Interaction of the Blood Components with Ascending Thoracic Aortic Aneurysm Wall: Biomechanical and Fluid Analyses
Abstract
:1. Introduction
2. Materials and Methods
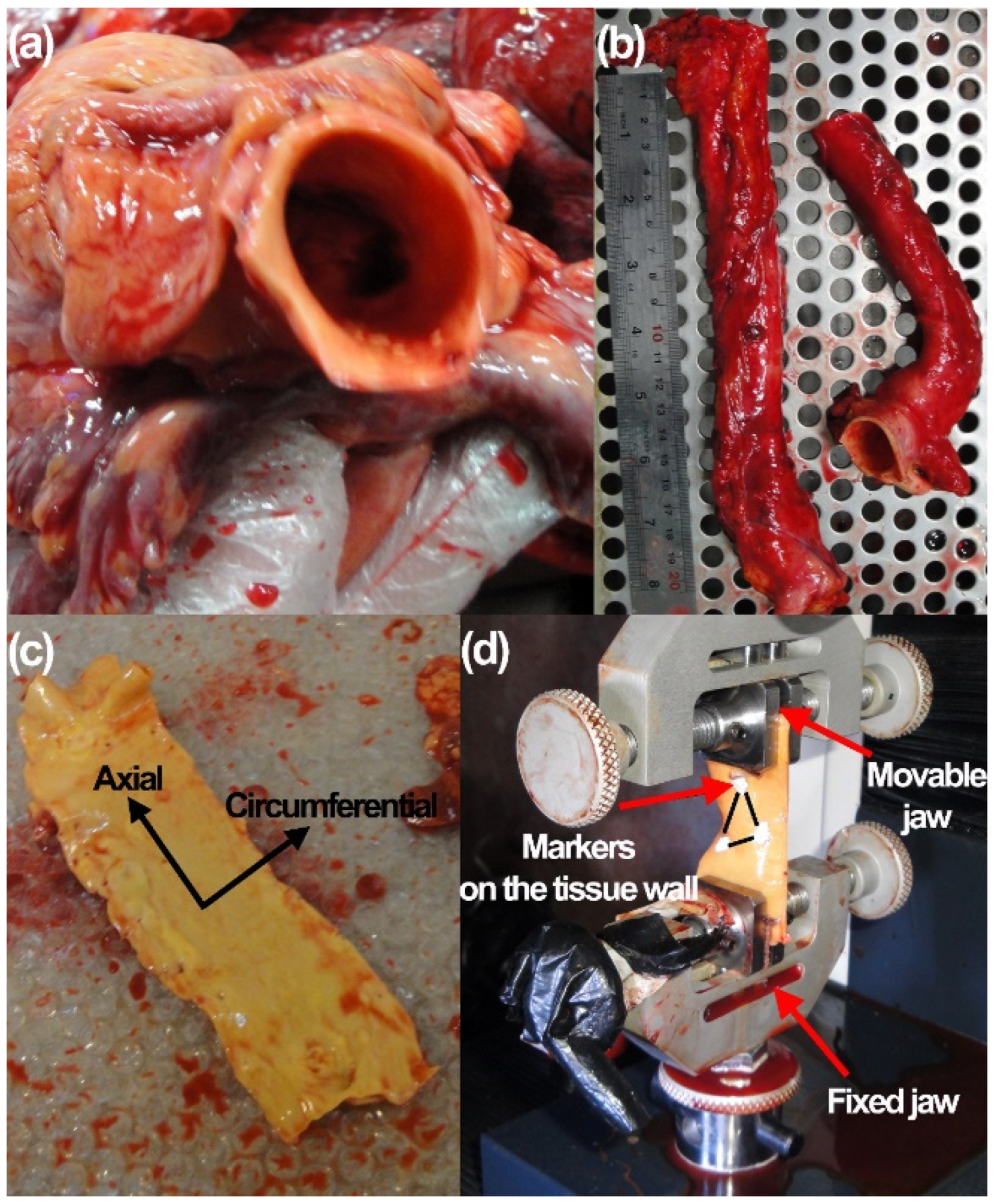
2.1. Human Donors and Mechanical Measurements
2.2. Statistical Analysis
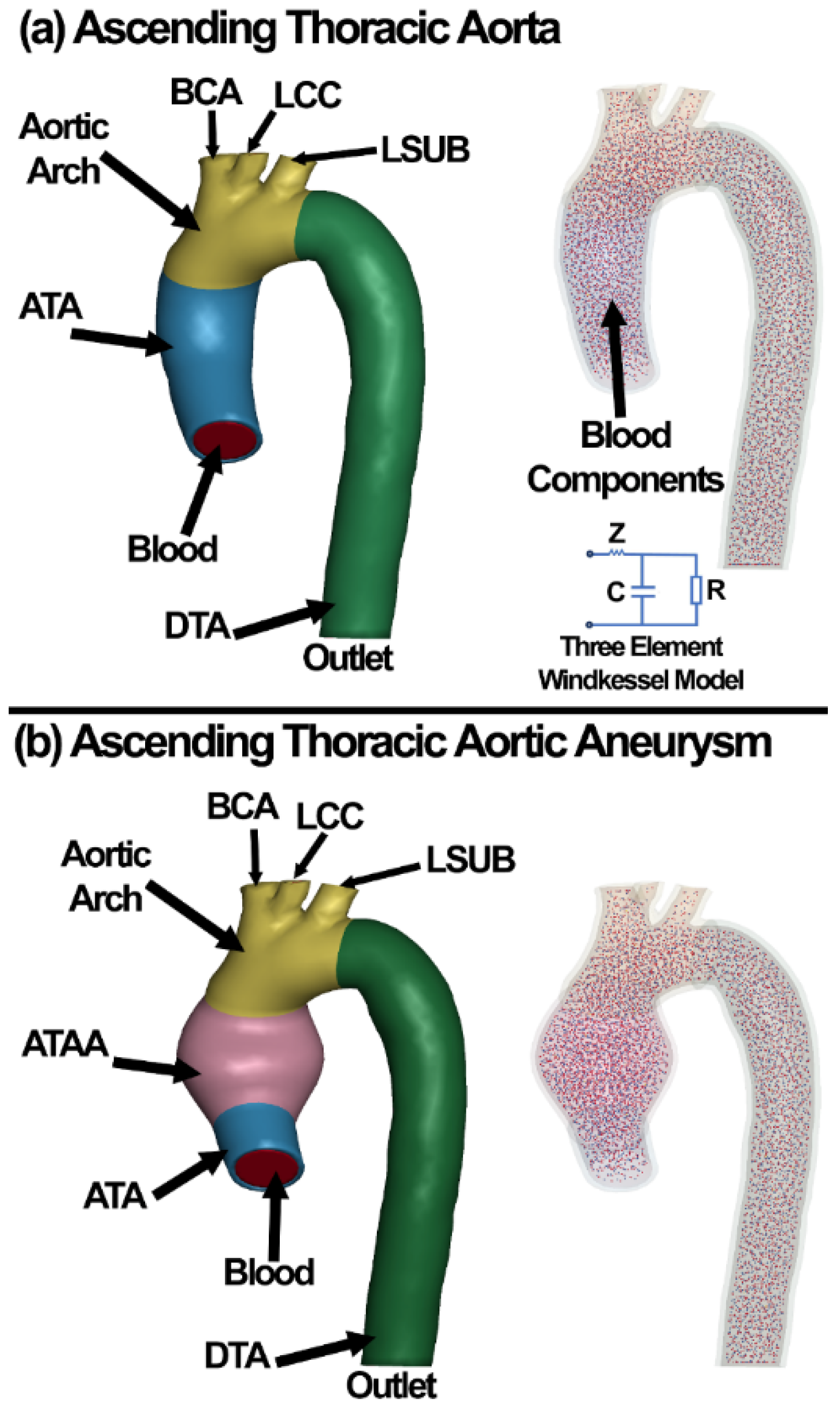
2.3. Finite Element Model of the Aortic Wall
2.4. Red Blood Cell, White Blood Cell, and Plasma Modeling Using Discrete Elements
2.5. Computational Fluid Dynamics
2.6. Fluid–Structure Interaction
3. Results
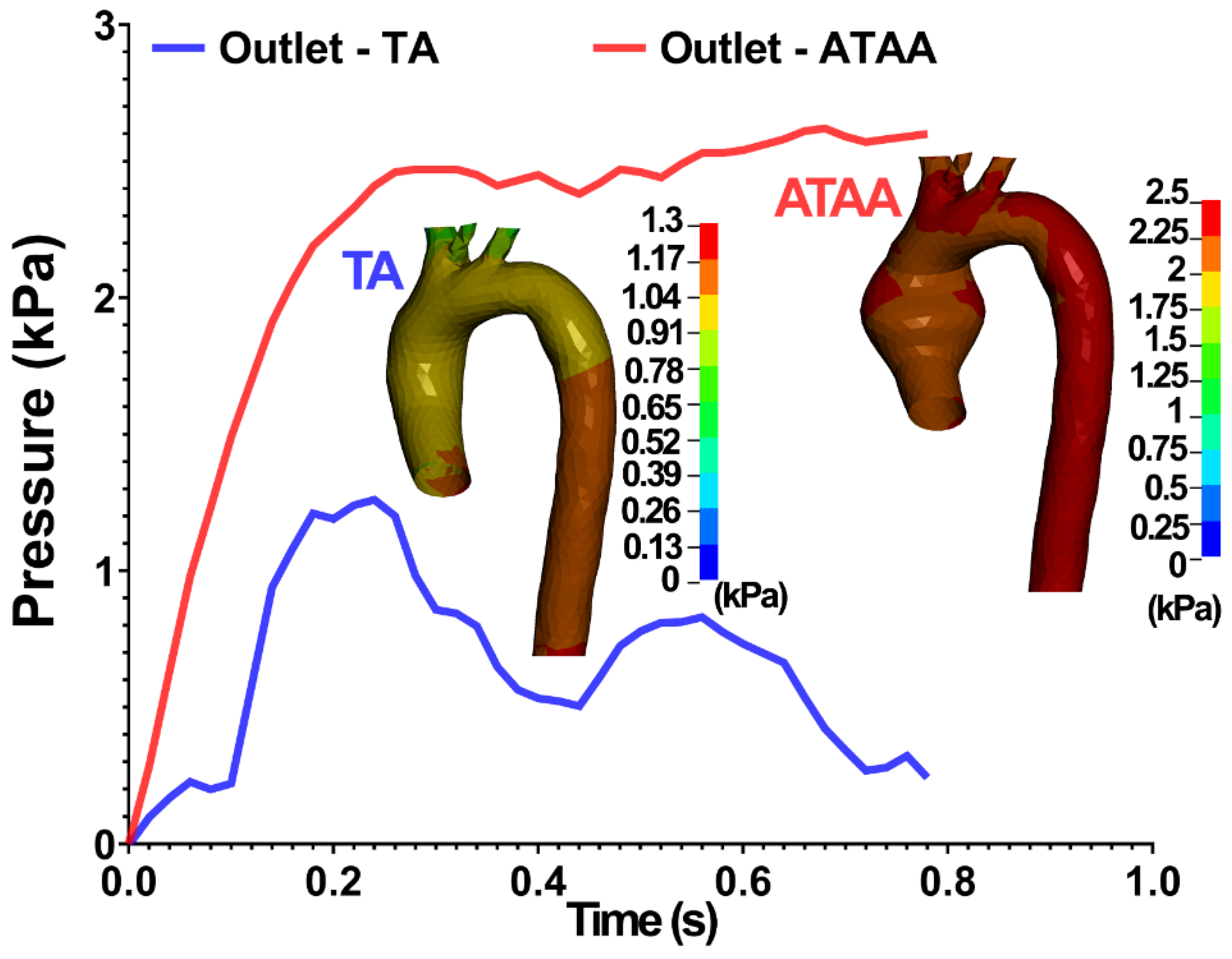
3.1. Experimental Results
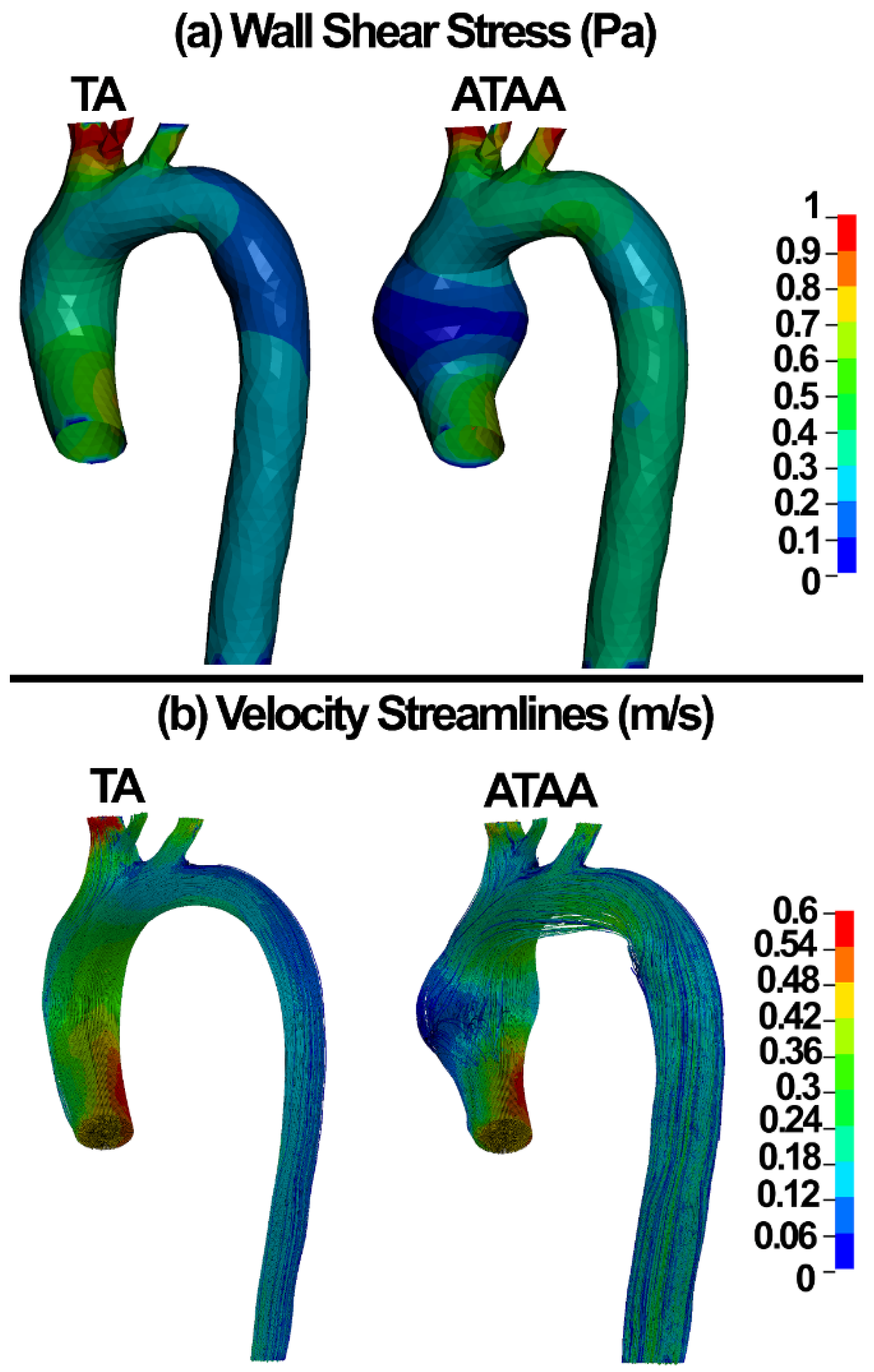
3.2. Numerical Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coady, M.A.; Rizzo, J.A.; Goldstein, L.J.; Elefteriades, J.A. Natural history, pathogenesis, and etiology of thoracic aortic aneurysms and dissections. Cardiol. Clin. 1999, 17, 615–635. [Google Scholar] [CrossRef]
- Beckman, J.A. Pathophysiology, Epidemiology, and Prognosis, Vascular Medicine; Elsevier: Amsterdam, The Netherlands, 2006; pp. 543–559. [Google Scholar]
- Davies, R.R.; Goldstein, L.J.; Coady, M.A.; Tittle, S.L.; Rizzo, J.A.; Kopf, G.S.; Elefteriades, J.A. Yearly rupture or dissection rates for thoracic aortic aneurysms: Simple prediction based on size. Ann. Thorac. Surg. 2002, 73, 17–28. [Google Scholar] [CrossRef]
- Martin, C.; Sun, W.; Elefteriades, J. Patient-specific finite element analysis of ascending aorta aneurysms. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H1306–H1316. [Google Scholar] [CrossRef] [Green Version]
- Schriefl, A.J.; Schmidt, T.; Balzani, D.; Sommer, G.; Holzapfel, G.A. Selective enzymatic removal of elastin and collagen from human abdominal aortas: Uniaxial mechanical response and constitutive modeling. Acta Biomater. 2015, 17, 125–136. [Google Scholar] [CrossRef]
- Weisbecker, H.; Viertler, C.; Pierce, D.M.; Holzapfel, G.A. The role of elastin and collagen in the softening behavior of the human thoracic aortic media. J. Biomech. 2013, 46, 1859–1865. [Google Scholar] [CrossRef]
- Shahmansouri, N.; Alreshidan, M.; Emmott, A.; Lachapelle, K.; Cartier, R.; Leask, R.L.; Mongrain, R. Evaluating ascending aortic aneurysm tissue toughness: Dependence on collagen and elastin contents. J. Mech. Behav. Biomed. Mater. 2016, 64, 262–271. [Google Scholar] [CrossRef]
- Raghavan, M.; Vorp, D.; Federle, M.P.; Makaroun, M.S.; Webster, M.W. Wall stress distribution on three-dimensionally reconstructed models of human abdominal aortic aneurysm. J. Vasc. Surg. 2000, 31, 760–769. [Google Scholar] [CrossRef] [Green Version]
- Vorp, D.A.; Geest, J.P.V. Biomechanical Determinants of Abdominal Aortic Aneurysm Rupture. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1558–1566. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, K.; Mitchell, R.N.; Libby, P. Inflammation and Cellular Immune Responses in Abdominal Aortic Aneurysms. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 987–994. [Google Scholar] [CrossRef]
- Shang, E.K.; Nathan, D.P.; Woo, E.Y.; Fairman, R.M.; Wang, G.J.; Gorman, R.C.; Gorman, J.H., III; Jackson, B.M. Local wall thickness in finite element models improves prediction of abdominal aortic aneurysm growth. J. Vasc. Surg. 2015, 61, 217–223. [Google Scholar] [CrossRef] [Green Version]
- Trabelsi, O.; Gutierrez, M.; Farzaneh, S.; Duprey, A.; Avril, S. A non-invasive methodology for ATAA rupture risk estimation. J. Biomech. 2018, 66, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-Y.; Tang, T.Y.; U-King-Im, J.; Bowden, D.J.; Sadat, U.; Gillard, J.H.; Hayes, P.D. Association Between Aneurysm Shoulder Stress and Abdominal Aortic Aneurysm Expansion: A Longitudinal Follow-Up Study. Circulation 2010, 122, 1815–1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conlisk, N.; Geers, A.J.; McBride, O.M.; Newby, D.E.; Hoskins, P.R. Patient-specific modelling of abdominal aortic aneurysms: The influence of wall thickness on predicted clinical outcomes. Med. Eng. Phys. 2016, 38, 526–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyle, B.J.; McGloughlin, T.M.; Miller, K.; Powell, J.T.; Norman, P.E. Regions of High Wall Stress Can Predict the Future Location of Rupture of Abdominal Aortic Aneurysm. Cardiovasc. Interv. Radiol. 2014, 37, 815–818. [Google Scholar] [CrossRef]
- Ge, L.; Krishnan, K.; Hope, M.; Saloner, D.; Guccione, J.; Tseng, E.E. Ascending thoracic aortic aneurysm wall stress analysis using patient-specific finite element modelling of in vivo magnetic resonance imaging. Interact. Cardiovasc. Thorac. Surg. 2015, 21, 471–480. [Google Scholar] [CrossRef] [Green Version]
- Pasta, S.; Rinaudo, A.; Luca, A.; Pilato, M.; Scardulla, C.; Gleason, T.G.; Vorp, D.A. Difference in hemodynamic and wall stress of ascending thoracic aortic aneurysms with bicuspid and tricuspid aortic valve. J. Biomech. 2013, 46, 1729–1738. [Google Scholar] [CrossRef] [Green Version]
- Roccabianca, S.; Figueroa, C.; Tellides, G.; Humphrey, J. Quantification of regional differences in aortic stiffness in the aging human. J. Mech. Behav. Biomed. Mater. 2013, 29, 618–634. [Google Scholar] [CrossRef] [Green Version]
- Paini, A.; Boutouyrie, P.; Calvet, D.; Tropeano, A.-I.; Laloux, B.; Laurent, S. Carotid and aortic stiffness: Determinants of discrepancies. Hypertension 2006, 47, 371–376. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, N.; Bouchot, O.; Rouleau, L.; Tremblay, D.; Cartier, R.; Butany, J.; Mongrain, R.; Leask, R.L. Local mechanical and structural properties of healthy and diseased human ascending aorta tissue. Cardiovasc. Pathol. 2008, 18, 83–91. [Google Scholar] [CrossRef]
- Geest, J.P.V.; Sacks, M.S.; Vorp, D.A. The effects of aneurysm on the biaxial mechanical behavior of human abdominal aorta. J. Biomech. 2006, 39, 1324–1334. [Google Scholar] [CrossRef]
- Okamoto, R.J.; Xu, H.; Kouchoukos, N.T.; Moon, M.R.; Sundt, T.M. The influence of mechanical properties on wall stress and distensibility of the dilated ascending aorta. J. Thorac. Cardiovasc. Surg. 2003, 126, 842–850. [Google Scholar] [CrossRef] [Green Version]
- Vorp, D.A.; Schiro, B.J.; Ehrlich, M.P.; Juvonen, T.S.; Ergin, M.; Griffith, B.P. Effect of aneurysm on the tensile strength and biomechanical behavior of the ascending thoracic aorta. Ann. Thorac. Surg. 2003, 75, 1210–1214. [Google Scholar] [CrossRef]
- Peterson, S.; Sundt, T.; Kouchoukos, N.; Yin, F.; Okamoto, R. Biaxial Mechanical Properties of Dilated Human Ascending Aortic Tissue. In Proceedings of the First Joint BMES/EMBS Conference, 1999 IEEE Engineering in Medicine and Biology 21st Annual Conference and the 1999 Annual Fall Meeting of the Biomedical Engineering Society, Atlanta, GA, USA, 13–16 October 1999; IEEE: Piscataway, NJ, USA, 1999; Volume 1, p. 195. [Google Scholar]
- Halloran, B.G.; Davis, V.A.; McManus, B.M.; Lynch, T.G.; Baxter, B. Localization of Aortic Disease Is Associated with Intrinsic Differences in Aortic Structure. J. Surg. Res. 1995, 59, 17–22. [Google Scholar] [CrossRef]
- Iliopoulos, D.C.; Kritharis, E.P.; Giagini, A.T.; Papadodima, S.A.; Sokolis, D.P. Ascending thoracic aortic aneurysms are associated with compositional remodeling and vessel stiffening but not weakening in age-matched subjects. J. Thorac. Cardiovasc. Surg. 2009, 137, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Azadani, A.N.; Chitsaz, S.; Mannion, A.; Mookhoek, A.; Wisneski, A.; Guccione, J.M.; Hope, M.D.; Ge, L.; Tseng, E.E. Biomechanical Properties of Human Ascending Thoracic Aortic Aneurysms. Ann. Thorac. Surg. 2013, 96, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, O.; Davis, F.M.; Rodriguez-Matas, J.F.; Duprey, A.; Avril, S. Patient specific stress and rupture analysis of ascending thoracic aneurysms. J. Biomech. 2015, 48, 1836–1843. [Google Scholar] [CrossRef] [PubMed]
- Suito, H.; Takizawa, K.; Huynh, V.Q.H.; Sze, D.; Ueda, T. FSI analysis of the blood flow and geometrical characteristics in the thoracic aorta. Comput. Mech. 2014, 54, 1035–1045. [Google Scholar] [CrossRef]
- Yeh, H.H.; Rabkin, S.W.; Grecov, D. Hemodynamic assessments of the ascending thoracic aortic aneurysm using fluid-structure interaction approach. Med. Biol. Eng. Comput. 2017, 56, 435–451. [Google Scholar] [CrossRef]
- Mousavi, S.J.; Jayendiran, R.; Farzaneh, S.; Campisi, S.; Viallon, M.; Croisille, P.; Avril, S. Coupling hemodynamics with mechanobiology in patient-specific computational models of ascending thoracic aortic aneurysms. Comput. Methods Programs Biomed. 2021, 205, 106107. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Chang, H.-Y.; Lykotrafitis, G.; Karniadakis, G.E. Computational Biomechanics of Human Red Blood Cells in Hematological Disorders. J. Biomech. Eng. 2017, 139, 021008. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.; Shin, S. Advances in the measurement of red blood cell deformability: A brief review. J. Cell. Biotechnol. 2015, 1, 63–79. [Google Scholar] [CrossRef] [Green Version]
- Fedosov, D.A.; Fornleitner, J.; Gompper, G. Margination of White Blood Cells in Microcapillary Flow. Phys. Rev. Lett. 2012, 108, 028104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marth, W.; Aland, S.; Voigt, A. Margination of white blood cells: A computational approach by a hydrodynamic phase field model. J. Fluid Mech. 2016, 790, 389–406. [Google Scholar] [CrossRef] [Green Version]
- Karimi, A.; Razaghi, R. Interaction of the blood components and plaque in a stenotic coronary artery. Artery Res. 2018, 24, 47–61. [Google Scholar] [CrossRef]
- McGloughlin, T. Biomechanics and Mechanobiology of Aneurysms; Springer: Berlin, Germany, 2011. [Google Scholar]
- Redheuil, A.; Yu, W.-C.; Mousseaux, E.; Harouni, A.A.; Kachenoura, N.; Wu, C.O.; Bluemke, D.; Lima, J.A. Age-Related Changes in Aortic Arch Geometry: Relationship with Proximal Aortic Function and Left Ventricular Mass and Remodeling. J. Am. Coll. Cardiol. 2011, 58, 1262–1270. [Google Scholar] [CrossRef] [Green Version]
- Karimi, A.; Navidbakhsh, M.; Razaghi, R. Plaque and arterial vulnerability investigation in a three-layer atherosclerotic human coronary artery using computational fluid-structure interaction method. J. Appl. Phys. 2014, 116, 064701. [Google Scholar] [CrossRef]
- Karimi, A.; Navidbakhsh, M.; Razaghi, R.; Haghpanahi, M. A computational fluid-structure interaction model for plaque vulnerability assessment in atherosclerotic human coronary arteries. J. Appl. Phys. 2014, 115, 144702. [Google Scholar] [CrossRef]
- Karimi, A.; Navidbakhsh, M.; Alizadeh, M.; Shojaei, A. A comparative study on the mechanical properties of the umbilical vein and umbilical artery under uniaxial loading. Artery Res. 2013, 8, 51–56. [Google Scholar] [CrossRef]
- Karimi, A.; Navidbakhsh, M. A comparative study on the uniaxial mechanical properties of the umbilical vein and umbilical artery using different stress–strain definitions. Australas. Phys. Eng. Sci. Med. 2014, 37, 645–654. [Google Scholar] [CrossRef]
- Scheffé, H. A method for judging all contrasts in the analysis of variance. Biometrika 1953, 40, 87–110. [Google Scholar]
- Gasser, T.; Auer, M.; Labruto, F.; Swedenborg, J.; Roy, J. Biomechanical Rupture Risk Assessment of Abdominal Aortic Aneurysms: Model Complexity versus Predictability of Finite Element Simulations. Eur. J. Vasc. Endovasc. Surg. 2010, 40, 176–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martufi, G.; Satriano, A.; Moore, R.D.; Vorp, D.A.; Di Martino, E. Local Quantification of Wall Thickness and Intraluminal Thrombus Offer Insight into the Mechanical Properties of the Aneurysmal Aorta. Ann. Biomed. Eng. 2015, 43, 1759–1771. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Grytz, R.; Rahmati, S.M.; Girkin, C.A.; Downs, J.C. Analysis of the effects of finite element type within a 3D biomechanical model of a human optic nerve head and posterior pole. Comput. Methods Programs Biomed. 2020, 198, 105794. [Google Scholar] [CrossRef]
- Karimi, A.; Rahmati, S.M.; Grytz, R.G.; Girkin, C.A.; Downs, J.C. Modeling the biomechanics of the lamina cribrosa microstructure in the human eye. Acta Biomater. 2021, 134, 357–378. [Google Scholar] [CrossRef] [PubMed]
- Chua, S.N.D.; Donald, B.J.M.; Hashmi, M.S.J. Finite element simulation of stent and balloon interaction. J. Mater. Process. Technol. 2003, 143–144, 591–597. [Google Scholar] [CrossRef]
- Lally, C.; Reid, A.J.; Prendergast, P.J. Elastic Behavior of Porcine Coronary Artery Tissue Under Uniaxial and Equibiaxial Tension. Ann. Biomed. Eng. 2004, 32, 1355–1364. [Google Scholar] [CrossRef]
- Karimi, A.; Navidbakhsh, M.; Shojaei, A.; Hassani, K.; Faghihi, S. Study of plaque vulnerability in coronary artery using mooney–rivlin model: A combination of finite element and experimental method. Biomed. Eng. Appl. Basis Commun. 2014, 26, 1450013. [Google Scholar] [CrossRef]
- Faghihi, S.; Gheysour, M.; Karimi, A.; Salarian, R. Fabrication and mechanical characterization of graphene oxide-reinforced poly (acrylic acid)/gelatin composite hydrogels. J. Appl. Phys. 2014, 115, 083513. [Google Scholar] [CrossRef]
- Jarrahi, A.; Karimi, A.; Navidbakhsh, M.; Ahmadi, H. Experimental/numerical study to assess mechanical properties of healthy and Marfan syndrome ascending thoracic aorta under axial and circumferential loading. Mater. Technol. 2016, 31, 247–254. [Google Scholar] [CrossRef]
- Karimi, A.; Sera, T.; Kudo, S.; Navidbakhsh, M. Experimental verification of the healthy and atherosclerotic coronary arteries incompressibility via Digital Image Correlation. Artery Res. 2016, 16, 1–7. [Google Scholar] [CrossRef]
- Faghihi, S.; Karimi, A.; Jamadi, M.; Imani, R.; Salarian, R. Graphene oxide/poly(acrylic acid)/gelatin nanocomposite hydrogel: Experimental and numerical validation of hyperelastic model. Mater. Sci. Eng. C 2014, 38, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Navidbakhsh, M.; Haghi, A.M.; Faghihi, S. Measurement of the uniaxial mechanical properties of rat brains infected by Plasmodium berghei ANKA. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2013, 227, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Navidbakhsh, M.; Shojaei, A. A combination of histological analyses and uniaxial tensile tests to determine the material coefficients of the healthy and atherosclerotic human coronary arteries. Tissue Cell 2015, 47, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Navidbakhsh, M. Mechanical properties of PVA material for tissue engineering applications. Mater. Technol. 2013, 29, 90–100. [Google Scholar] [CrossRef]
- Karimi, A.; Rahmati, S.M.A.; Razaghi, R.; Downs, J.C.; Acott, T.S.; Wang, R.K.; Johnstone, M. Biomechanics of human trabecular meshwork in healthy and glaucoma eyes via dynamic Schlemm’s canal pressurization. Comput. Methods Programs Biomed. 2022, 221, 106921–106938. [Google Scholar] [CrossRef]
- Karimi, A.; Rahmati, S.M.; Navidbakhsh, M. Mechanical characterization of the rat and mice skin tissues using histostructural and uniaxial data. Bioengineered 2015, 6, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Hallquist, J. Recent developments in LS-DYNA. In Proceedings of the 7th European LS-DYNA Conference, Salzburg, Austria, 14–15 May 2009; Livermore Software Technology Corporation: Livermore, CA, USA; pp. 1–52. [Google Scholar]
- Fedosov, D.A.; Caswell, B.; Karniadakis, G.E. A Multiscale Red Blood Cell Model with Accurate Mechanics, Rheology, and Dynamics. Biophys. J. 2010, 98, 2215–2225. [Google Scholar] [CrossRef] [Green Version]
- Johnston, B.M.; Johnston, P.R.; Corney, S.; Kilpatrick, D. Non-Newtonian blood flow in human right coronary arteries: Transient simulations. J. Biomech. 2006, 39, 1116–1128. [Google Scholar] [CrossRef] [Green Version]
- Joshi, A.K.; Leask, R.; Myers, J.G.; Ojha, M.; Butany, J.; Ethier, C.R. Intimal Thickness Is Not Associated with Wall Shear Stress Patterns in the Human Right Coronary Artery. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2408–2413. [Google Scholar] [CrossRef] [Green Version]
- Del Pin, F. Advances on the Incompressible CFD Solver in LS-DYNA®. In Proceedings of the 11th International LS-DYNA Users Conference, Detroit, MI, USA, 6–8 June 2010; pp. 1–6. [Google Scholar]
- Nader, E.; Skinner, S.; Romana, M.; Fort, R.; Lemonne, N.; Guillot, N.; Gauthier, A.; Antoine-Jonville, S.; Renoux, C.; Hardy-Dessources, M.-D.; et al. Blood Rheology: Key Parameters, Impact on Blood Flow, Role in Sickle Cell Disease and Effects of Exercise. Front. Physiol. 2019, 10, 1329. [Google Scholar] [CrossRef] [Green Version]
- Souli, M. ALE Incompressible Fluid in LS-DYNA. In Proceedings of the 11th International LS-DYNA Conference, Detroit, MI, USA, 6–8 June 2010; pp. 29–36. [Google Scholar]
- Aquelet, N.; Souli, M. ALE Incompressible Fluid in LS-DYNA®. In Proceedings of the 12th International LS-DYNA Users Conference, Dearborn, MI, USA, 3–5 June 2012; Volume 21. [Google Scholar]
- Hughes, T.J.; Liu, W.K.; Zimmermann, T.K. Lagrangian-Eulerian finite element formulation for incompressible viscous flows. Comput. Methods Appl. Mech. Eng. 1981, 29, 329–349. [Google Scholar] [CrossRef]
- Souli, M.; Ouahsine, A.; Lewin, L. ALE formulation for fluid–structure interaction problems. Comput. Methods Appl. Mech. Eng. 2000, 190, 659–675. [Google Scholar] [CrossRef]
- Dzwinel, W.; Boryczko, K.; Yuen, D.A. A discrete-particle model of blood dynamics in capillary vessels. J. Colloid Interface Sci. 2003, 258, 163–173. [Google Scholar] [CrossRef]
- Filipovic, N.; Ravnic, D.; Kojic, M.; Mentzer, S.; Haber, S.; Tsuda, A. Interactions of blood cell constituents: Experimental investigation and computational modeling by discrete particle dynamics algorithm. Microvasc. Res. 2008, 75, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Vorp, D.A.; Lee, P.C.; Wang, D.H.; Makaroun, M.S.; Nemoto, E.M.; Ogawa, S.; Webster, M.W. Association of intraluminal thrombus in abdominal aortic aneurysm with local hypoxia and wall weakening. J. Vasc. Surg. 2001, 34, 291–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mongrain, R.; Rodés-Cabau, J. Role of Shear Stress in Atherosclerosis and Restenosis After Coronary Stent Implantation. Rev. Esp. Cardiol. 2006, 59, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Tse, K.M.; Chiu, P.; Lee, H.P.; Ho, P. Investigation of hemodynamics in the development of dissecting aneurysm within patient-specific dissecting aneurismal aortas using computational fluid dynamics (CFD) simulations. J. Biomech. 2011, 44, 827–836. [Google Scholar] [CrossRef]
- Condemi, F.; Campisi, S.; Viallon, M.; Croisille, P.; Fuzelier, J.-F.; Avril, S. Ascending thoracic aorta aneurysm repair induces positive hemodynamic outcomes in a patient with unchanged bicuspid aortic valve. J. Biomech. 2018, 81, 145–148. [Google Scholar] [CrossRef]

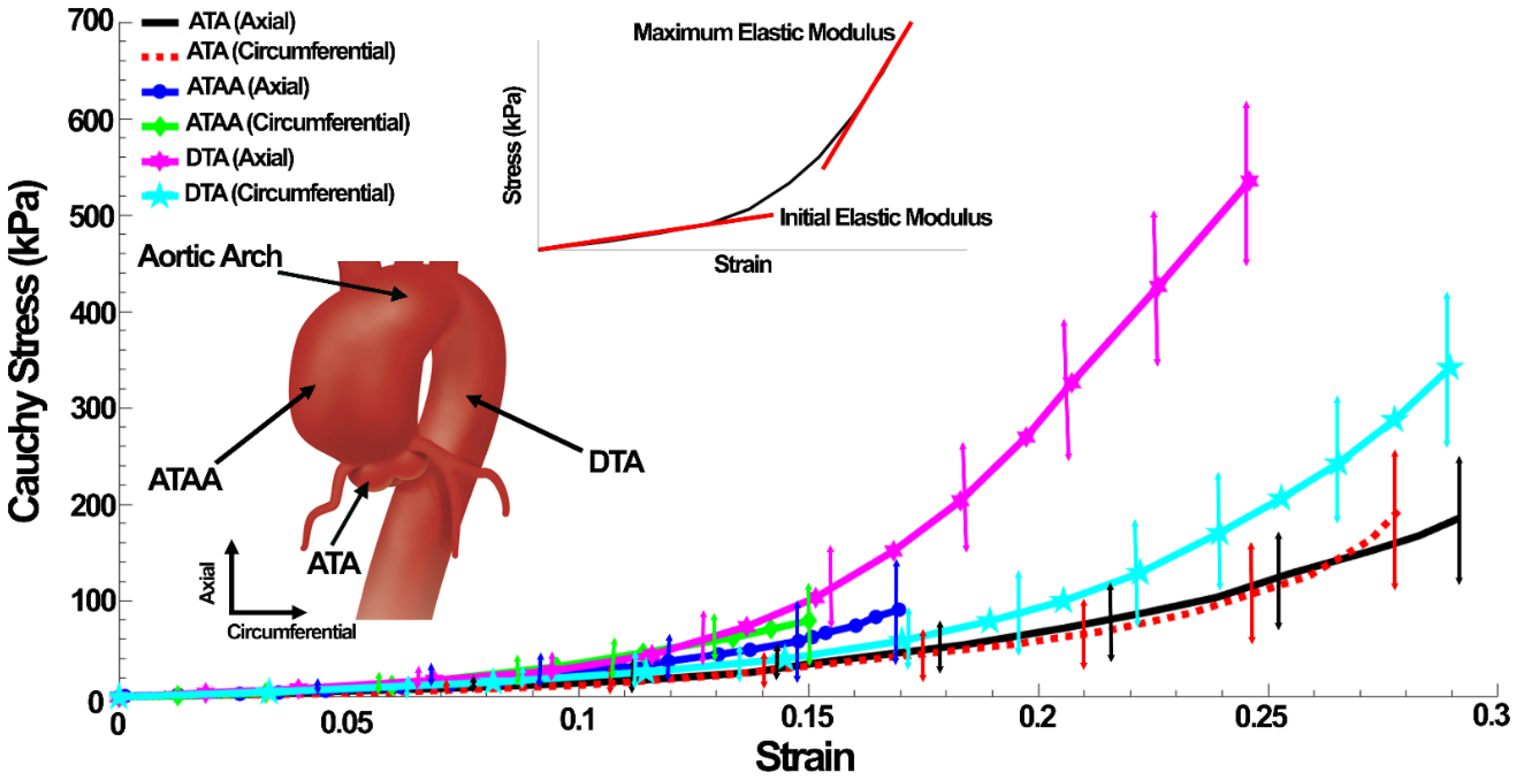


| Tissue | Initial Elastic Modulus (kPa) | Maximum Elastic Modulus (kPa) | Failure Stress (kPa) | Failure Strain (%) | Thickness (mm) | Lumen Diameter (mm) |
|---|---|---|---|---|---|---|
| ATA (Axial) | 126.19 ± 16.15 | 591.08 ± 58.29 | 185.79 ± 28.14 | 29.16 ± 6.59 | 2.31 ± 0.31 | 28.14 ± 5.12 |
| ATA (Circumferential) | 78.78 ± 9.55 | 600.44 ± 79.16 | 194.79 ± 41.98 | 27.86 ± 6.28 | ||
| DTA (Axial) | 254.74 ± 45.89 | 1884.64 ± 169.14 | 535.33 ± 81.11 | 24.59 ± 9.21 | 2.25 ± 0.19 | 29.49 ± 6.08 |
| DTA (Circumferential) | 188.95 ± 25.19 | 1038.15 ± 101.46 | 341.71 ± 71.69 | 28.95 ± 8.87 | ||
| ATAA (Axial) | 145.22 ± 20.85 | 506.64 ± 70.79 | 90.68 ± 25.65 | 16.96 ± 5.79 | 1.91 ± 0.21 | 52.14 ± 6.88 |
| ATAA (Circumferential) | 131.03 ± 22.58 | 498.79 ± 65.98 | 79.25 ± 32.14 | 15.00 ± 4.39 |
| Tissue | C10 (kPa) | C01 | C02 | C20 | C11 |
|---|---|---|---|---|---|
| ATA (Axial) | 82.38 | −67.50 | −3633.55 | −2176.87 | 5743.45 |
| ATA (Circumferential) | −1244.49 | 1283.22 | 50,754.36 | 32,263.82 | −79,953.43 |
| DTA (Axial) | 1129.80 | −1110.20 | −95,766.17 | −61,503.26 | 153,585.58 |
| DTA (Circumferential) | 271.69 | −251.54 | −3754.28 | −671.36 | 3981.76 |
| ATAA (Axial) | −1500.43 | 1546.61 | 172,626.85 | 127,687.77 | −295,095.53 |
| ATAA (Circumferential) | −311.13 | 331.29 | 70,892.11 | 51,270.33 | −119,772.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taheri, R.A.; Razaghi, R.; Bahramifar, A.; Morshedi, M.; Mafi, M.; Karimi, A. Interaction of the Blood Components with Ascending Thoracic Aortic Aneurysm Wall: Biomechanical and Fluid Analyses. Life 2022, 12, 1296. https://doi.org/10.3390/life12091296
Taheri RA, Razaghi R, Bahramifar A, Morshedi M, Mafi M, Karimi A. Interaction of the Blood Components with Ascending Thoracic Aortic Aneurysm Wall: Biomechanical and Fluid Analyses. Life. 2022; 12(9):1296. https://doi.org/10.3390/life12091296
Chicago/Turabian StyleTaheri, Ramezan Ali, Reza Razaghi, Ali Bahramifar, Mahdi Morshedi, Majid Mafi, and Alireza Karimi. 2022. "Interaction of the Blood Components with Ascending Thoracic Aortic Aneurysm Wall: Biomechanical and Fluid Analyses" Life 12, no. 9: 1296. https://doi.org/10.3390/life12091296






