef]
- Kühn, K.D.; Höntzsch, D. [Augmentation with PMMA Cement]. Unfallchirurg 2015, 118, 737–748. [Google Scholar] [CrossRef]
- Phakatkar, A.H.; Shirdar, M.R.; Qi, M.L.; Taheri, M.M.; Narayanan, S.; Foroozan, T.; Sharifi-Asl, S.; Huang, Z.; Agrawal, M.; Lu, Y.P.; et al. Novel PMMA Bone Cement Nanocomposites Containing Magnesium Phosphate Nanosheets and Hydroxyapatite Nanofibers. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110497. [Google Scholar] [CrossRef]
- Liu, J.; Liao, J.; Li, Y.; Yang, Z.; Ying, Q.; Xie, Y.; Zhou, A. Bioactive Tetracalcium Phosphate/Magnesium Phosphate Composite Bone Cement for Bone Repair. J. Biomater. Appl. 2019, 34, 239–249. [Google Scholar] [CrossRef]
- Wang, S.; Xu, C.; Yu, S.; Wu, X.; Jie, Z.; Dai, H. Citric Acid Enhances the Physical Properties, Cytocompatibility and Osteogenesis of Magnesium Calcium Phosphate Cement. J. Mech. Behav. Biomed. Mater. 2019, 94, 42–50. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewald, A.; Kreczy, D.; Brückner, T.; Gbureck, U.; Bengel, M.; Hoess, A.; Nies, B.; Bator, J.; Klammert, U.; Fuchs, A. Development and Bone Regeneration Capacity of Premixed Magnesium Phosphate Cement Pastes. Materials 2019, 12, 2119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Xu, C.; Dai, H. Preparation and Characterization of a Degradable Magnesium Phosphate Bone Cement. Regen. Biomater. 2016, 3, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Kanter, B.; Vikman, A.; Brückner, T.; Schamel, M.; Gbureck, U.; Ignatius, A. Bone Regeneration Capacity of Magnesium Phosphate Cements in a Large Animal Model. Acta Biomater. 2018, 69, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Waselau, M.; Samii, V.F.; Weisbrode, S.E.; Litsky, A.S.; Bertone, A.L. Effects of a Magnesium Adhesive Cement on Bone Stability and Healing Following a Metatarsal Osteotomy in Horses. Am. J. Vet. Res. 2007, 68, 370–378. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Wang, J.; Liu, C.; Zhang, B.; Chen, H.; Guo, H.; Zhong, G.; Qu, W.; Jiang, S.; Huang, H. Evaluation of Inherent Toxicology and Biocompatibility of Magnesium Phosphate Bone Cement. Colloids Surf. B Biointerfaces 2010, 76, 496–504. [Google Scholar] [CrossRef]
- Yoshizawa, S.; Brown, A.; Barchowsky, A.; Sfeir, C. Magnesium Ion Stimulation of Bone Marrow Stromal Cells Enhances Osteogenic Activity, Simulating the Effect of Magnesium Alloy Degradation. Acta Biomater. 2014, 10, 2834–2842. [Google Scholar] [CrossRef]
- Wu, L.; Feyerabend, F.; Schilling, A.F.; Willumeit-Römer, R.; Luthringer, B.J.C. Effects of Extracellular Magnesium Extract on the Proliferation and Differentiation of Human Osteoblasts and Osteoclasts in Coculture. Acta Biomater. 2015, 27, 294–304. [Google Scholar] [CrossRef]
- Mestres, G.; Fernandez-Yague, M.A.; Pastorino, D.; Montufar, E.B.; Canal, C.; Manzanares-Céspedes, M.C.; Ginebra, M.P. In Vivo Efficiency of Antimicrobial Inorganic Bone Grafts in Osteomyelitis Treatments. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 84–95. [Google Scholar] [CrossRef]
- Fan, S.; Chen, B. Experimental Study of Phosphate Salts Influencing Properties of Magnesium Phosphate Cement. Constr. Build. Mater. 2014, 65, 480–486. [Google Scholar] [CrossRef]
- White, A.A.; Best, S.M.; Kinloch, I.A. Hydroxyapatite–Carbon Nanotube Composites for Biomedical Applications: A Review. Int. J. Appl. Ceram. Technol. 2010, 4, 1–13. [Google Scholar] [CrossRef]
- Wang, A.J.; Yuan, Z.L.; Zhang, J.; Liu, L.T.; Li, J.M.; Liu, Z. Effect of Raw Material Ratios on the Compressive Strength of Magnesium Potassium Phosphate Chemically Bonded Ceramics. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 5058–5063. [Google Scholar] [CrossRef]
- Li, H.; Chang, J. Fabrication and Characterization of Bioactive Wollastonite/PHBV Composite Scaffolds. Biomaterials. 2004, 25, 5473–5480. [Google Scholar] [CrossRef] [PubMed]
- Kafchitsas, K.; Geiger, F.; Rauschmann, M.; Schmidt, S. Cement Distribution in Vertebroplasty Pedicle Screws With Different Designs. Orthopade 2010, 39, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Wilke, H.J.; Wenger, K.; Claes, L. Testing Criteria for Spinal Implants: Recommendations for the Standardization of In Vitro Stability Testing of Spinal Implants. Eur. Spine J. 1998, 7, 148–154. [Google Scholar] [CrossRef] [Green Version]
- Ginebra, M.P.; Fernández, E.; De Maeyer, E.A.; Verbeeck, R.M.; Boltong, M.G.; Ginebra, J.; Driessens, F.C.; Planell, J.A. Setting Reaction and Hardening of an Apatitic Calcium Phosphate Cement. J. Dent. Res. 1997, 76, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, C.; Atalay, B.; Caner, H.; Altinors, N. Augmentation of a Loosened Sacral Pedicle Screw With Percutaneous Polymethylmethacrylate Injection. J. Spinal Disord. Tech. 2006, 19, 373–375. [Google Scholar] [CrossRef]
- Mestres, G.; Abdolhosseini, M.; Bowles, W.; Huang, S.H.; Aparicio, C.; Gorr, S.U.; Ginebra, M.P. Antimicrobial Properties and Dentin Bonding Strength of Magnesium Phosphate Cements. Acta Biomater. 2013, 9, 8384–8393. [Google Scholar] [CrossRef]
- Mestres, G.; Ginebra, M.P. Novel Magnesium Phosphate Cements With High Early Strength and Antibacterial Properties. Acta Biomater. 2011, 7, 1853–1861. [Google Scholar] [CrossRef]
- Augustin, G.; Zigman, T.; Davila, S.; Udilljak, T.; Staroveski, T.; Brezak, D.; Babic, S. Cortical Bone Drilling and Thermal Osteonecrosis. Clin. Biomech. 2012, 27, 313–325. [Google Scholar] [CrossRef]
- Deramond, H.; Wright, N.T.; Belkoff, S.M. Temperature Elevation Caused by Bone Cement Polymerization During Vertebroplasty. Bone 1999, 25, 17S–21S. [Google Scholar] [CrossRef]
- Anselmetti, G.C.; Manca, A.; Kanika, K.; Murphy, K.; Eminefendic, H.; Masala, S.; Regge, D. Temperature Measurement During Polymerization of Bone Cement in Percutaneous Vertebroplasty: An In Vivo Study in Humans. Cardiovasc. Intervent. Rad. 2009, 32, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Hirvinen, L.J.; Litsky, A.S.; Samii, V.F.; Weisbrode, S.E.; Bertone, A.L. Influence of Bone Cements on Bone-Screw Interfaces in the Third Metacarpal and Third Metatarsal Bones of Horses. Am. J. Vet. Res. 2009, 70, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Chendel, S.A.; Peauroi, J. Magnesium-Based Bone Cement and Bone Void Filler: Preliminary Experimental Studies. J. Craniofac. Surg. 2009, 20, 461–464. [Google Scholar] [CrossRef]
- Sehlke, B.M.; Wilson, T.G.; Jones, A.A.; Yamashita, M.; Cochran, D.L. The Use of a Magnesium-Based Bone Cement to Secure Immediate Dental Implants. Int. J. Oral Maxillofac. Implants. 2013, 28, e357–e367. [Google Scholar] [CrossRef] [Green Version]
- OSTEOPAL® V User Manual, See. Available online: https://www.heraeus.com/en/group/home/home.html (accessed on 1 March 2022).
- Patel, P.S.; Shepherd, D.E.; Hukins, D.W. Compressive Properties of Commercially Available Polyurethane Foams as Mechanical Models for Osteoporotic Human Cancellous Bone. BMC Musculoskelet. Disord. 2008, 9, 137. [Google Scholar] [CrossRef] [Green Version]
- Frankel, B.M.; Jones, T.; Wang, C. Segmental Polymethylmethacrylate-Augmented Pedicle Screw Fixation in Patients With Bone Softening Caused by Osteoporosis and Metastatic Tumor Involvement: A Clinical Evaluation. Neurosurgery 2007, 61, 531–537. [Google Scholar] [CrossRef]


| Test Grouping | Cement Setting Time | |||
|---|---|---|---|---|
| 30 min | 12 h | 24 h | 72 h | |
| Pull-out testing (n = 6) | M/P | M/P | M/P | M/P |
| Torsion testing (n = 6) | M/P | |||
| Fatigue testing (n = 6) | M/P | |||
| Vertebral body testing (n = 5) | M/P | |||
| Samples (n = 6) | 1 | 2 | 3 | 4 | 5 | 6 | Mean ± SD |
|---|---|---|---|---|---|---|---|
| Compressive strength (MPa) | 56.04 | 43.10 | 46.06 | 44.52 | 50.76 | 49.23 | 48.29 ± 4.76 |
| Exothermic temperature (°C) | 45.20 | 43.70 | 47.50 | 44.90 | 46.70 | 45.30 | 45.55 ± 1.35 |
| Setting time (min) | 7.58 | 7.83 | 8.50 | 8.17 | 7.58 | 7.67 | 7.89 ± 0.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Z.; Wang, B.; Ren, B.; Liu, Y.; Zhang, N.; Wang, Z.; Liu, J.; Mao, K. Characterization and Biomechanical Study of a Novel Magnesium Potassium Phosphate Cement. Life 2022, 12, 997. https://doi.org/10.3390/life12070997
Han Z, Wang B, Ren B, Liu Y, Zhang N, Wang Z, Liu J, Mao K. Characterization and Biomechanical Study of a Novel Magnesium Potassium Phosphate Cement. Life. 2022; 12(7):997. https://doi.org/10.3390/life12070997
Chicago/Turabian StyleHan, Zhenchuan, Bo Wang, Bowen Ren, Yihao Liu, Nan Zhang, Zheng Wang, Jianheng Liu, and Keya Mao. 2022. "Characterization and Biomechanical Study of a Novel Magnesium Potassium Phosphate Cement" Life 12, no. 7: 997. https://doi.org/10.3390/life12070997
APA StyleHan, Z., Wang, B., Ren, B., Liu, Y., Zhang, N., Wang, Z., Liu, J., & Mao, K. (2022). Characterization and Biomechanical Study of a Novel Magnesium Potassium Phosphate Cement. Life, 12(7), 997. https://doi.org/10.3390/life12070997





