Association of NT-proBNP and hs-cTnT with Imaging Markers of Diastolic Dysfunction and Focal Myocardial Fibrosis in Hypertrophic Cardiomyopathy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Echocardiography
2.3. CMR Protocol and Analysis
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Regression Analysis
3.2.1. Association of NT-proBNP with Echocardiographic Parameters of Diastolic Dysfunction
3.2.2. Association of hs-cTnT with Focal Myocardial Fibrosis
4. Discussion
4.1. NT-proBNP and Diastolic Dysfunction in HCM
4.2. Hs-cTnT and Fibrosis in HCM
4.3. Overlap between Associations
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wigle, E.D.; Silver, M.D. Myocardial fiber disarray and ventricular septal hypertrophy in asymmetrical hypertrophy of the heart. Circulation 1978, 58, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Arteaga, E.; Araujo, A.Q.; Buck, P.; Ianni, B.M.; Rabello, R.; Mady, C. Plasma amino-terminal pro-B-type natriuretic peptide quantification in hypertrophic cardiomyopathy. Am. Heart J. 2005, 150, 1228–1232. [Google Scholar] [CrossRef] [PubMed]
- Cramer, G.; Bakker, J.; Gommans, F.; Brouwer, M.; Kurvers, M.; Fouraux, M.; Verheugt, F.; Kofflard, M. Relation of highly sensitive cardiac troponin T in hypertrophic cardiomyopathy to left ventricular mass and cardiovascular risk. Am. J. Cardiol. 2014, 113, 1240–1245. [Google Scholar] [CrossRef]
- Hasegawa, K.; Fujiwara, H.; Doyama, K.; Miyamae, M.; Fujiwara, T.; Suga, S.; Mukoyama, M.; Nakao, K.; Imura, H.; Sasayama, S. Ventricular expression of brain natriuretic peptide in hypertrophic cardiomyopathy. Circulation 1993, 88, 372–380. [Google Scholar] [CrossRef]
- Kahveci, G.; Bayrak, F.; Mutlu, B.; Basaran, Y. Determinants of elevated NT-proBNP levels in patients with hypertrophic cardiomyopathy: An echocardiographic study. Heart Lung Circ. 2009, 18, 266–270. [Google Scholar] [CrossRef]
- Kawasaki, T.; Sakai, C.; Harimoto, K.; Yamano, M.; Miki, S.; Kamitani, T. Usefulness of high-sensitivity cardiac troponin T and brain natriuretic peptide as biomarkers of myocardial fibrosis in patients with hypertrophic cardiomyopathy. Am. J. Cardiol. 2013, 112, 867–872. [Google Scholar] [CrossRef]
- Kubo, T.; Kitaoka, H.; Yamanaka, S.; Hirota, T.; Baba, Y.; Hayashi, K.; Iiyama, T.; Kumagai, N.; Tanioka, K.; Yamasaki, N.; et al. Significance of high-sensitivity cardiac troponin T in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2013, 62, 1252–1259. [Google Scholar] [CrossRef]
- Moreno, V.; Hernández-Romero, D.; Vilchez, J.A.; García-Honrubia, A.; Cambronero, F.; Casas, T.; González, J.; Martínez, P.; Climent, V.; de la Morena, G.; et al. Serum levels of high-sensitivity troponin T: A novel marker for cardiac remodeling in hypertrophic cardiomyopathy. J. Card. Fail. 2010, 16, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Park, J.R.; Choi, J.O.; Han, H.J.; Chang, S.A.; Park, S.J.; Lee, S.C.; Choe, Y.H.; Park, S.W.; Oh, J.K. Degree and distribution of left ventricular hypertrophy as a determining factor for elevated natriuretic peptide levels in patients with hypertrophic cardiomyopathy: Insights from cardiac magnetic resonance imaging. Int. J. Cardiovasc. Imaging 2012, 28, 763–772. [Google Scholar] [CrossRef]
- Tesic, M.; Seferovic, J.; Trifunovic, D.; Djordjevic-Dikic, A.; Giga, V.; Jovanovic, I.; Petrovic, O.; Marinkovic, J.; Stankovic, S.; Stepanovic, J.; et al. N-terminal pro-brain natriuretic peptide is related with coronary flow velocity reserve and diastolic dysfunction in patients with asymmetric hypertrophic cardiomyopathy. J. Cardiol. 2017, 70, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, R.; Yuan, J.; Cui, J.; Hu, F.; Yang, W.; Zhang, Y.; Chen, Y.; Qiao, S. Predictive values of N-terminal pro-B-type natriuretic peptide and cardiac troponin I for myocardial fibrosis in hypertrophic obstructive cardiomyopathy. PLoS ONE 2016, 11, e0146572. [Google Scholar] [CrossRef]
- Gawor, M.; Śpiewak, M.; Kubik, A.; Wróbel, A.; Lutyńska, A.; Marczak, M.; Grzybowski, J. Circulating biomarkers of hypertrophy and fibrosis in patients with hypertrophic cardiomyopathy assessed by cardiac magnetic resonance. Biomarkers 2018, 23, 676–682. [Google Scholar] [CrossRef]
- Jenab, Y.; Pourjafari, M.; Darabi, F.; Boroumand, M.A.; Zoroufian, A.; Jalali, A. Prevalence and determinants of elevated high-sensitivity cardiac troponin T in hypertrophic cardiomyopathy. J. Cardiol. 2014, 63, 140–144. [Google Scholar] [CrossRef]
- Kaliyappan, L.; Watson, S.; Fiend, E.; Norrish, G.; Cervi, E.; Kaski, J. 7 Relation between N-terminal pro B-type natriuretic peptide (NT-probnp) and disease severity in paediatric hypertrophic cardiomyopathy. Heart 2021, 107, A6. [Google Scholar] [CrossRef]
- Kim, S.W.; Park, S.W.; Lim, S.H.; Kwon, S.U.; Choi, Y.J.; Park, M.K.; Lee, S.C.; Lee, S.H.; Park, J.E.; Jeon, E.S. Amount of left ventricular hypertrophy determines the plasma N-terminal pro-brain natriuretic peptide level in patients with hypertrophic cardiomyopathy and normal left ventricular ejection fraction. Clin. Cardiol. 2006, 29, 155–160. [Google Scholar] [CrossRef]
- Kubo, T.; Kitaoka, H.; Okawa, M.; Yamanaka, S.; Hirota, T.; Hoshikawa, E.; Hayato, K.; Yamasaki, N.; Matsumura, Y.; Yasuda, N.; et al. Serum cardiac troponin I is related to increased left ventricular wall thickness, left ventricular dysfunction, and male gender in hypertrophic cardiomyopathy. Clin. Cardiol. 2010, 33, E1–E7. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Cao, Y.; Han, X.; Shao, G.; Zhou, X.; Gu, J.; Liu, T.; Cui, Y.; Shi, H. Predictive values of multiple non-invasive markers for myocardial fibrosis in hypertrophic cardiomyopathy patients with preserved ejection fraction. Sci. Rep. 2021, 11, 4297. [Google Scholar] [CrossRef] [PubMed]
- Coats, C.J.; Gallagher, M.J.; Foley, M.; O’Mahony, C.; Critoph, C.; Gimeno, J.; Dawnay, A.; McKenna, W.J.; Elliott, P.M. Relation between serum N-terminal pro-brain natriuretic peptide and prognosis in patients with hypertrophic cardiomyopathy. Eur. Heart J. 2013, 34, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- Panou, F.K.; Kotseroglou, V.K.; Lakoumentas, J.A.; Chrysanthopoulou, S.A.; Armeniakos, J.A.; Stratigou, T.; Veve, H.; Zacharoulis, A.A. Significance of brain natriuretic peptide in the evaluation of symptoms and the degree of left ventricular diastolic dysfunction in patients with hypertrophic cardiomyopathy. Hell. J. Cardiol. 2006, 47, 344–351. [Google Scholar]
- Iles, L.M.; Ellims, A.H.; Llewellyn, H.; Hare, J.L.; Kaye, D.M.; McLean, C.A.; Taylor, A.J. Histological validation of cardiac magnetic resonance analysis of regional and diffuse interstitial myocardial fibrosis. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 14–22. [Google Scholar] [CrossRef]
- Elliott, P.M.; Gimeno, J.R.; Tomé, M.T.; Shah, J.; Ward, D.; Thaman, R.; Mogensen, J.; McKenna, W.J. Left ventricular outflow tract obstruction and sudden death risk in patients with hypertrophic cardiomyopathy. Eur. Heart J. 2006, 27, 1933–1941. [Google Scholar] [CrossRef] [PubMed]
- Maron, M.S.; Olivotto, I.; Betocchi, S.; Casey, S.A.; Lesser, J.R.; Losi, M.A.; Cecchi, F.; Maron, B.J. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N. Engl. J. Med. 2003, 348, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Spirito, P.; Seidman, C.E.; McKenna, W.J.; Maron, B.J. The management of hypertrophic cardiomyopathy. N. Engl. J. Med. 1997, 336, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Wigle, E.D.; Sasson, Z.; Henderson, M.A.; Ruddy, T.D.; Fulop, J.; Rakowski, H.; Williams, W.G. Hypertrophic cardiomyopathy. The importance of the site and the extent of hypertrophy. A review. Prog. Cardiovasc. Dis. 1985, 28, 1–83. [Google Scholar] [CrossRef]
- Bruder, O.; Wagner, A.; Jensen, C.J.; Schneider, S.; Ong, P.; Kispert, E.M.; Nassenstein, K.; Schlosser, T.; Sabin, G.V.; Sechtem, U.; et al. Myocardial scar visualized by cardiovascular magnetic resonance imaging predicts major adverse events in patients with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2010, 56, 875–887. [Google Scholar] [CrossRef]
- Chan, R.H.; Maron, B.J.; Olivotto, I.; Pencina, M.J.; Assenza, G.E.; Haas, T.; Lesser, J.R.; Gruner, C.; Crean, A.M.; Rakowski, H.; et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation 2014, 130, 484–495. [Google Scholar] [CrossRef]
- Weng, Z.; Yao, J.; Chan, R.H.; He, J.; Yang, X.; Zhou, Y.; He, Y. Prognostic value of LGE-CMR in HCM: A meta-analysis. JACC Cardiovasc. Imaging 2016, 9, 1392–1402. [Google Scholar] [CrossRef]
- Finocchiaro, G.; Haddad, F.; Pavlovic, A.; Magavern, E.; Sinagra, G.; Knowles, J.W.; Myers, J.; Ashley, E.A. How does morphology impact on diastolic function in hypertrophic cardiomyopathy? A single centre experience. BMJ Open 2014, 4, e004814. [Google Scholar] [CrossRef]
- Shah, P.M. Hypertrophic cardiomyopathy and diastolic dysfunction. J. Am. Coll. Cardiol. 2003, 42, 286–287. [Google Scholar] [CrossRef]
- Maron, B.J.; Maron, M.S. Hypertrophic cardiomyopathy. Lancet 2013, 381, 242–255. [Google Scholar] [CrossRef]
- Biagini, E.; Spirito, P.; Rocchi, G.; Ferlito, M.; Rosmini, S.; Lai, F.; Lorenzini, M.; Terzi, F.; Bacchi-Reggiani, L.; Boriani, G.; et al. Prognostic implications of the Doppler restrictive filling pattern in hypertrophic cardiomyopathy. Am. J. Cardiol. 2009, 104, 1727–1731. [Google Scholar] [CrossRef] [PubMed]
- Ismail, T.F.; Jabbour, A.; Gulati, A.; Mallorie, A.; Raza, S.; Cowling, T.E.; Das, B.; Khwaja, J.; Alpendurada, F.D.; Wage, R.; et al. Role of late gadolinium enhancement cardiovascular magnetic resonance in the risk stratification of hypertrophic cardiomyopathy. Heart 2014, 100, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Pinamonti, B.; Merlo, M.; Nangah, R.; Korcova, R.; Di Lenarda, A.; Barbati, G.; Sinagra, G. The progression of left ventricular systolic and diastolic dysfunctions in hypertrophic cardiomyopathy: Clinical and prognostic significance. J. Cardiovasc. Med. 2010, 11, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., III; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1321–1360. [Google Scholar] [CrossRef] [PubMed]
- Hundley, W.G.; Bluemke, D.A.; Finn, J.P.; Flamm, S.D.; Fogel, M.A.; Friedrich, M.G.; Ho, V.B.; Jerosch-Herold, M.; Kramer, C.M.; Manning, W.J.; et al. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: A report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J. Am. Coll. Cardiol. 2010, 55, 2614–2662. [Google Scholar] [CrossRef] [PubMed]
- Elliott, P.M.; Anastasakis, A.; Borger, M.A.; Borggrefe, M.; Cecchi, F.; Charron, P.; Hagege, A.A.; Lafont, A.; Limongelli, G.; Mahrholdt, H.; et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: The Task Force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2733–2779. [Google Scholar] [CrossRef]
- Mikami, Y.; Kolman, L.; Joncas, S.X.; Stirrat, J.; Scholl, D.; Rajchl, M.; Lydell, C.P.; Weeks, S.G.; Howarth, A.G.; White, J.A. Accuracy and reproducibility of semi-automated late gadolinium enhancement quantification techniques in patients with hypertrophic cardiomyopathy. J. Cardiovasc. Magn. Reson. 2014, 16, 85. [Google Scholar] [CrossRef]
- Cheung, B.M.; Kumana, C.R. Natriuretic peptides–relevance in cardiovascular disease. JAMA 1998, 280, 1983–1984. [Google Scholar] [CrossRef]
- Nishikimi, T.; Kuwahara, K.; Nakao, K. Current biochemistry, molecular biology, and clinical relevance of natriuretic peptides. J. Cardiol. 2011, 57, 131–140. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Fernández, A.; Vigliano, C.A.; Casabé, J.H.; Diez, M.; Favaloro, L.E.; Guevara, E.; Favaloro, R.R.; Laguens, R.P. Comparison of prevalence, clinical course, and pathological findings of left ventricular systolic impairment versus normal systolic function in patients with hypertrophic cardiomyopathy. Am. J. Cardiol. 2011, 108, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Marstrand, P.; Han, L.; Day, S.M.; Olivotto, I.; Ashley, E.A.; Michels, M.; Pereira, A.C.; Wittekind, S.G.; Helms, A.; Saberi, S.; et al. Hypertrophic cardiomyopathy with left ventricular systolic dysfunction. Circulation 2020, 141, 1371–1383. [Google Scholar] [CrossRef] [PubMed]
- Spirito, P.; Maron, B.J.; Bonow, R.O.; Epstein, S.E. Occurrence and significance of progressive left ventricular wall thinning and relative cavity dilatation in hypertrophic cardiomyopathy. Am. J. Cardiol. 1987, 60, 123–129. [Google Scholar] [CrossRef]
- Thaman, R.; Gimeno, J.R.; Murphy, R.T.; Kubo, T.; Sachdev, B.; Mogensen, J.; Elliott, P.M.; McKenna, W.J. Prevalence and clinical significance of systolic impairment in hypertrophic cardiomyopathy. Heart 2005, 91, 920. [Google Scholar] [CrossRef]
- Ashrafian, H.; McKenna, W.J.; Watkins, H. Disease pathways and novel therapeutic targets in hypertrophic cardiomyopathy. Circ. Res. 2011, 109, 86–96. [Google Scholar] [CrossRef]
- D’Amato, R.; Tomberli, B.; Castelli, G.; Spoladore, R.; Girolami, F.; Fornaro, A.; Caldini, A.; Torricelli, F.; Camici, P.; Gensini, G.F.; et al. Prognostic value of N-terminal pro-brain natriuretic Peptide in outpatients with hypertrophic cardiomyopathy. Am. J. Cardiol. 2013, 112, 1190–1196. [Google Scholar] [CrossRef]
- Kubo, T.; Kitaoka, H.; Okawa, M.; Yamanaka, S.; Hirota, T.; Baba, Y.; Hayato, K.; Yamasaki, N.; Matsumura, Y.; Yasuda, N.; et al. Combined measurements of cardiac troponin I and brain natriuretic peptide are useful for predicting adverse outcomes in hypertrophic cardiomyopathy. Circ. J. 2011, 75, 919–926. [Google Scholar] [CrossRef]
- Maron, B.J.; Tholakanahalli, V.N.; Zenovich, A.G.; Casey, S.A.; Duprez, D.; Aeppli, D.M.; Cohn, J.N. Usefulness of B-type natriuretic peptide assay in the assessment of symptomatic state in hypertrophic cardiomyopathy. Circulation 2004, 109, 984–989. [Google Scholar] [CrossRef]
- Binder, J.; Ommen, S.R.; Chen, H.H.; Ackerman, M.J.; Tajik, A.J.; Jaffe, A.S. Usefulness of brain natriuretic peptide levels in the clinical evaluation of patients with hypertrophic cardiomyopathy. Am. J. Cardiol. 2007, 100, 712–714. [Google Scholar] [CrossRef]
- Payá, E.; Marín, F.; González, J.; Gimeno, J.R.; Feliu, E.; Romero, A.; Ruiz-Espejo, F.; Roldán, V.; Climent, V.; de la Morena, G.; et al. Variables associated with contrast-enhanced cardiovascular magnetic resonance in hypertrophic cardiomyopathy: Clinical implications. J. Card. Fail. 2008, 14, 414–419. [Google Scholar] [CrossRef]
- Nakamura, S.; Takano, H.; Matsuda, J.; Chinen, D.; Kitamura, M.; Murai, K.; Asai, K.; Yasutake, M.; Takayama, M.; Shimizu, W. Prognostic values of highly sensitive cardiac troponin T and B-type natriuretic peptide for clinical features in hypertrophic obstructive cardiomyopathy: A cross-sectional study. BMJ Open 2014, 4, e005968. [Google Scholar] [CrossRef] [PubMed]
- Voigt, C.; Münch, J.; Avanesov, M.; Suling, A.; Witzel, K.; Lund, G.; Patten, M. Early segmental relaxation abnormalities in hypertrophic cardiomyopathy for differential diagnostic of patients with left ventricular hypertrophy. Clin. Cardiol. 2017, 40, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Marian, A.J.; Roberts, R. The molecular genetic basis for hypertrophic cardiomyopathy. J. Mol. Cell. Cardiol. 2001, 33, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Nishigaki, K.; Tomita, M.; Kagawa, K.; Noda, T.; Minatoguchi, S.; Oda, H.; Watanabe, S.; Morita, N.; Nakao, K.; Fujiwara, H. Marked expression of plasma brain natriuretic peptide is a special feature of hypertrophic obstructive cardiomyopathy. J. Am. Coll. Cardiol. 1996, 28, 1234–1242. [Google Scholar] [CrossRef]
- Briguori, C.; Betocchi, S.; Manganelli, F.; Gigante, B.; Losi, M.A.; Ciampi, Q.; Gottilla, R.; Violante, A.; Tocchetti, C.G.; Volpe, M.; et al. Determinants and clinical significance of natriuretic peptides and hypertrophic cardiomyopathy. Eur. Heart J. 2001, 22, 1328–1336. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, R.; Yuan, J.; Cui, J.; Hu, F.; Yang, W.; Zhang, Y.; Yang, C.; Qiao, S. Significance and determinants of cardiac troponin i in patients with obstructive hypertrophic cardiomyopathy. Am. J. Cardiol. 2015, 116, 1744–1751. [Google Scholar] [CrossRef]
- Rubinshtein, R.; Glockner, J.F.; Ommen, S.R.; Araoz, P.A.; Ackerman, M.J.; Sorajja, P.; Bos, J.M.; Tajik, A.J.; Valeti, U.S.; Nishimura, R.A.; et al. Characteristics and clinical significance of late gadolinium enhancement by contrast-enhanced magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Circ. Heart Fail. 2010, 3, 51–58. [Google Scholar] [CrossRef]
- O’Hanlon, R.; Grasso, A.; Roughton, M.; Moon, J.C.; Clark, S.; Wage, R.; Webb, J.; Kulkarni, M.; Dawson, D.; Sulaibeekh, L.; et al. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2010, 56, 867–874. [Google Scholar] [CrossRef]
- Christenson, R.H.; Phillips, D. Sensitive and high sensitivity next generation cardiac troponin assays: More than just a name. Pathology 2011, 43, 213–219. [Google Scholar] [CrossRef]
- Gomes, A.V.; Barnes, J.A.; Harada, K.; Potter, J.D. Role of troponin T in disease. Mol. Cell. Biochem. 2004, 263, 115–129. [Google Scholar] [CrossRef]
- Gommans, F.; Bakker, J.; Cramer, E.; Fouraux, M.A.; Kurvers, M.J.; Verheugt, F.W.; Brouwer, M.A.; Kofflard, M. Elevated high-sensitivity cardiac troponin is associated with hypertrophy and fibrosis assessed with CMR in patients with hypertrophic cardiomyopathy. J. Cardiovasc. Magn. Reson. 2013, 15, P144. [Google Scholar] [CrossRef]
- Kubo, T.; Ochi, Y.; Baba, Y.; Sugiura, K.; Takahashi, A.; Hirota, T.; Yamanaka, S.; Yamasaki, N.; Doi, Y.L.; Kitaoka, H. Elevation of high-sensitivity cardiac troponin T and left ventricular remodelling in hypertrophic cardiomyopathy. ESC Heart Fail. 2020, 7, 3593–3600. [Google Scholar] [CrossRef]
- Petersen, S.E.; Jerosch-Herold, M.; Hudsmith, L.E.; Robson, M.D.; Francis, J.M.; Doll, H.A.; Selvanayagam, J.B.; Neubauer, S.; Watkins, H. Evidence for microvascular dysfunction in hypertrophic cardiomyopathy: New insights from multiparametric magnetic resonance imaging. Circulation 2007, 115, 2418–2425. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, V.; Wijnker, P.J.; Nijenkamp, L.L.; Kuster, D.W.; Najafi, A.; Witjas-Paalberends, E.R.; Regan, J.A.; Boontje, N.; Ten Cate, F.J.; Germans, T.; et al. Perturbed length-dependent activation in human hypertrophic cardiomyopathy with missense sarcomeric gene mutations. Circ. Res. 2013, 112, 1491–1505. [Google Scholar] [CrossRef] [PubMed]
- Hessel, M.H.; Atsma, D.E.; van der Valk, E.J.; Bax, W.H.; Schalij, M.J.; van der Laarse, A. Release of cardiac troponin I from viable cardiomyocytes is mediated by integrin stimulation. Pflug. Arch. 2008, 455, 979–986. [Google Scholar] [CrossRef]
- Gommans, D.F.; Cramer, G.E.; Bakker, J.; Michels, M.; Dieker, H.J.; Timmermans, J.; Fouraux, M.A.; Marcelis, C.L.; Verheugt, F.W.; Brouwer, M.A.; et al. High T2-weighted signal intensity is associated with elevated troponin T in hypertrophic cardiomyopathy. Heart 2017, 103, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Osmanska, J.; Connelly, A.; Nordin, S.; Vega, A.; Simpson, J.; Anusas, J.; Findlay, I.; Coats, C. High sensitivity troponin I in hypertrophic cardiomyopathy. Eur. Heart J. 2020, 41, ehaa946.2078. [Google Scholar] [CrossRef]
- Makavos, G.; Κairis, C.; Tselegkidi, M.E.; Karamitsos, T.; Rigopoulos, A.G.; Noutsias, M.; Ikonomidis, I. Hypertrophic cardiomyopathy: An updated review on diagnosis, prognosis, and treatment. Heart Fail. Rev. 2019, 24, 439–459. [Google Scholar] [CrossRef]
- Shim, C.Y.; Ha, J.W.; Choi, E.Y.; Lee, H.J.; Moon, S.H.; Kim, J.M.; Rim, S.J.; Chung, N. Relationship between serum biochemical markers of myocardial fibrosis and diastolic function at rest and with exercise in hypertrophic cardiomyopathy. Korean Circ. J. 2009, 39, 519–524. [Google Scholar] [CrossRef]
- Tsuruda, T.; Boerrigter, G.; Huntley, B.K.; Noser, J.A.; Cataliotti, A.; Costello-Boerrigter, L.C.; Chen, H.H.; Burnett, J.C., Jr. Brain natriuretic Peptide is produced in cardiac fibroblasts and induces matrix metalloproteinases. Circ. Res. 2002, 91, 1127–1134. [Google Scholar] [CrossRef]
- Sato, Y.; Fujiwara, H.; Takatsu, Y. Cardiac troponin and heart failure in the era of high-sensitivity assays. J. Cardiol. 2012, 60, 160–167. [Google Scholar] [CrossRef] [PubMed]


| Clinical Parameters (Unit) | Median (IQR)/n-Number (Percentage)/Mean (±SD) | Range | N |
|---|---|---|---|
| Age (years) | 54 (±16) | 16–87 | 366 |
| Sex (males, n) | 206 (56%) | 366 | |
| BMI (kg/m2) | 27 (±5) | 14–49 | 361 |
| Arterial hypertension (n) | 170 (47%) | 365 | |
| Diabetes mellitus (n) | 40 (11%) | 364 | |
| AF (n) | 104 (28%) | 366 | |
| Ventricular tachycardia (medical history, n) | 82 (22%) | 365 | |
| Sudden cardiac death (family history, n) | 92 (25%) | 361 | |
| Syncope (n) | 71 (20%) | 359 | |
| SCD Risk Score | 3 (2–5) | 0.72–22.23 | 336 |
| <4% (n) | 221 (66%) | ||
| ≥4, <6% (n) | 56 (17%) | ||
| ≥6% (n) | 59 (17%) | ||
| ICD (n) | 54 (15%) | 366 | |
| CAD (n) | 59 (16%) | 365 | |
| Myocardial infarction (n) | 8 (2%) | 366 | |
| NYHA functional class (n) | 366 | ||
| I | 112 (31%) | ||
| II | 154 (42%) | ||
| III | 99 (27%) | ||
| IV | 1 (<1%) | ||
| eGFR (mL/min/1.73 m2) | 80 (±24) | 31–200 | 364 |
| eGFR of 30–50 mL/min/1.73 m2 (n) | 26 (7%) | ||
| Hs-cTnT (pg/mL) | 12 (7–21) | 3–416 | 358 |
| >14 pg/mL (n) | 146 (41%) | ||
| NT-proBNP (ng/L) | 663 (234–1534) | 5–31,505 | 362 |
| >125 ng/L (n) | 311 (86%) |
| Imaging Parameters (Unit) | Median (IQR)/n-Number (Percentage)/Mean (±SD) | Range | N |
|---|---|---|---|
| Echocardiography | |||
| LVEF (n) | 366 | ||
| Normal (>50%) | 336 (92%) | ||
| Mildly reduced (41–49%) | 20 (5%) | ||
| Moderately reduced (30–40%) | 4 (1%) | ||
| Severely reduced (<30%) | 6 (2%) | ||
| SW thickness (mm) | 21 (±5) | 9–48 | 366 |
| LW thickness (mm) | 14 (±3) | 5–32 | 347 |
| LA diameter (mm) | 46 (±10) | 24–97 | 365 |
| Resting LVOT flow gradient (mmHg) | 11 (5–30) | 2–210 | 365 |
| Provoked LVOT flow gradient (mmHg) | 20 (8–50) | 1–234 | 288 |
| Obstruction (n) | 365 | ||
| HNOCM | 245 (67%) | ||
| HLOCM | 28 (8%) | ||
| HOCM | 92 (25%) | ||
| Diastolic Dysfunction (n) | 361 | ||
| No DD | 105 (29%) | ||
| Mild DD | 119 (33%) | ||
| Moderate or severe DD | 137 (38%) | ||
| IVRT septal (ms) | 132 (±40) | 60–363 | 351 |
| IVRT lateral (ms) | 107 (±33) | 46–299 | 353 |
| E/A | 1.2 (0.8–1.6) | 0.1–4.6 | 347 |
| E/E′ mean | 12.3 (9.1–17.6) | 1.7–57.2 | 355 |
| E/E′ septal | 15.0 (11.5–21.9) | 5.1–71.5 | 357 |
| E/E′ lateral | 10.4 (7.7–15.6) | 0.9–47.7 | 357 |
| Cardiovascular Magnetic Resonance Imaging | |||
| LGE (n) | 150 (78%) | 193 | |
| LGE size (g) | 4.7 (0.9–9.7) | 0–169.3 | 193 |
| LGE size (% of LV mass) | 3.7 (0.8–6.9) | 0–43.9 | 193 |
| Variables (Unit) | b | 95 % CI | p-Value | N | R2 |
|---|---|---|---|---|---|
| SCD Risk Score (%) | 0.006 | −0.001–0.012 | 0.095 | 329 | 0.11 |
| LA diameter (mm) | 0.026 | 0.006–0.046 | 0.010 | 355 | 0.18 |
| Resting LVOT flow gradient (ln *) | 1.005 | 1.003–1.007 | <0.001 | 355 | 0.13 |
| Provoked LVOT flow gradient (ln *) | 1.003 | 0.999–1.006 | 0.101 | 278 | 0.11 |
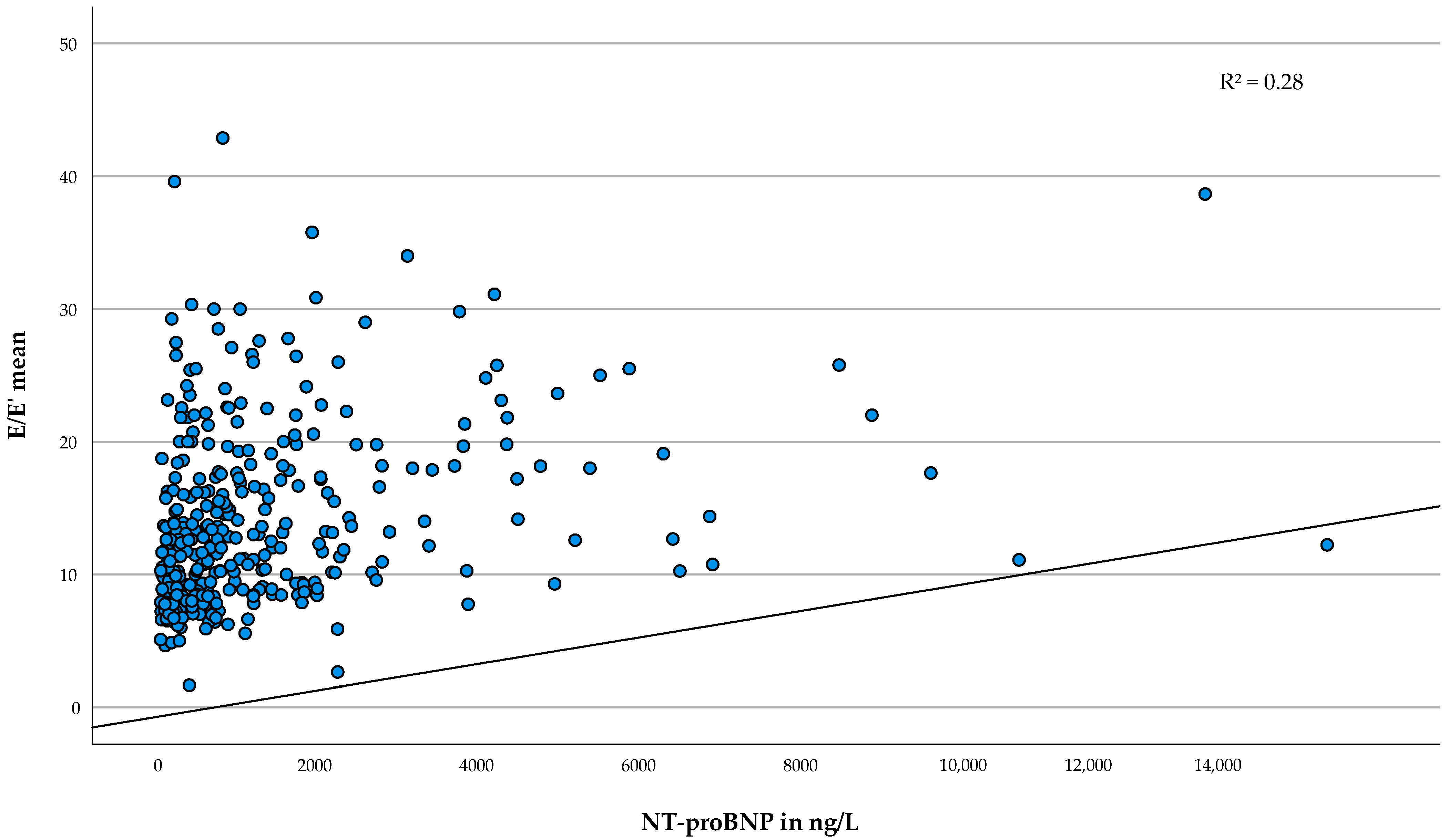
| E/E′ mean | 0.058 | 0.045–0.072 | <0.001 | 347 | 0.28 |
| E/E′ septal | 0.080 | 0.060–0.100 | <0.001 | 349 | 0.25 |
| E/E′ lateral | 0.049 | 0.036–0.062 | <0.001 | 349 | 0.22 |
| IVRT septal | 0.165 | 0.078–0.252 | <0.001 | 343 | 0.09 |
| IVRT lateral | 0.218 | 0.146–0.290 | <0.001 | 345 | 0.12 |
| SW thickness (mm) | 0.035 | 0.024–0.047 | <0.001 | 356 | 0.11 |
| LGE size (g) | 0.123 | 0.068–0.179 | <0.001 | 190 | 0.11 |
| Variables (Unit) | b | 95% CI | p-Value | N | R2 |
|---|---|---|---|---|---|
| SCD Risk Score (%) | 0.016 | 0.005–0.027 | 0.004 | 325 | 0.13 |
| LA diameter (mm) | 0.043 | 0.016–0.071 | 0.002 | 351 | 0.19 |
| Resting LVOT flow gradient (ln *) | 1.002 | 0.999–1.005 | 0.195 | 351 | 0.08 |
| Provoked LVOT flow gradient (ln *) | 1.001 | 0.997–1.004 | 0.616 | 274 | 0.11 |
| E/E′ mean | 0.022 | 0.001–0.042 | 0.041 | 342 | 0.14 |
| E/E′ septal | 0.037 | 0.007–0.067 | 0.016 | 344 | 0.12 |
| E/E′ lateral | 0.014 | −0.006–0.034 | 0.161 | 344 | 0.12 |
| IVRT septal | 0.233 | 0.114–0.352 | <0.001 | 338 | 0.09 |
| IVRT lateral | 0.180 | 0.078–0.281 | <0.001 | 340 | 0.06 |
| SW thickness (mm) | 0.049 | 0.033–0.066 | <0.001 | 352 | 0.11 |
| LGE size (g) | 0.196 | 0.147–0.244 | <0.001 | 186 | 0.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chevalier, C.; Wendner, M.; Suling, A.; Cavus, E.; Muellerleile, K.; Lund, G.; Kirchhof, P.; Patten, M. Association of NT-proBNP and hs-cTnT with Imaging Markers of Diastolic Dysfunction and Focal Myocardial Fibrosis in Hypertrophic Cardiomyopathy. Life 2022, 12, 1241. https://doi.org/10.3390/life12081241
Chevalier C, Wendner M, Suling A, Cavus E, Muellerleile K, Lund G, Kirchhof P, Patten M. Association of NT-proBNP and hs-cTnT with Imaging Markers of Diastolic Dysfunction and Focal Myocardial Fibrosis in Hypertrophic Cardiomyopathy. Life. 2022; 12(8):1241. https://doi.org/10.3390/life12081241
Chicago/Turabian StyleChevalier, Céleste, Miriam Wendner, Anna Suling, Ersin Cavus, Kai Muellerleile, Gunnar Lund, Paulus Kirchhof, and Monica Patten. 2022. "Association of NT-proBNP and hs-cTnT with Imaging Markers of Diastolic Dysfunction and Focal Myocardial Fibrosis in Hypertrophic Cardiomyopathy" Life 12, no. 8: 1241. https://doi.org/10.3390/life12081241
APA StyleChevalier, C., Wendner, M., Suling, A., Cavus, E., Muellerleile, K., Lund, G., Kirchhof, P., & Patten, M. (2022). Association of NT-proBNP and hs-cTnT with Imaging Markers of Diastolic Dysfunction and Focal Myocardial Fibrosis in Hypertrophic Cardiomyopathy. Life, 12(8), 1241. https://doi.org/10.3390/life12081241






