Suppression of Pepper mild mottle virus (PMMoV) by Modified Whey Proteins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Modification of Protein with Quercetin Dihydrate and Onion Extract
2.2. Determination of the Change in the Structure of Modified Proteins
2.2.1. The Change in Tryptophan Fluorescence Intensity and the Hydrophilic and Hydrophobic Character of Proteins
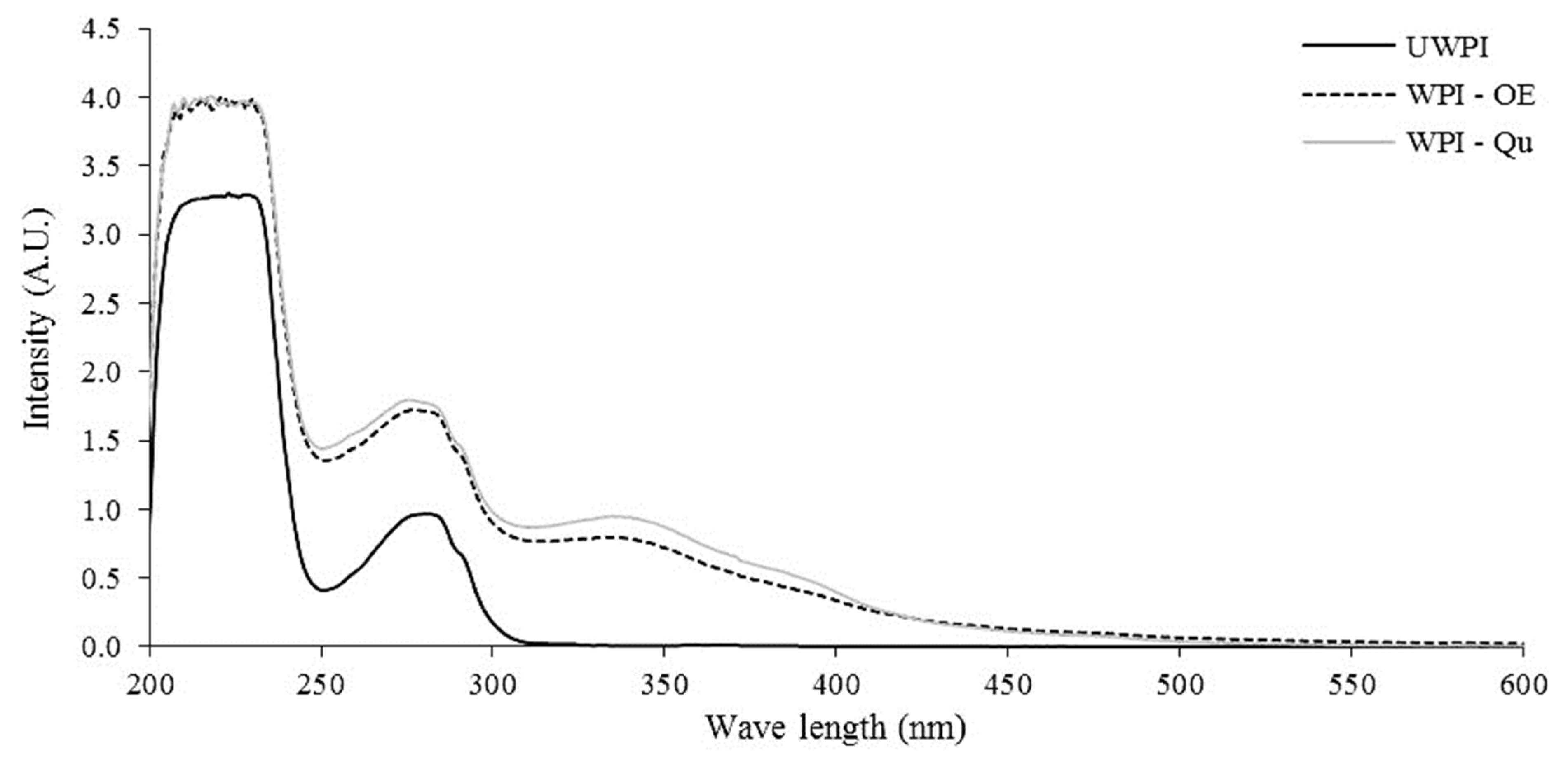
2.2.2. UV-Vis Spectrometry Measurements
2.3. Determination of Chemical Changes of Modified Proteins Using UHPLC-ESI-Q-TOF Mass Spectrometer Analysis
2.4. Effect of Modified Whey Proteins on Pepper mild mottle virus (PMMoV)
2.4.1. Plants and Pathogen
2.4.2. Effect of Modified Protein Treatments on PMMoV Disease Incidence and Severity in Pepper
2.4.3. Effect of Modified Whey Proteins on PMMoV Concentration in Pepper Plants
2.4.4. Quantitative RT-PCR (qRT-PCR)
2.4.5. Assessment of Defense Enzymes and Total Phenols
2.4.6. Assessments of Total Antioxidant Status (TAS) and Vitamin C
2.5. Statistical Analysis
3. Results
3.1. Characterization of the Change in the Structure of Modified Proteins
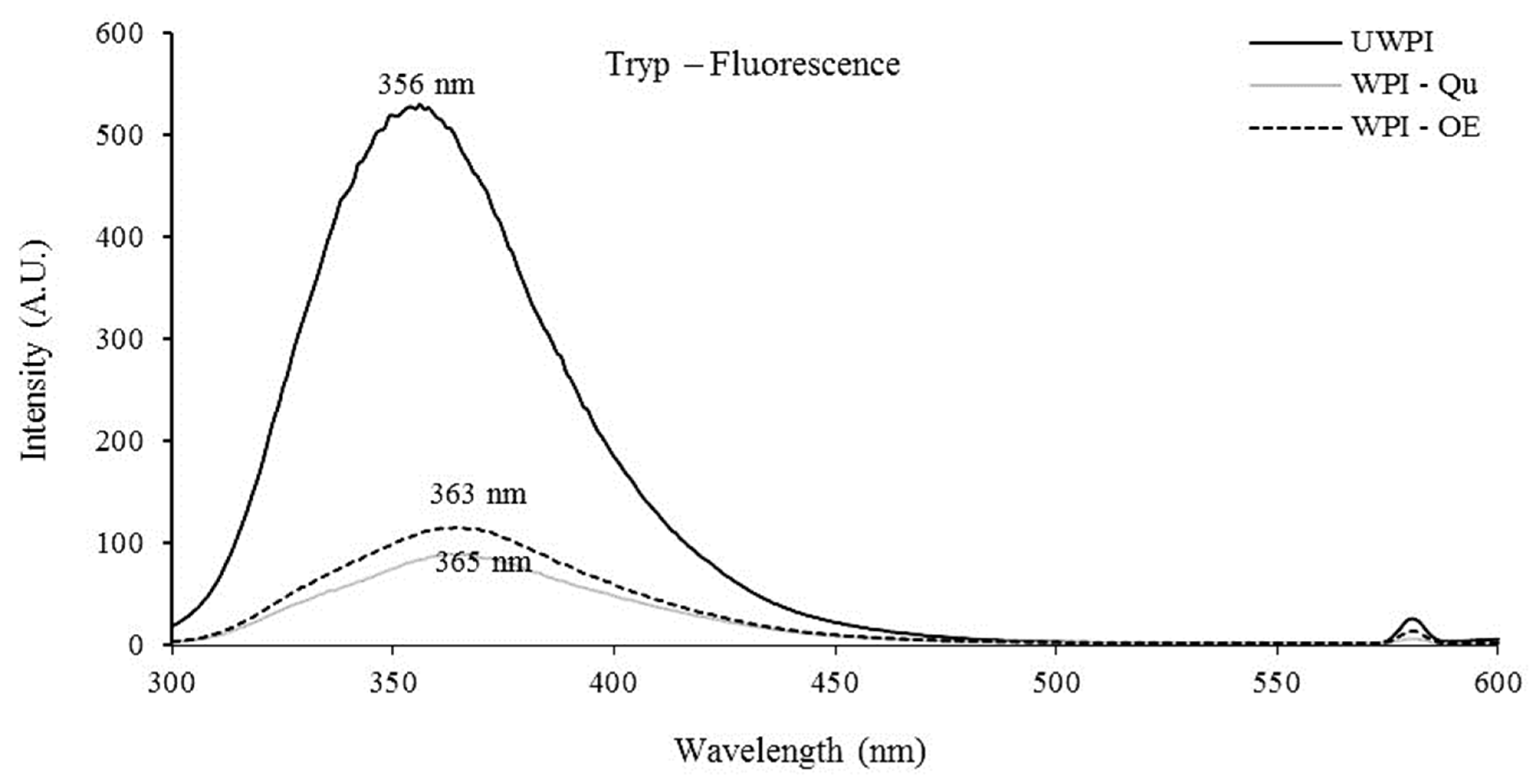
3.1.1. The Change in Tryptophan Fluorescence Scans and Hydrophilic/Hydrophobic Character of Modified Proteins
3.1.2. The Change in UV-Vis Spectra of Proteins
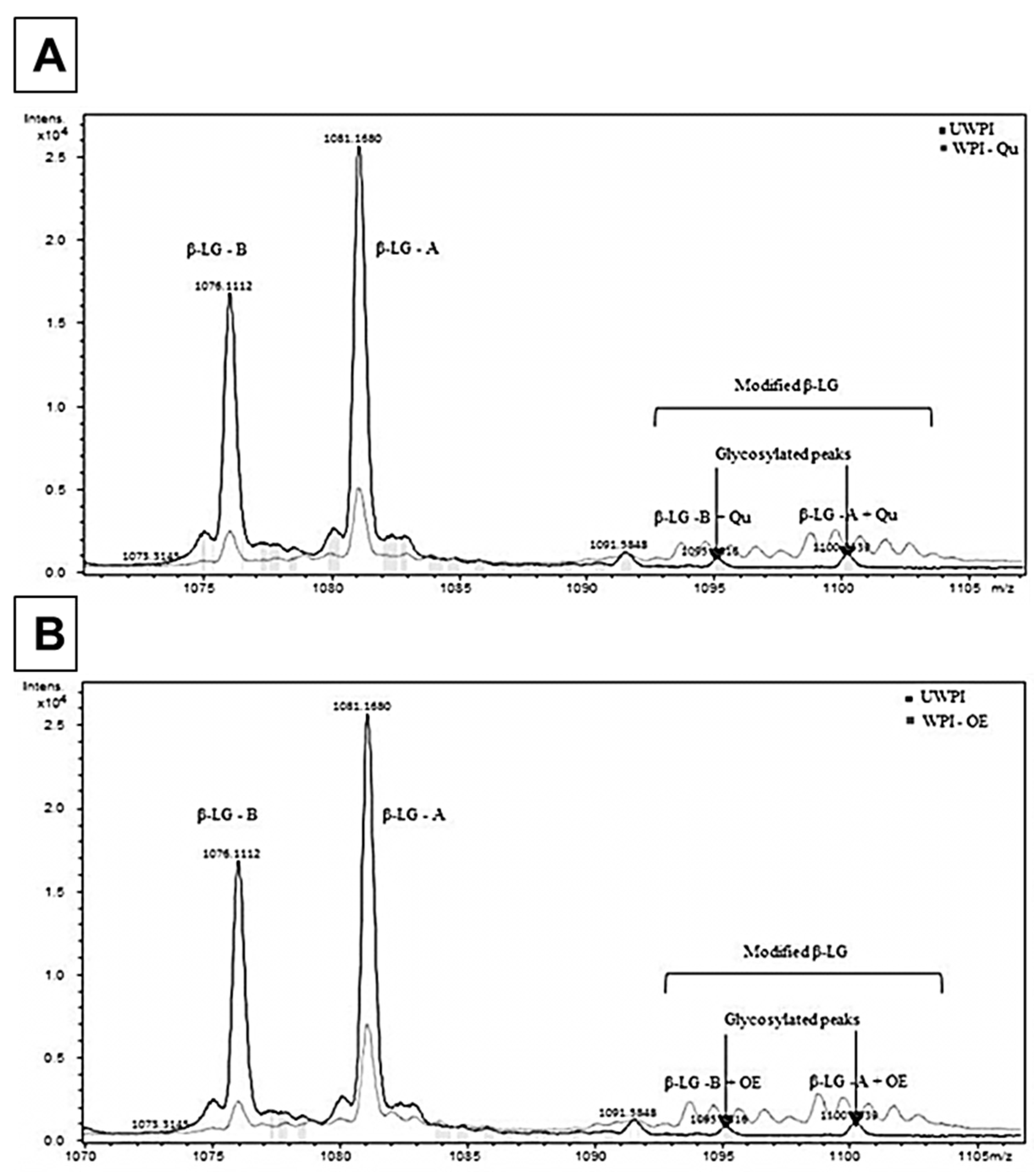
3.2. Characterization of the Change in the Chemical Properties of Modified Proteins
3.3. Systemic Resistance Induced by Modified Proteins against Pepper mild mottle virus (PMMoV)
3.3.1. Effects of Modified Proteins on the Severity and Concentration of PMMoV
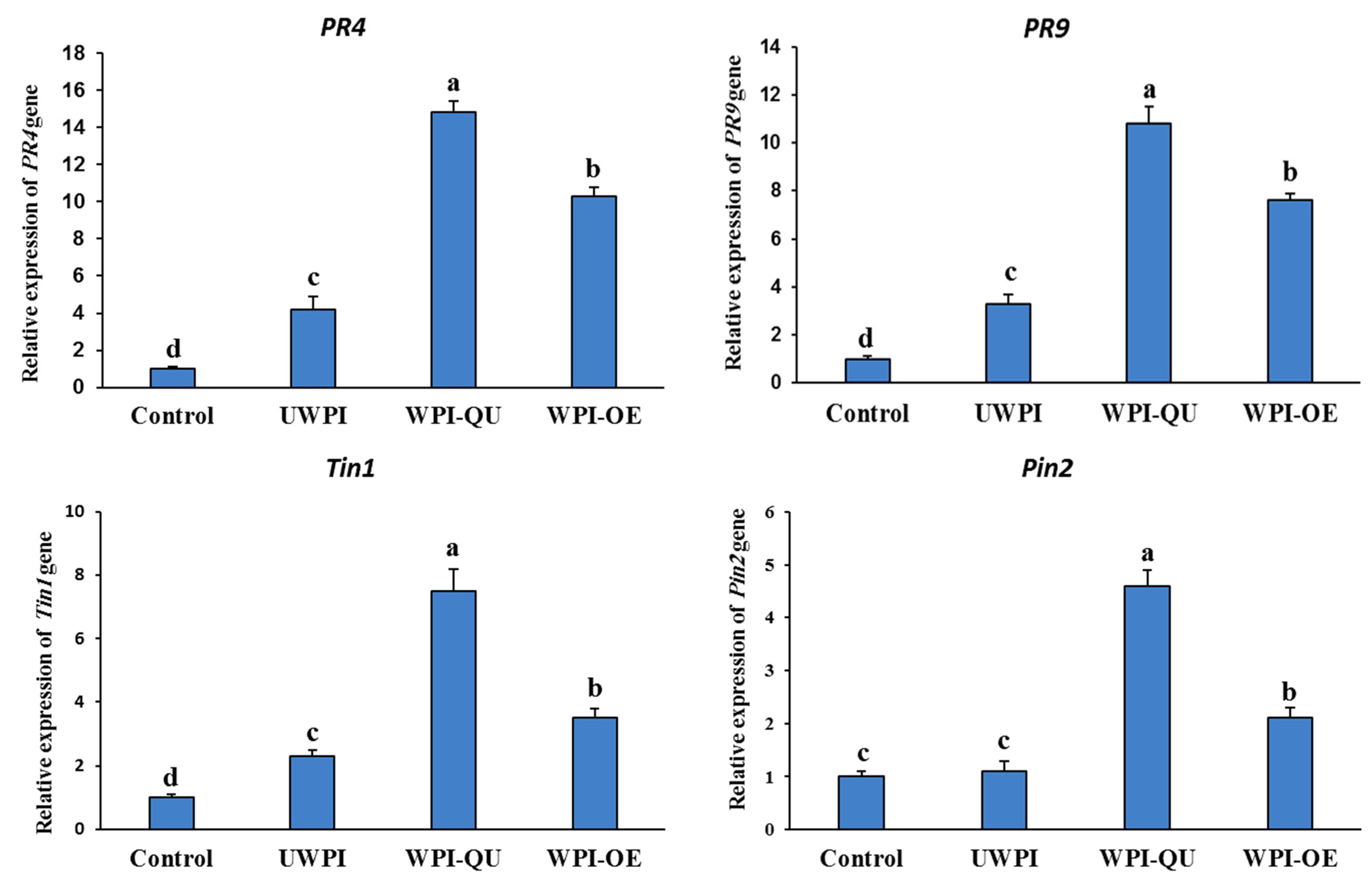
3.3.2. Effect of Modified Whey Protein Treatments on the Expression of Defense-Related Genes
3.3.3. Effect of Modified Whey Proteins on Total Phenol Contents and Defense Enzyme Activities
3.3.4. Effect of Modified Whey Proteins on Certain Plant Growth Parameters
3.3.5. Effects of Modified Whey Proteins on Antioxidative Parameter and Vitamin C Contents
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gui, F.; Wu, J.; Chen, F.; Liao, X.; Hu, X.; Zhang, Z.; Wang, Z. Change of polyphenol oxidase activity, color, and browning degree during storage of cloudy apple juice treated by supercritical carbon dioxide. Eur. Food Res. Technol. 2006, 223, 427–432. [Google Scholar] [CrossRef]
- Crozier, A.; Clifford, M.N.; Ashihara, H. Plant Secondary Metabolites: Occurrence, Structure and Role in the Human Diet; John Wiley & Sons: Hoboken, NJ, USA, 2008; 384p. [Google Scholar]
- Rohn, S.; Rawel, H.M.; Kroll, J. Antioxidant activity of protein-bound quercetin. J. Agric. Food Chem. 2004, 52, 4725–4729. [Google Scholar] [CrossRef] [PubMed]
- Von Staszewski, M.; Pilosof, A.M.; Jagus, R.J. Antioxidant and antimicrobial performance of different Argentinean green tea varieties as affected by whey proteins. Food Chem. 2011, 125, 186–192. [Google Scholar] [CrossRef]
- Anandakumar, P.; Kamaraj, S.; Jagan, S.; Ramakrishnan, G.; Vinodhkumar, R.; Devaki, D. Capsaicin modulates pulmonary antioxidant defense system during benzo(a) pyrene-induced lung cancer in Swiss Albino mice. Phytoter. Res. 2008, 22, 529–533. [Google Scholar] [CrossRef]
- Aycicek, A.; Erel, O.; Kocyigit, A.; Selek, S.; Demirkol, M.R. Breast milk provides better antioxidant power than does formula. Nutrition 2006, 22, 616–619. [Google Scholar] [CrossRef]
- Güldür, M.E.; Çağlar, B.K. Outbreaks of pepper mild mottled virus in greenhouses in Sanliurfa, Turkey. J. Plant Pathol. 2006, 88, 339–342. [Google Scholar]
- Jarret, R.L.; Gillaspie, A.G.; Barkley, N.A.; Pinnow, D.L. The Occurrence and Control of Pepper mild mottle virus (PMMoV) in the USDA/ARS Capsicum germplasm collection. Seed Technol. J. 2008, 30, 26–36. [Google Scholar]
- Colson, P.; Richet, H.; Desnues, C.; Balique, F.; Moal, V.; Grob, J.J.; Berbis, P.; Lecoq, H.; Harlé, J.R.; Berland, Y.; et al. Pepper mild mottle virus, a plant virus associated with specific immune responses, Fever, abdominal pains, and pruritus in humans. PLoS ONE 2010, 5, e10041. [Google Scholar] [CrossRef]
- Pan, Y.; Lee, A.; Wan, J.; Coventry, M.; Michalsk, W.; Shiell, B.; Roginski, H. Antiviral properties of milk proteins and peptides (A Review). Int. Dairy J. 2006, 16, 1252–1261. [Google Scholar] [CrossRef]
- Chobert, J.; Sitohy, M.; Billaudel, S.; Michele, D.; Haertle, T. Anticytomegaloviral activity of esterified milk proteins and l-Polylysines. Mol. Microbiol. Biotechnol. 2007, 13, 255–258. [Google Scholar] [CrossRef]
- Ali, M.; Keppler, J.K.; Coenye, T.; Schwarz, K. Covalent Whey protein-rosmarinic acid interactions: A Comparison of alkaline and enzymatic modifications on physicochemical, antioxidative, and antibacterial properties. J. Food Sci. 2018, 83, 2092–2100. [Google Scholar] [CrossRef]
- Elsharkawy, M.M.; Shimizu, M.; Takahashi, H.; Hyakumachi, M. Induction of systemic resistance against Cucumber mosaic virus by Penicillium simplicissimum GP17-2 in Arabidopsis and tobacco. Plant Pathol. 2012, 61, 964–976. [Google Scholar] [CrossRef]
- Elsharkawy, M.M.; Mousa, K.M. Induction of systemic resistance against Papaya ring spot virus (PRSV) and its vector Myzus persicae by Penicillium simplicissimum GP17-2 and silica (Sio2) nanopowder. Int. J. Pest Manag. 2015, 61, 353–358. [Google Scholar] [CrossRef]
- Song, R.; Sponer, N.; He, L. Methods to quantify microRNAs in the Myc gene network for posttranscriptional gene repression. Methods Mol. Biol. 2013, 1012, 135–144. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Terefe, N.S.; Yang, Y.H.; Knoerzer, K.; Buckow, R.; Versteeg, C. High Pressure and Thermal Inactivation kinetics of polyphenol oxidase and peroxidase in strawberry puree. Innov. Food Sci. Emerg. Technol. 2010, 11, 52–60. [Google Scholar] [CrossRef]
- Deng, M.; Deng, Y.Y.; Dong, L.H.; Ma, Y.X.; Liu, L.; Huang, F.; Wei, Z.C.; Zhang, Y.; Zhang, M.W.; Zhang, R.F. Effect of storage conditions on phenolic profiles and antioxidant activity of litchi pericarp. Molecules 2018, 23, 2276. [Google Scholar] [CrossRef] [Green Version]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Erel, O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin. Biochem. 2004, 37, 112–119. [Google Scholar] [CrossRef]
- Dikilitas, M.; Guldur, M.E.; Deryaoglu, A.; Erel, O. Antioxidant and Oxidant Levels of Pepper (Capsicum annuum cv. ‘Charlee’) Infected with Pepper Mild Mottle Virus. Not. Bot. Horti Agrobot. 2011, 39, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Abdelbacki, A.M.; Taha, S.H.; Sitohy, M.Z.; Abou Dawood, A.I.; Abd-EL Hamid, M.M.; Rezk, A.A. Inhibition of Tomato Yellow Leaf Curl Virus (TYLCV) using whey proteins. Virol. J. 2010, 7, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waarts, B.; Aneke, O.J.; Smit, J.M.; Kimata, K.; Bittman, R.; Meijer, D.K.F. Antiviral activity of human lactoferrin: Inhibition of alphavirus interaction with heparan sulfate. Virology 2005, 333, 284–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papadopoulou, A.; Green, R.J.; Frazier, R.A. Interaction of flavonoids with bovine serum albumin: A fluorescence quenching study. J. Agric. Food Chem. 2005, 53, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Homann, T.; Khalil, M.; Kruse, H.-P.; Rawel, H. Milk whey protein modification by coffee-specific phenolics: Effect on structural and functional properties. J. Agric. Food Chem. 2013, 61, 6911–6920. [Google Scholar] [CrossRef]
- Cao, Y.; Xiong, Y.L. Interaction of Whey Proteins with Phenolic Derivatives Under Neutral and Acidic pH Conditions. J. Food Sci. 2017, 82, 409–419. [Google Scholar] [CrossRef]
- Keppler, J.K.; Koudelka, T.; Palani, K.; Stuhldreier, M.C.; Temps, F.; Tholey, A.; Schwarz, K. Characterization of the covalent binding of allyl isothiocyanate to β-lactoglobulin by fluorescence quenching, equilibrium measurement, and mass spectrometry. J. Biomol. Struct. Dyn. 2014, 32, 1103–1117. [Google Scholar] [CrossRef]
- Wilde, S.C.; Treitz, C.; Keppler, J.K.; Koudelka, T.; Palani, K.; Tholey, A.; Rawel, H.M.; Schwarz, K. β-Lactoglobulin as nanotransporter—Part II: Characterization of the covalent protein modification by allicin and diallyl disulfide. Food Chem. 2016, 197, 1022–1029. [Google Scholar] [CrossRef]
- McGrath, M.T.; Shiskoff, N. Evaluation of biocompatible products for managing cucurbit powdery mildew. Crop Prot. 1999, 18, 471–478. [Google Scholar] [CrossRef]
- Bettiol, W.; Harllen, S.A.; Reis, R.C. Effectiveness of whey against zucchini squash and cucumber powdery mildew. Sci. Hortic. 2008, 117, 82–84. [Google Scholar] [CrossRef] [Green Version]
- Crisp, P.; Wicks, T.J.; Troup, G.; Scott, E.S. Mode of action of milk and whey in the control of grapevine powdery mildew. Australas. Plant Pathol. 2006, 35, 487–493. [Google Scholar] [CrossRef]
- Harish, S.; Kavino, M.; Kumar, N.; Saravanakumar, D.; Soorianathasundaram, K.; Samiyappan, R. Biohardening with plant growth promoting rhizosphere and endophytic bacteria induces systemic resistance against Banana bunchy top virus’. Appl. Soil Ecol. 2008, 39, 187–200. [Google Scholar] [CrossRef]
- Hernández-Ruiz, K.L.; Ruiz-Cruz, S.; Cira-Chávez, L.A.; Gassos-Ortega, L.E.; Ornelas-Paz, J.D.J.; Del-ToroSánchez, C.L.; Márquez-Ríos, E.; López-Mata, M.A.; Rodríguez-Félix, F. Evaluation of Antioxidant Capacity, Protective Effect on Human Erythrocytes and Phenolic Compound Identification in Two Varieties of Plum Fruit (Spondias spp.) By UPLC-MS. Molecules 2018, 23, 3200. [Google Scholar] [CrossRef] [Green Version]
- Elsharkawy, M.M.; El-Khateeb, N.M. Antifungal activity and resistance induction against Sclerotium cepivorum by plant growth-promoting fungi in onion plants. Egypt. J. Biol. Pest Control 2019, 29, 68. [Google Scholar] [CrossRef]
- El-kazzaz, M.K.; Ghoneim, K.E.; Agha, M.K.M.; Daif, A.H.; Elsharkawy, M.M. Utilization of non-traditional control methods of pepper root rot and wilt diseases caused by Fusarium oxysporum and Rhizoctonia solani. Egy. J. Plant Pro. Res. 2019, 7, 17–39. [Google Scholar]
- Krishna, A.G.G.; Lokesh, B.R.; Sugasini, D.; Kancheva, V.D. Evaluation of the antiradical and antioxidant properties of extracts from Indian red chili and black pepper by in vitro models. Bulg. Chem Communic 2010, 42, 62–69. [Google Scholar]
- Navarro, J.M.; Flores, P.; Garrido, C.; Martinez, V. Changes in the contents of antioxidant compounds in pepper fruits as different ripening stages, as affected by salinity. Food Chem. 2006, 96, 66–73. [Google Scholar] [CrossRef]
- Diwan, H.; Khan, I.; Ahmad, A.; Iqbal, M. Induction of phytochelatins and antioxidant defence system in Brassica juncea and Vigna radiata in response to chromium treatments. Plant Growth Regul. 2010, 61, 97–107. [Google Scholar] [CrossRef]
- Mishra, J.; Srivastava, R.; Trivedi, P.K.; Verma, P.C. Effect of virus infection on the secondary metabolite production and phytohormone biosynthesis in plants. 3 Biotech 2020, 10, 547. [Google Scholar] [CrossRef]


| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Tin1 | AGCCTGAAATAGAAGAAACGGAGATGGAGATGAG | GGAACCAGAATTGGTTACTCATGGCTACCTGAAC |
| PIN2 | TGGGACTTTCATTTGTGAAGGAGAG | GACACAGTGAATAGGCAATATTTGG |
| PR4 | AACTGGGATTGAGAACTGCCAGC | ATCCAAGGTACATATAGAGCTTCC |
| PR9 | GACTAGTTTCAAGAGCATCA | AATTGTATAGCCTGTAGCTG |
| Actin | CACTGAAGCACCCTTGAACCC | GAGACAACACCGCCTGAATAGC |
| Treatment | 2 WPI | 3WPI | ||
|---|---|---|---|---|
| DI (%) | DS | DI (%) | DS | |
| UWPI | 25b | 2.4b | 40b | 2.9b |
| WPI-QU | 12d | 1.1d | 20d | 1.4d |
| WPI-OE | 18c | 1.8c | 28c | 2.1c |
| Control | 68a | 4.3a | 89a | 5a |
| Treatment | 2 WPI | 3WPI |
|---|---|---|
| UWPI | 0.54b | 0.81b |
| WPI-QU | 0.21d | 0.47d |
| WPI-OE | 0.39c | 0.60c |
| Control | 1.04a | 1.49a |
| Treatment | Phenol (mg g−1 FW) | POX (U g−1 FW) | PPO (U g−1 FW) |
|---|---|---|---|
| UWPI | 0.509c | 0.562c | 0.282c |
| WPI-QU | 0.738a | 0.896a | 0.401a |
| WPI-OE | 0.643b | 0.782b | 0.312b |
| Control | 0.311d | 0.414d | 0.131d |
| Treatment | Plant Height (cm) | No. Leaves/Plant | Shoot Weight (g) | |
|---|---|---|---|---|
| Fresh | Dry | |||
| Control | 19.52d | 9.03c | 6.84d | 2.37d |
| UWPI | 24.26c | 13.46b | 8.14c | 2.98c |
| WPI-QU | 31.00a | 15.32a | 9.35a | 3.96a |
| WPI-OE | 28.16b | 14.93a | 8.61b | 3.45b |
| Treatment | TAS, µmol Trolox Eq g −1 Fwt | Vitamin C, µmol g −1 Fwt |
|---|---|---|
| UWPI | 4.6c | 3.1c |
| WPI-QU | 6.8a | 4.2a |
| WPI-OE | 5.5b | 3.9b |
| Control | 3.5d | 2.4d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsharkawy, M.M.; Al-Askar, A.A.; Abdelkhalek, A.; Behiry, S.I.; Kamran, M.; Ali, M. Suppression of Pepper mild mottle virus (PMMoV) by Modified Whey Proteins. Life 2022, 12, 1165. https://doi.org/10.3390/life12081165
Elsharkawy MM, Al-Askar AA, Abdelkhalek A, Behiry SI, Kamran M, Ali M. Suppression of Pepper mild mottle virus (PMMoV) by Modified Whey Proteins. Life. 2022; 12(8):1165. https://doi.org/10.3390/life12081165
Chicago/Turabian StyleElsharkawy, Mohsen Mohamed, Abdulaziz A. Al-Askar, Ahmed Abdelkhalek, Said I. Behiry, Muhammad Kamran, and Mostafa Ali. 2022. "Suppression of Pepper mild mottle virus (PMMoV) by Modified Whey Proteins" Life 12, no. 8: 1165. https://doi.org/10.3390/life12081165
APA StyleElsharkawy, M. M., Al-Askar, A. A., Abdelkhalek, A., Behiry, S. I., Kamran, M., & Ali, M. (2022). Suppression of Pepper mild mottle virus (PMMoV) by Modified Whey Proteins. Life, 12(8), 1165. https://doi.org/10.3390/life12081165










