Management of Patients with Adhesive Capsulitis via Ultrasound-Guided Hydrodilatation without Concomitant Intra-Articular Lidocaine Infusion: A Single-Center Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
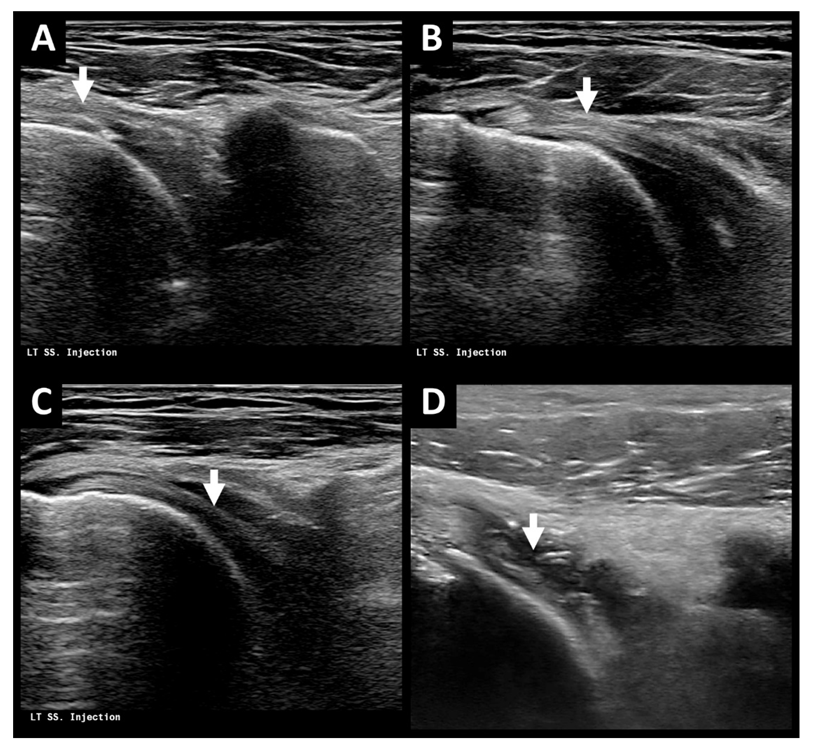
2.2. Treatment Process
2.3. Statistical Analysis
3. Results
3.1. Patient Selection and Demographics
3.2. Treatment Outcome of Ultrasound-Guided Hydrodilatation with and without Lidocaine
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neviaser, A.S.; Neviaser, R.J. Adhesive capsulitis of the shoulder. J. Am. Acad. Orthop. Surg. 2011, 19, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Hand, G.C.; Athanasou, N.A.; Matthews, T.; Carr, A.J. The pathology of frozen shoulder. J. Bone Joint. Surg. Br. 2007, 89, 928–932. [Google Scholar] [CrossRef] [PubMed]
- Le, H.V.; Lee, S.J.; Nazarian, A.; Rodriguez, E.K. Adhesive capsulitis of the shoulder: Review of pathophysiology and current clinical treatments. Shoulder Elbow 2017, 9, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Challoumas, D.; Biddle, M.; McLean, M.; Millar, N.L. Comparison of treatments for frozen shoulder: A systematic review and meta-analysis. JAMA Netw. Open. 2020, 3, e2029581. [Google Scholar] [CrossRef] [PubMed]
- Piper, S.L.; Kramer, J.D.; Kim, H.T.; Feeley, B.T. Effects of local anesthetics on articular cartilage. Am. J. Sports Med. 2011, 39k, 2245–2253. [Google Scholar] [CrossRef] [PubMed]
- Gulihar, A.; Robati, S.; Twaij, H.; Salih, A.; Taylor, G.J. Articular cartilage and local anaesthetic: A systematic review of the current literature. J. Orthop. 2015, 12, S200–S210. [Google Scholar] [CrossRef]
- Hynes, J.P.; Kavanagh, E.C. Complications in image-guided musculoskeletal injections. Skeletal Radiol. 2022, 1–8. [Google Scholar] [CrossRef]
- Dragoo, J.L.; Braun, H.J.; Kim, H.J.; Phan, H.D.; Golish, S.R. The in vitro chondrotoxicity of single-dose local anesthetics. Am. J. Sports Med. 2012, 40, 794–799. [Google Scholar] [CrossRef]
- Rymaruk, S.; Peach, C. Indications for hydrodilatation for frozen shoulder. EFORT Open Rev. 2017, 2, 462–468. [Google Scholar] [CrossRef]
- Catapano, M.; Mittal, N.; Adamich, J.; Kumbhare, D.; Sangha, H. Hydrodilatation with corticosteroid for the treatment of adhesive capsulitis: A systematic review. PM R 2018, 10, 623–635. [Google Scholar] [CrossRef]
- Fama, G.; Tagliapietra, J.; Belluzzi, E.; Pozzuoli, A.; Biz, C.; Ruggieri, P. Mid-Term Outcomes after Arthroscopic “Tear Completion Repair” of Partial Thickness Rotator Cuff Tears. Medicina 2021, 57, 74. [Google Scholar] [CrossRef] [PubMed]
- Ryan, V.; Brown, H.; Minns Lowe, C.J.; Lewis, J.S. The pathophysiology associated with primary (idiopathic) frozen shoulder: A systematic review. BMC Musculoskelet. Disord. 2016, 17, 340. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Lewis, M. Shoulder adhesive capsulitis: Systematic review of randomised trials using multiple corticosteroid injections. Br. J. Gen. Pract. 2007, 57, 662–667. [Google Scholar] [PubMed]
- Wu, W.T.; Chang, K.V.; Han, D.S.; Chang, C.H.; Yang, F.S.; Lin, C.P. Effectiveness of glenohumeral joint dilatation for treatment of frozen shoulder: A systematic review and meta-analysis of randomized controlled trials. Sci. Rep. 2017, 7, 10507. [Google Scholar] [CrossRef]
- Lin, M.T.; Hsiao, M.Y.; Tu, Y.K.; Wang, T.G. Comparative efficacy of intra-articular steroid injection and distension in patients with frozen shoulder: A systematic review and network meta-analysis. Arch. Phys. Med. Rehabil. 2018, 99, 1383–1394.e6. [Google Scholar] [CrossRef]
- Rex, S.S.; Kottam, L.; McDaid, C.; Brealey, S.; Dias, J.; Hewitt, C.E.; Keding, A.; Lamb, S.E.; Wright, K.; Rangan, A. Effectiveness of interventions for the management of primary frozen shoulder: A systematic review of randomized trials. Bone Jt. Open. 2021, 2, 773–784. [Google Scholar] [CrossRef]
- Makki, D.; Al-Yaseen, M.; Almari, F.; Monga, P.; Funk, L.; Basu, S.; Walton, M. Shoulder hydrodilatation for primary, post-traumatic and post-operative adhesive capsulitis. Shoulder Elbow 2021, 13, 649–655. [Google Scholar] [CrossRef]
- Jayaram, P.; Kennedy, D.J.; Yeh, P.L.; Dragoo, J. Chondrotoxic effects of local anesthetics on human knee articular cartilage: A systematic review. PM R 2019, 11, 379–400. [Google Scholar] [CrossRef]
- Kreuz, P.C.; Steinwachs, M.; Angele, P. Single-dose local anesthetics exhibit a type-, dose-, and time-dependent chondrotoxic effect on chondrocytes and cartilage: A systematic review of the current literature. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 819–830. [Google Scholar] [CrossRef]
- Karpie, J.C.; Chu, C.R. Lidocaine exhibits dose- and time-dependent cytotoxic effects on bovine articular chondrocytes in vitro. Am. J. Sports Med. 2007, 35, 1621–1627. [Google Scholar] [CrossRef]
- Braun, H.J.; Wilcox-Fogel, N.; Kim, H.J.; Pouliot, M.A.; Harris, A.H.; Dragoo, J.L. The effect of local anesthetic and corticosteroid combinations on chondrocyte viability. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 1689–1695. [Google Scholar] [CrossRef] [PubMed]
- Ravnihar, K.; Marš, T.; Pirkmajer, S.; Alibegović, A.; Koželj, G.; Stožer, A.; Drobnič, M. The influence of a single intra-articular lidocaine injection on the viability of articular cartilage in the knee. Cartilage 2021, 13, 456S–463S. [Google Scholar] [CrossRef] [PubMed]
- Ravnihar, K.; Barlič, A.; Drobnič, M. Effect of intra-articular local anesthesia on articular cartilage in the knee. Arthroscopy 2014, 30, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Matziolis, G.; Roehner, E.; Windisch, C.; Wagner, A. The volume of the human knee joint. Arch. Orthop. Trauma. Surg. 2015, 135, 1401–1403. [Google Scholar] [CrossRef]
- Nagy, M.T.; Macfarlane, R.J.; Khan, Y.; Waseem, M. The frozen shoulder: Myths and realities. Open Orthop. J. 2013, 7, 352–355. [Google Scholar] [CrossRef][Green Version]
- Baumgarten, K.M.; Helsper, E. Does chondrolysis occur after corticosteroid-analgesic injections? An analysis of patients treated for adhesive capsulitis of the shoulder. J. Shoulder Elbow Surg. 2016, 25, 890–897. [Google Scholar] [CrossRef]
- Wang, J.C.; Tsai, P.Y.; Hsu, P.C.; Huang, J.R.; Wang, K.A.; Chou, C.L.; Chang, K.V. Ultrasound-Guided Hydrodilatation with triamcinolone acetonide for adhesive capsulitis: A randomized controlled trial comparing the posterior glenohumeral recess and the rotator cuff interval approaches. Front. Pharmacol. 2021, 12, 686139. [Google Scholar] [CrossRef]
- Cho, J.H. Updates on the treatment of adhesive capsulitis with hydraulic distension. Yeungnam Univ. J. Med. 2021, 38, 19–26. [Google Scholar] [CrossRef]
- Lockard, C.A.; Nolte, P.C.; Gawronski, K.; Elrick, B.P.; Goldenberg, B.T.; Horan, M.P.; Dornan, G.J.; Ho, C.P.; Millett, P.J. Quantitative T2 mapping of the glenohumeral joint cartilage in asymptomatic shoulders and shoulders with increasing severity of rotator cuff pathology. Eur. J. Radiol. Open 2021, 8, 100329. [Google Scholar] [CrossRef]


| Total (n = 104) | Corticosteroid Only (n = 59, 56.7%) | Corticosteroid + Lidocaine (n = 45, 43.3%) | p-Value | |
|---|---|---|---|---|
| Age (years) * | 55.7 ± 9.5 | 56.7 ± 10.3 | 54.4 ± 8.2 | 0.589 |
| Gender (Male/Female, Female %) | 46/58 (55.8%) | 30/29 (49.2%) | 16/29 (64.4%) | 0.120 |
| Laterality (Left/Right, Left %) | 59/45 (56.7%) | 34/25 (57.6%) | 25/20 (55.6%) | 0.833 |
| Treatment outcome (number, %) | ||||
| Poor | 9 (8.7%) | 6 (10.2%) | 3 (6.7%) | 0.689 |
| Moderate | 54 (51.9%) | 28 (47.4%) | 26 (57.8%) | |
| Good | 41 (37.4%) | 25 (42.4%) | 16 (35.5%) | |
| No. of hydrodilatation (number, %) | ||||
| 1 | 44 (42.3%) | 27 (45.8%) | 17 (37.8%) | 0.146 |
| 2 | 44 (42.3%) | 27 (45.8%) | 17 (37.8%) | |
| 3 | 9 (8.7%) | 2 (3.4%) | 7 (15.5%) | |
| ≥4 | 7 (6.7%) | 3 (5%) | 4 (8.9%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-C.; Shen, S.-H.; Chiou, H.-J.; Wan, Y.-L. Management of Patients with Adhesive Capsulitis via Ultrasound-Guided Hydrodilatation without Concomitant Intra-Articular Lidocaine Infusion: A Single-Center Experience. Life 2022, 12, 1293. https://doi.org/10.3390/life12091293
Chen Y-C, Shen S-H, Chiou H-J, Wan Y-L. Management of Patients with Adhesive Capsulitis via Ultrasound-Guided Hydrodilatation without Concomitant Intra-Articular Lidocaine Infusion: A Single-Center Experience. Life. 2022; 12(9):1293. https://doi.org/10.3390/life12091293
Chicago/Turabian StyleChen, Yung-Chieh, Shu-Huei Shen, Hong-Jen Chiou, and Yung-Liang Wan. 2022. "Management of Patients with Adhesive Capsulitis via Ultrasound-Guided Hydrodilatation without Concomitant Intra-Articular Lidocaine Infusion: A Single-Center Experience" Life 12, no. 9: 1293. https://doi.org/10.3390/life12091293
APA StyleChen, Y.-C., Shen, S.-H., Chiou, H.-J., & Wan, Y.-L. (2022). Management of Patients with Adhesive Capsulitis via Ultrasound-Guided Hydrodilatation without Concomitant Intra-Articular Lidocaine Infusion: A Single-Center Experience. Life, 12(9), 1293. https://doi.org/10.3390/life12091293






