Preterm Labor and Preterm-PROM at a Lower Gestational Age Are Associated with a Longer Latency-to-Delivery Even in Patients with the Same Intensity of Intra-Amniotic Inflammation: “Carroll-Model” Revisited
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Clinical Characteristics and Pregnancy Outcomes
2.3. Amniotic Fluid (AF)
2.4. Diagnosis of Acute Histologic Chorioamnionitis (Acute-HCA) and Funisitis
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics and Pregnancy Outcomes According to Gestational Age at Amniocentesis in Either Preterm Labor and Intact Membranes (PTL) or Preterm Premature Rupture of Membranes (Preterm-PROM) with the Same Intensity of Intra-Amniotic Inflammation (IAI)
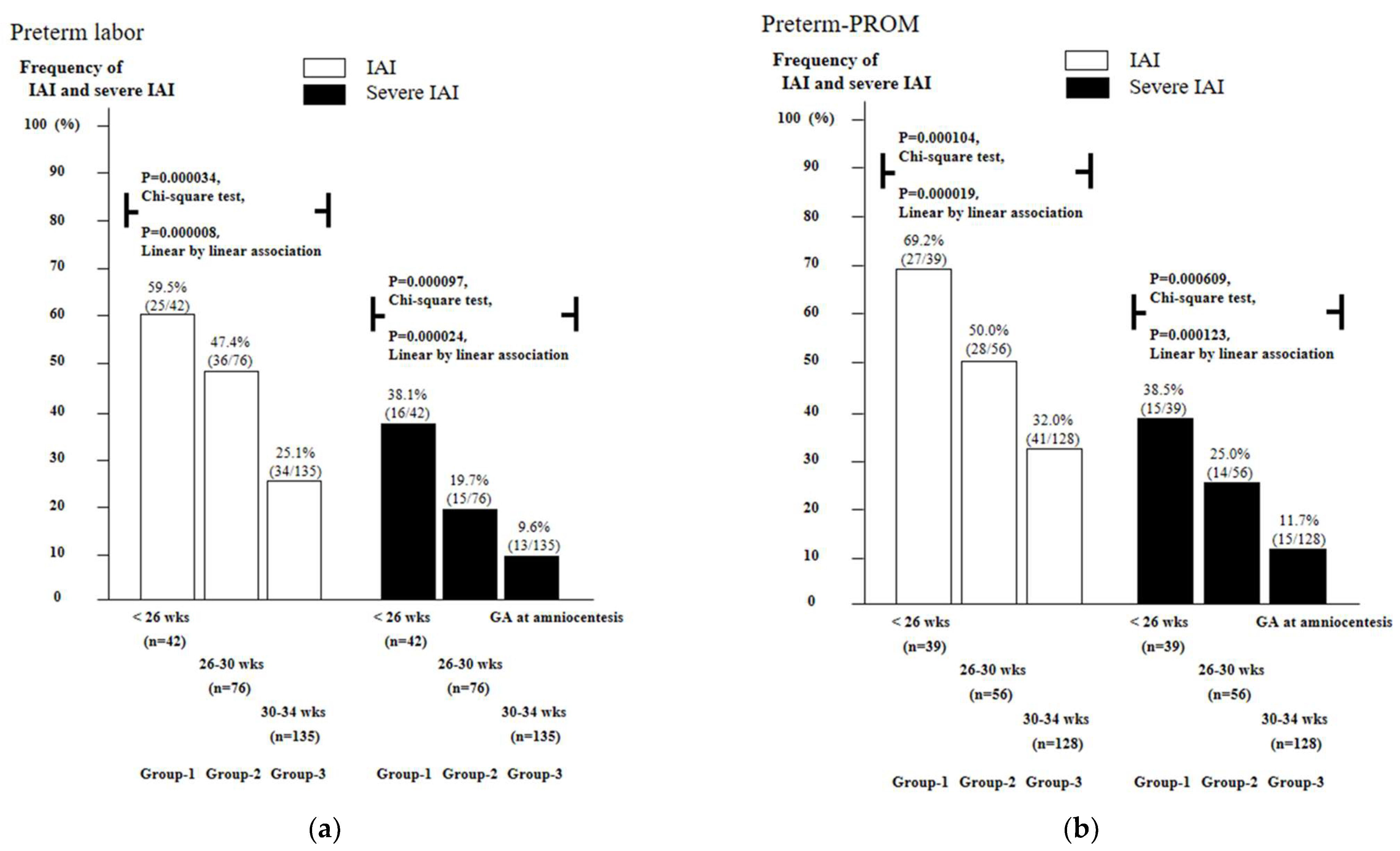
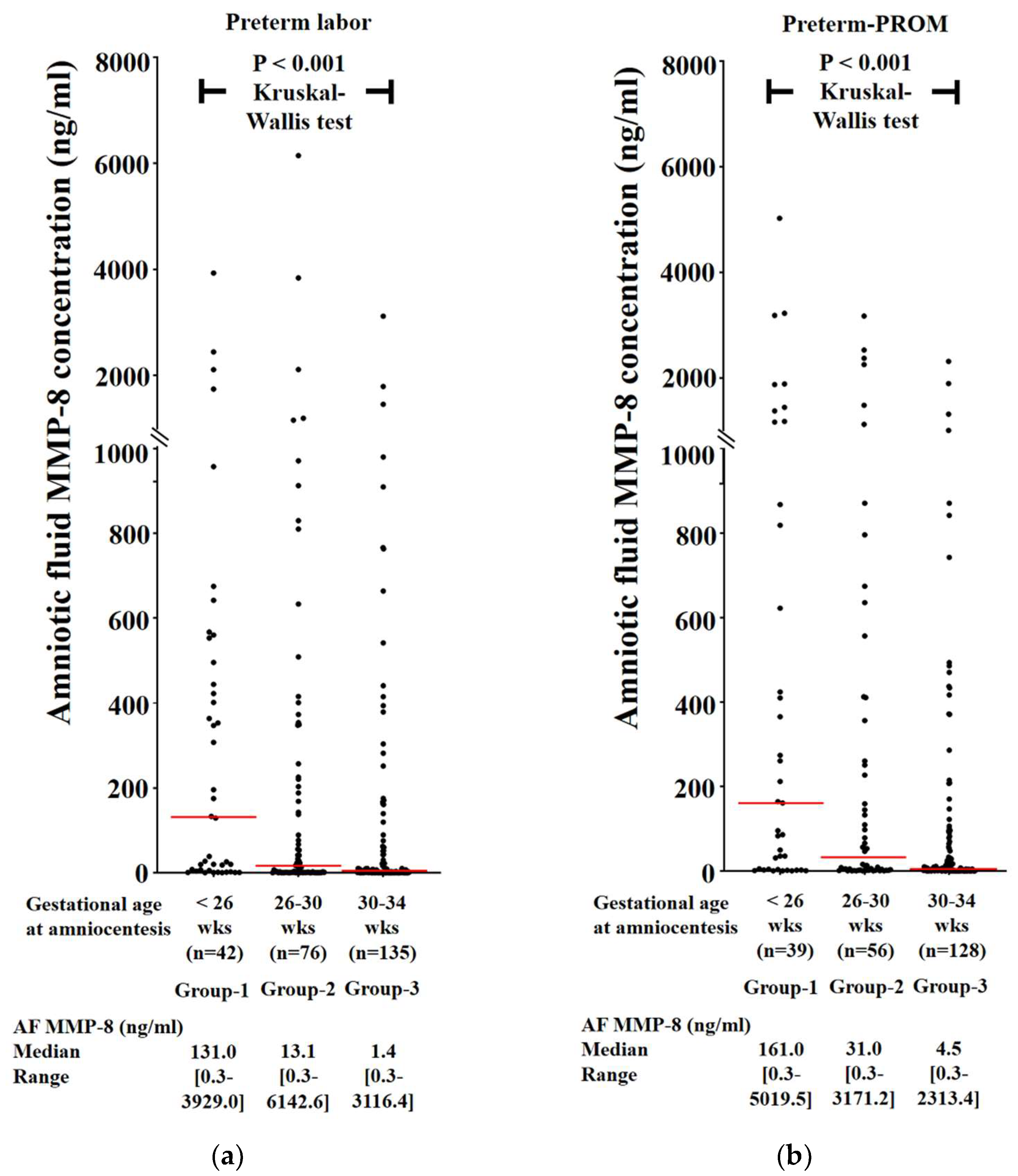
3.2. Frequency of Intra-Amniotic Inflammation (IAI) and Severe IAI as a Function of Gestational Age (GA) in Cases with PTL and Preterm-PROM
3.3. Frequency of Intra-Amniotic Inflammation (IAI) and Severe IAI as a Function of Gestational Age (GA) in Cases with PTL and Preterm-PROM
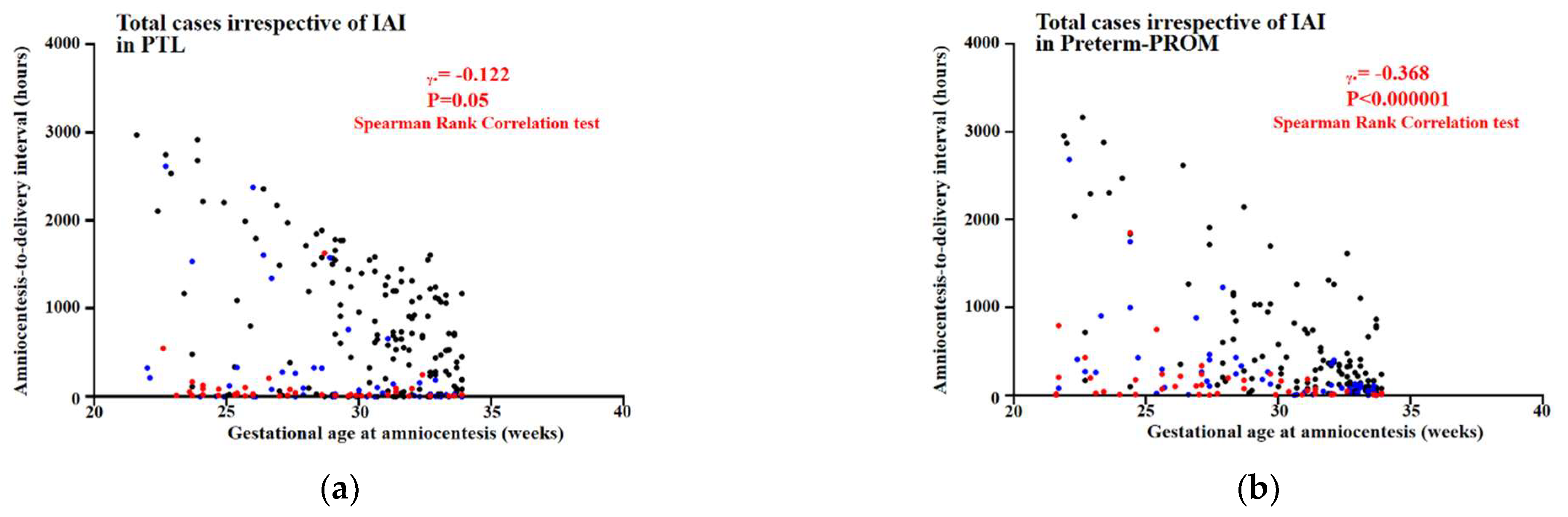
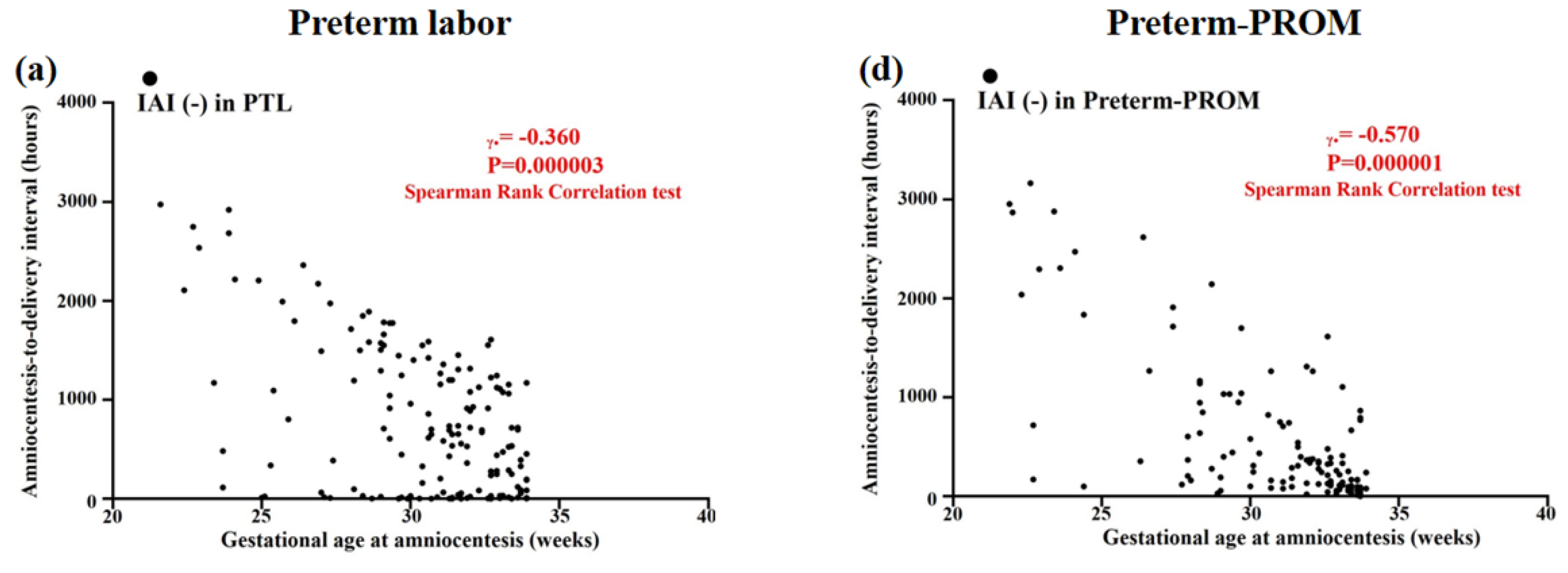
3.4. Relationships between Gestational Age (GA) at Amniocentesis and Amniocentesis-to-Delivery (ATD) Interval in PTL and Preterm-PROM
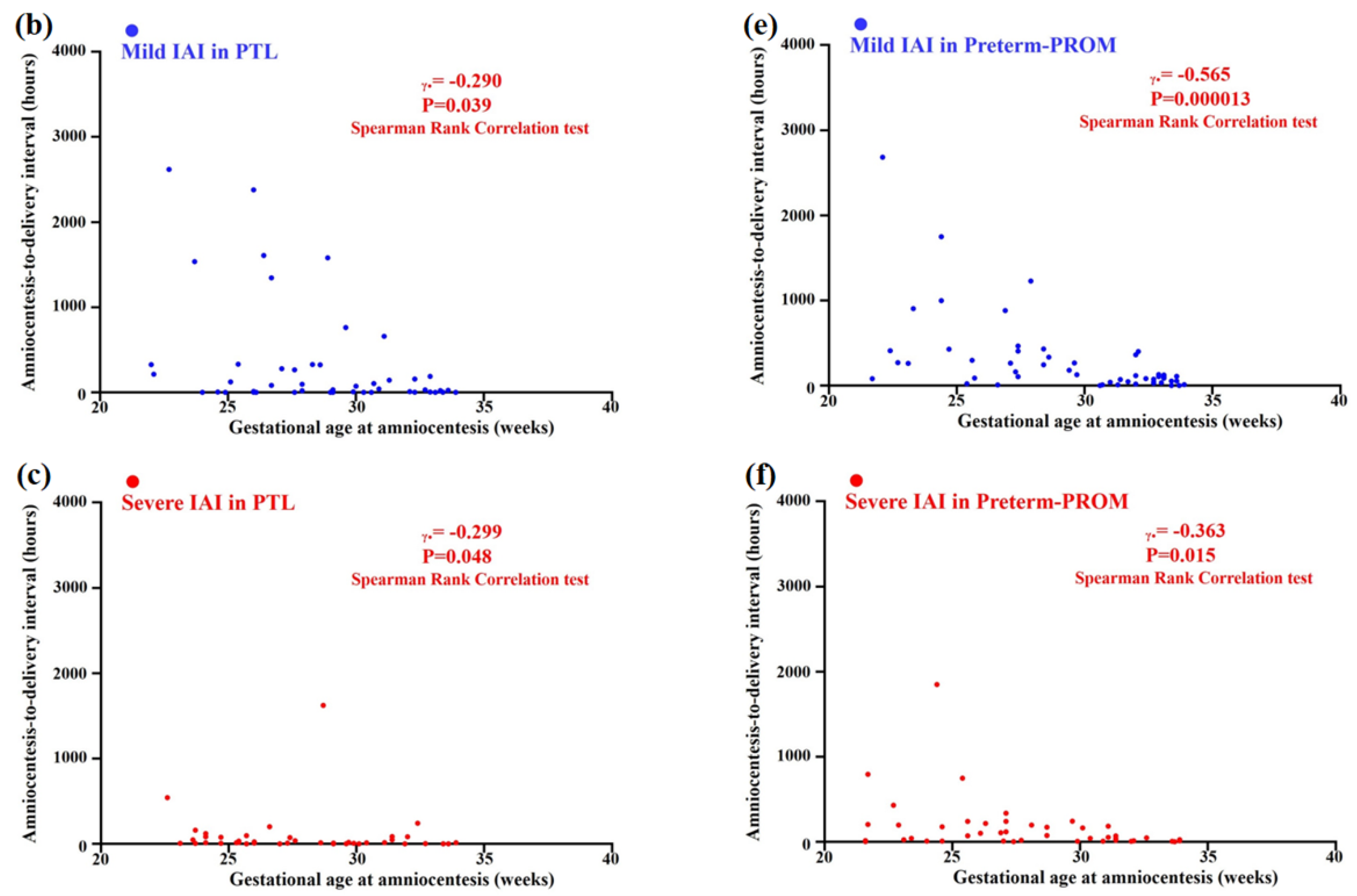
3.5. Relationships between Gestational Age (GA) at Amniocentesis and Amniocentesis-to-Delivery (ATD) Interval in the Context of the Same Intensity of Intra-Amniotic Inflammation (IAI) in PTL and Preterm-PROM
4. Discussion
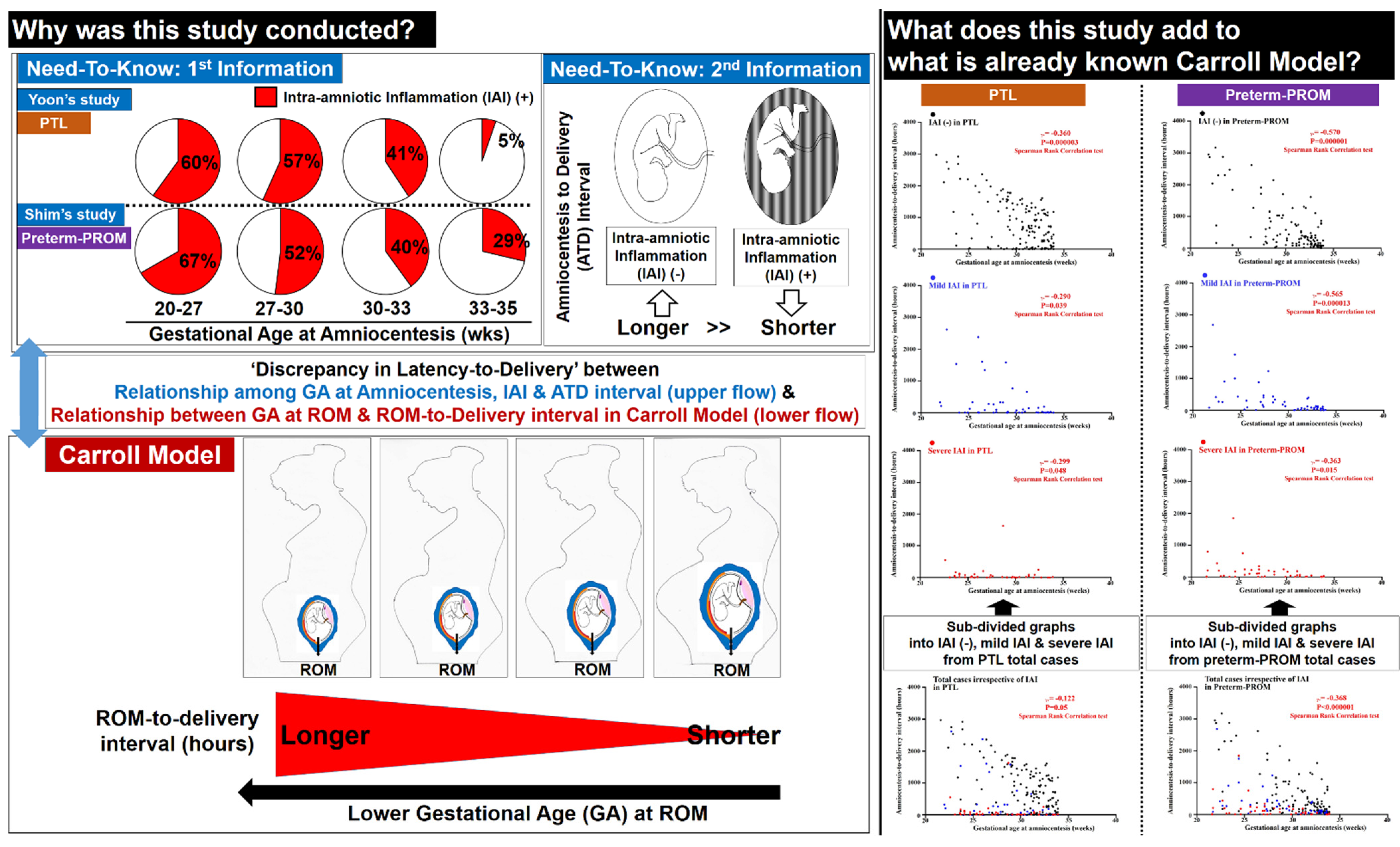
4.1. The Principal Finding of the Study
4.2. Limitations of Previous Studies (Figure 6)
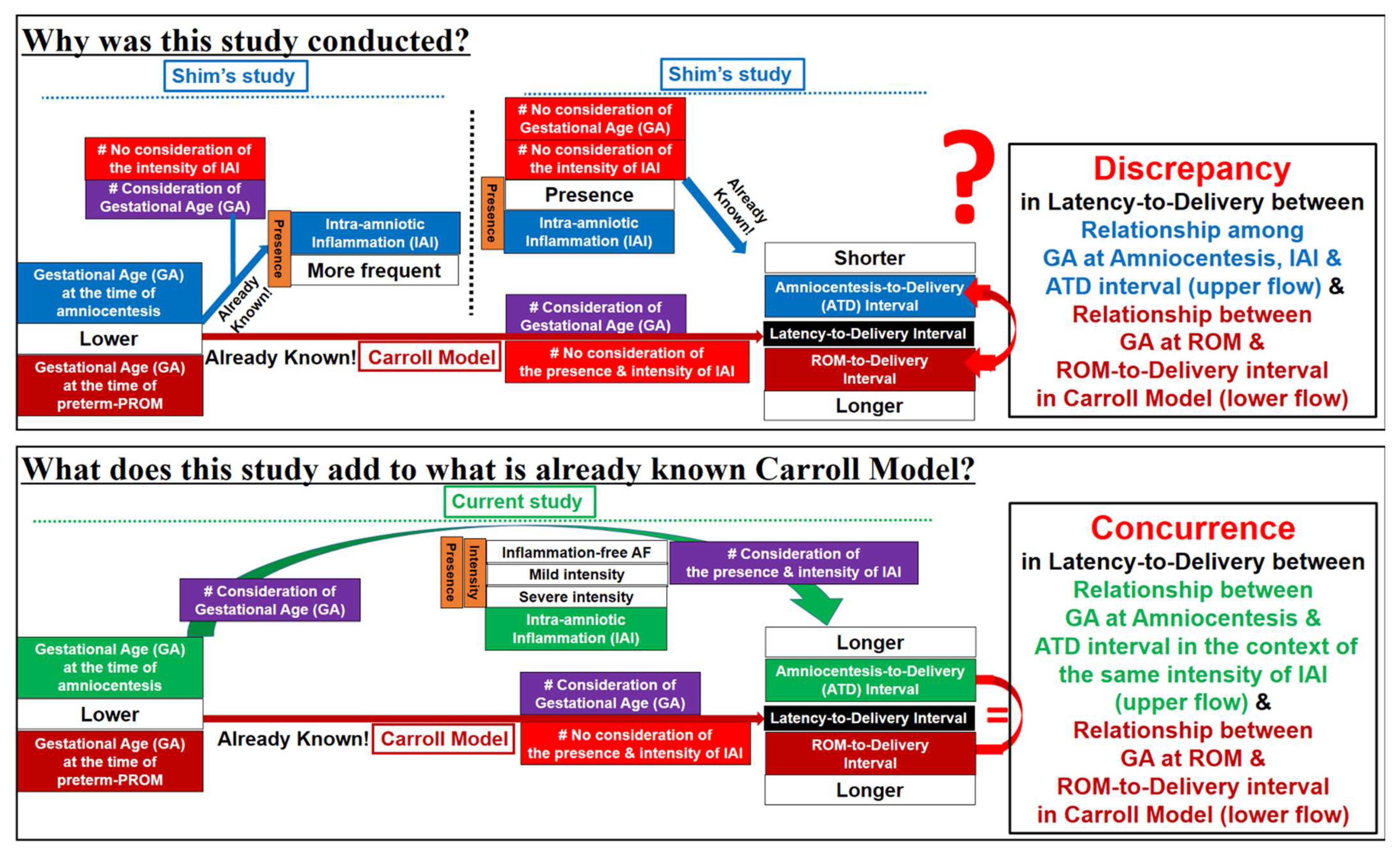
4.3. Why Are PTL and Preterm-PROM at a Lower Gestational Age (GA) Associated with a Longer Latency to Delivery?
4.4. Prostaglandins (PGs) Change in Amniotic Fluid (AF) and Amnion According to the Development of Inflammation and Advancing Gestational Age (GA) Ultimately Leading to Labor and Delivery
4.5. Biologic Plausibility about the Current Study’s Findings That PTL and Preterm-PROM at a Lower Gestational Age (GA) Are Associated with a Longer Latency to Delivery Even in the Context of the Same Intensity of IAI
4.6. Major Strengths and Weakness of Current Study
4.7. Clinical Implications of Current Study
4.8. Unanswered Questions and Proposals for Further Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Goldenberg, R.L.; Hauth, J.C.; Andrews, W.W. Intrauterine infection and preterm delivery. N. Engl. J. Med. 2000, 342, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Mazor, M. Infection and preterm labor. Clin. Obstet. Gynecol. 1988, 31, 553–584. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, L.F.; Chaiworapongsa, T.; Romero, R. Intrauterine infection and prematurity. Ment. Retard. Dev. Disabil. Res. Rev. 2002, 8, 3–13. [Google Scholar] [CrossRef]
- Yoon, B.H.; Romero, R.; Moon, J.B.; Shim, S.S.; Kim, M.; Kim, G.; Jun, J.K. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am. J. Obstet. Gynecol. 2001, 185, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Miranda, J.; Chaiworapongsa, T.; Korzeniewski, S.J.; Chaemsaithong, P.; Gotsch, F.; Dong, Z.; Ahmed, A.I.; Yoon, B.H.; Hassan, S.S.; et al. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am. J. Reprod. Immunol. 2014, 72, 458–474. [Google Scholar] [CrossRef]
- Shim, S.S.; Romero, R.; Hong, J.S.; Park, C.W.; Jun, J.K.; Kim, B.I.; Yoon, B.H. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am. J. Obstet. Gynecol. 2004, 191, 1339–1345. [Google Scholar] [CrossRef]
- Romero, R.; Miranda, J.; Chaemsaithong, P.; Chaiworapongsa, T.; Kusanovic, J.P.; Dong, Z.; Ahmed, A.I.; Shaman, M.; Lannaman, K.; Yoon, B.H.; et al. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J. Matern. Fetal. Neonatal. Med. 2015, 28, 1394–1409. [Google Scholar] [CrossRef]
- Carroll, S.G.; Blott, M.; Nicolaides, K.H. Preterm prelabor amniorrhexis: Outcome of live births. Obstet. Gynecol. 1995, 86, 18–25. [Google Scholar] [CrossRef]
- Park, C.W.; Park, J.S.; Jun, J.K.; Yoon, B.H. The inflammatory milieu of amniotic fluid in acute-chorioamnionitis decreases with increasing gestational age. Placenta 2015, 36, 1283–1290. [Google Scholar] [CrossRef]
- Yoon, B.H.; Yang, S.H.; Jun, J.K.; Park, K.H.; Kim, C.J.; Romero, R. Maternal blood C-reactive protein, white blood cell count, and temperature in preterm labor: A comparison with amniotic fluid white blood cell count. Obstet. Gynecol. 1996, 87, 231–237. [Google Scholar] [CrossRef]
- Yoon, B.H.; Jun, J.K.; Park, K.H.; Syn, H.C.; Gomez, R.; Romero, R. Serum C-reactive protein, white blood cell count, and amniotic fluid white blood cell count in women with preterm premature rupture of membranes. Obstet. Gynecol. 1996, 88, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Romero, R.; Yoon, B.H.; Moon, J.B.; Oh, S.Y.; Han, S.Y.; Ko, E.M. The relationship between amniotic fluid matrix metalloproteinase-8 and funisitis. Am. J. Obstet. Gynecol. 2001, 185, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.H.; Romero, R.; Kim, C.J.; Jun, J.K.; Gomez, R.; Choi, J.H.; Syn, H.C. Amniotic fluid interleukin-6: A sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am. J. Obstet. Gynecol. 1995, 172, 960–970. [Google Scholar] [CrossRef]
- Heron, M. Deaths: Leading Causes for 2015. Natl. Vital. Stat. Rep. 2017, 66, 1–76. [Google Scholar]
- Kim, Y.M.; Park, J.Y.; Sung, J.H.; Choi, S.J.; Oh, S.Y.; Roh, C.R.; Kim, J.H. Predicting factors for success of vaginal delivery in preterm induction with prostaglandin E2. Obstet. Gynecol. Sci. 2017, 60, 163–169. [Google Scholar] [CrossRef]
- Nielsen, P.E.; Howard, B.C.; Crabtree, T.; Batig, A.L.; Pates, J.A. The distribution and predictive value of Bishop scores in nulliparas between 37 and 42 weeks gestation. J. Matern. Fetal. Neonatal. Med. 2012, 25, 281–285. [Google Scholar] [CrossRef]
- Cook, J.L.; Zaragoza, D.B.; Sung, D.H.; Olson, D.M. Expression of myometrial activation and stimulation genes in a mouse model of preterm labor: Myometrial activation, stimulation, and preterm labor. Endocrinology 2000, 141, 1718–1728. [Google Scholar] [CrossRef]
- Kim, S.C.; Lee, J.E.; Kang, S.S.; Yang, H.S.; Kim, S.S.; An, B.S. The regulation of oxytocin and oxytocin receptor in human placenta according to gestational age. J. Mol. Endocrinol. 2017, 59, 235–243. [Google Scholar] [CrossRef]
- Fuchs, A.R.; Fuchs, F.; Husslein, P.; Soloff, M.S.; Fernström, M.J. Oxytocin receptors and human parturition: A dual role for oxytocin in the initiation of labor. Science 1982, 215, 1396–1398. [Google Scholar] [CrossRef]
- Maggi, M.; Del Carlo, P.; Fantoni, G.; Giannini, S.; Torrisi, C.; Casparis, D.; Massi, G.; Serio, M. Human myometrium during pregnancy contains and responds to V1 vasopressin receptors as well as oxytocin receptors. J. Clin. Endocrinol. Metab. 1990, 70, 1142–1154. [Google Scholar] [CrossRef]
- Lye, S.J.; Challis, J.R.G. Research in perinatal medicine. In Paracrine and Endocrine Control of Myo Metrial Activity; Gluckman, P.D., Johnston, B.M., Nathanielsz, P.W., Eds.; Perinatology Press: Ithaca, NY, USA, 1989; pp. 361–375. [Google Scholar]
- Challis, J.R.G.; Lye, S.J. The Physiology of Reproduction. In Parturition; Knobil, E., Neill, J.D., Eds.; Raven Press: New York, NY, USA, 1994; pp. 985–1031. [Google Scholar]
- Xu, C.; You, X.; Liu, W.; Sun, Q.; Ding, X.; Huang, Y.; Ni, X. Prostaglandin F2α regulates the expression of uterine activation proteins via multiple signalling pathways. Reproduction 2015, 149, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.E.; Park, I.S.; Romero, R.; Yoon, B.H. Amniotic fluid prostaglandin F2 increases even in sterile amniotic fluid and is an independent predictor of impending delivery in preterm premature rupture of membranes. J. Matern. Fetal. Neonatal. Med. 2009, 22, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.F.; Anthony, F.; Myatt, L.; Booth, L.; Furr, P.M.; Taylor-Robinson, D. Production of prostaglandin E2 by human amnion in vitro in response to addition of media conditioned by microorganisms associated with chorioamnionitis and preterm labor. Am. J. Obstet. Gynecol. 1990, 162, 819–825. [Google Scholar] [CrossRef]
- Bennett, P.R.; Rose, M.P.; Myatt, L.; Elder, M.G. Preterm labor: Stimulation of arachidonic acid metabolism in human amnion cells by bacterial products. Am. J. Obstet. Gynecol. 1987, 156, 649–655. [Google Scholar] [CrossRef]
- Lee, S.E.; Romero, R.; Park, I.S.; Seong, H.S.; Park, C.W.; Yoon, B.H. Amniotic fluid prostaglandin concentrations increase before the onset of spontaneous labor at term. J. Matern. Fetal. Neonatal. Med. 2008, 21, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Mijovic, J.E.; Zakar, T.; Angelova, J.; Olson, D.M. Prostaglandin endoperoxide H synthase mRNA expression in the human amnion and decidua during pregnancy and in the amnion at preterm labour. Mol. Hum. Reprod. 1999, 5, 182–187. [Google Scholar] [CrossRef]
- Chow, L.; Lye, S.J. Expression of the gap junction protein connexin-43 is increased in the human myometrium toward term and with the onset of labor. Am. J. Obstet. Gynecol. 1994, 170, 788–795. [Google Scholar] [CrossRef]
- Kim, B.J.; Romero, R.; Kang, K.H.; Shim, S.S.; Park, J.S.; Yoon, B.H. Is there a relationship between the interval between rupture of membranes and the timing of amniocentesis on the rate of intraamniotic inflammation in preterm premature rupture of membranes? Am. J. Obstet. Gynecol. 2005, 193, S55. [Google Scholar] [CrossRef]
| GA at Amniocentesis | <26 Wks Group-1 | 26 Wks~30 Wks Group-2 | 30 Wks~34 Wks Group-3 | p † |
|---|---|---|---|---|
| (n = 42) | (n = 76) | (n = 135) | ||
| IAI (-) [n = 158] | (n = 17) | (n = 40) | (n = 101) | |
| Maternal age, years (mean ± SD) | 31.53 ± 4.32 | 32.03 ± 5.05 | 30.37 ± 4.13 | NS (0.169) |
| Nulliparity | 64.7% (11/17) | 57.5% (23/40) | 53.5% (54/101) | NS (0.665) |
| GA at delivery, wks (mean ± SD) | 33.42 ± 5.99 | 34.58 ± 4.50 | 35.51 ± 2.90 | NS (0.539) |
| Birth weight, g (mean ± SD) | 2240 ± 987 | 2384 ± 859 | 2517 ± 677 | NS (0.642) |
| Male newborn | 47.1% (8/17) | 55.0% (22/40) | 50.5% (51/101) | NS (0.832) |
| Cesarean section | 29.4% (5/17) | 55.0% (22/40) | 45.5% (46/101) | NS (0.440) |
| Acute-HCA * or funisitis | 35.3% (6/17) | 27.5% (11/40) | 21.8% (22/101) | NS (0.439) |
| Mild IAI [n = 51] | (n = 9) | (n = 21) | (n = 21) | |
| Maternal age, years (mean ± SD) | 29.56 ± 3.09 | 29.48 ± 4.51 | 30.71 ± 4.17 | NS (0.578) |
| Nulliparity | 66.7% (6/9) | 47.6% (10/21) | 47.6% (10/21) | NS (0.584) |
| GA at delivery, wks (mean ± SD) | 27.29 ± 5.04 | 30.43 ± 3.95 | 32.50 ± 1.39 | <0.001 |
| Birth weight, g (mean ± SD) | 1199 ± 829 | 1655 ± 810 | 1966 ± 389 | 0.006 |
| Male newborn | 66.7% (6/9) | 57.1% (12/21) | 38.1% (8/21) | NS (0.272) |
| Cesarean section | 33.3% (3/9) | 47.6% (10/21) | 38.1% (8/21) | NS (0.715) |
| Acute-HCA * or funisitis | 66.7% (6/9) | 71.4% (15/21) | 61.9% (13/21) | NS (0.807) |
| Severe IAI [n = 44] | (n = 16) | (n = 15) | (n = 13) | |
| Maternal age, years (mean ± SD) | 31.69 ± 4.60 | 29.80 ± 3.80 | 30.54 ± 5.13 | NS (0.707) |
| Nulliparity | 37.5% (6/16) | 40.0% (6/15) | 38.5% (5/13) | NS (0.990) |
| GA at delivery, wks (mean ± SD) | 25.01 ± 0.91 | 28.92 ± 2.89 | 32.20 ± 1.28 | <0.001 |
| Birth weight, g (mean ± SD) | 759 ± 167 | 1286 ± 468 | 1771 ± 553 | <0.001 |
| Male newborn | 43.8% (7/16) | 53.3% (8/15) | 69.2% (9/13) | NS (0.388) |
| Cesarean section | 37.5% (6/16) | 20.0% (3/15) | 38.5% (5/13) | NS (0.480) |
| Acute-HCA * or funisitis | 87.5% (14/16) | 93.3% (14/15) | 84.6% (11/13) | NS (0.757) |
| GA at Amniocentesis | <26 Wks Group-1 | 26 Wks~30 Wks Group-2 | 30 Wks~34 Wks Group-3 | p † |
|---|---|---|---|---|
| (n = 39) | (n = 56) | (n = 128) | ||
| IAI (-) [n = 127] | (n = 12) | (n = 28) | (n = 87) | |
| Maternal age, years (mean ± SD) | 29.83 ± 2.59 | 30.29 ± 5.26 | 30.84 ± 4.53 | NS (0.573) |
| Nulliparity | 83.3% (10/12) | 57.1% (16/28) | 51.7% (45/87) | NS (0.117) |
| GA at delivery, wks (mean ± SD) | 35.00 ± 6.25 | 33.37 ± 4.02 | 34.32 ± 2.00 | NS (0.071) |
| Birth weight, g (mean ± SD) | 2581 ± 1344 | 2047 ± 767 | 2287 ± 485 | 0.027 |
| Male newborn | 50.0% (6/12) | 60.7% (17/28) | 58.6% (51/87) | NS (0.814) |
| Cesarean section | 50.0% (6/12) | 35.7% (10/28) | 34.5% (30/87) | NS (0.576) |
| Acute-HCA * or funisitis | 41.7% (5/12) | 46.4% (13/28) | 44.8% (39/87) | NS (0.962) |
| Mild IAI [n = 52] | (n = 12) | (n = 14) | (n = 26) | |
| Maternal age, years (mean ± SD) | 33.25 ± 3.89 | 30.07 ± 4.20 | 29.92 ± 3.67 | NS (0.075) |
| Nulliparity | 16.7% (2/12) | 21.4% (3/14) | 53.8% (14/26) | 0.034 |
| GA at delivery, wks (mean ± SD) | 27.87 ± 4.58 | 30.16 ± 2.03 | 33.01 ± 1.10 | <0.001 |
| Birth weight, g (mean ± SD) | 1234 ± 792 | 1500 ± 514 | 1957 ± 257 | <0.001 |
| Male newborn | 66.7% (8/12) | 42.9% (6/14) | 53.8% (14/26) | NS (0.479) |
| Cesarean section | 33.3% (4/12) | 42.9% (6/14) | 50.0% (13/26) | NS (0.625) |
| Acute-HCA * or funisitis | 75.0% (9/12) | 92.9% (13/14) | 84.6% (22/26) | NS (0.453) |
| Severe IAI [n = 44] | (n = 15) | (n = 14) | (n = 15) | |
| Maternal age, years (mean ± SD) | 31.67 ± 4.62 | 31.86 ± 5.60 | 31.13 ± 3.40 | NS (0.987) |
| Nulliparity | 46.7% (7/15) | 35.7% (5/14) | 40.0% (6/15) | NS (0.832) |
| GA at delivery, wks (mean ± SD) | 25.47 ± 3.51 | 28.48 ± 1.27 | 32.27 ± 1.13 | <0.001 |
| Birth weight, g (mean ± SD) | 914 ± 730 | 1208 ± 182 | 1892 ± 308 | <0.001 |
| Male newborn | 73.3% (11/15) | 71.4% (10/14) | 53.3% (8/15) | NS (0.446) |
| Cesarean section | 26.7% (4/15) | 57.1% (8/14) | 26.7% (4/15) | NS (0.147) |
| Acute-HCA * or funisitis | 86.7% (13/15) | 78.6% (11/14) | 100% (15/15) | NS (0.184) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sohn, J.-W.; Choi, E.-S.; Park, C.-W.; Moon, K.-C.; Park, J.-S.; Jun, J.-K. Preterm Labor and Preterm-PROM at a Lower Gestational Age Are Associated with a Longer Latency-to-Delivery Even in Patients with the Same Intensity of Intra-Amniotic Inflammation: “Carroll-Model” Revisited. Life 2022, 12, 1329. https://doi.org/10.3390/life12091329
Sohn J-W, Choi E-S, Park C-W, Moon K-C, Park J-S, Jun J-K. Preterm Labor and Preterm-PROM at a Lower Gestational Age Are Associated with a Longer Latency-to-Delivery Even in Patients with the Same Intensity of Intra-Amniotic Inflammation: “Carroll-Model” Revisited. Life. 2022; 12(9):1329. https://doi.org/10.3390/life12091329
Chicago/Turabian StyleSohn, Jeong-Won, Eun-Saem Choi, Chan-Wook Park, Kyung-Chul Moon, Joong-Shin Park, and Jong-Kwan Jun. 2022. "Preterm Labor and Preterm-PROM at a Lower Gestational Age Are Associated with a Longer Latency-to-Delivery Even in Patients with the Same Intensity of Intra-Amniotic Inflammation: “Carroll-Model” Revisited" Life 12, no. 9: 1329. https://doi.org/10.3390/life12091329
APA StyleSohn, J.-W., Choi, E.-S., Park, C.-W., Moon, K.-C., Park, J.-S., & Jun, J.-K. (2022). Preterm Labor and Preterm-PROM at a Lower Gestational Age Are Associated with a Longer Latency-to-Delivery Even in Patients with the Same Intensity of Intra-Amniotic Inflammation: “Carroll-Model” Revisited. Life, 12(9), 1329. https://doi.org/10.3390/life12091329







