Abstract
Flammulina filiformis is a popular edible mushroom that easily suffers from heat and oxidative stresses. The cyclic adenylate-dependent protein kinase A (cAMP/PKA) pathway is the main signaling pathway in response to environmental stress, and the PKAC is the terminal catalytic subunit of this pathway. In this study, the Pkac gene was identified in F. filiformis, which was highly conserved in basidiomycetes and ascomycetes. The transcription analysis showed that the Pkac gene was involved in the mycelial growth and the fruiting body development of fungi. In Neurospora crassa, the Pkac gene deletion (ΔPkac) resulted in the slower growth of the mycelia. We complemented the F. filiformis FfPkac to N. crassa ΔPkac mutant to obtain the CPkac strain. The mycelial growth in the CPkac strain was restored to the same level as the WT strain. In addition, the FfPkac gene showed significantly up-regulated expression under heat and oxidative stresses. By analyzing the differentially expressed genes of ΔPkac and Cpkac with WT, respectively, seven downstream genes regulated by Pkac were identified and may be related to mycelial growth. They were mainly focused on microbial metabolism in diverse environments, mitochondrial biogenesis, protein translation and nucleocytoplasmic transport. RT-qPCR results confirmed that the expression patterns of these seven genes were consistent with FfPkac under heat and oxidative stresses. The results revealed the conserved functions of PKAC in filamentous fungi and its regulatory mechanism in response to heat and oxidative stresses.
1. Introduction
The cyclic adenylate-dependent protein kinase A (cAMP/PKA) signal pathway is known as a major signal transduction pathway in eukaryotes in response to extracellular signal stimulation and plays an important role in regulating the growth and development of an organism [1,2]. Adenylate cyclase (AC) and PKA are two major catalytic enzymes in the cAMP/PKA signal pathway. AC is activated by the active G protein α subunit and catalyzes ATP to form the second messenger cyclic adenylate (cAMP) [1,3,4,5]. PKA is composed of two PKA catalytic subunits (PKAc) and two PKA regulatory subunits (PKAr) [6,7]. The cAMP binds to two PKAr when its concentration reaches a certain level, resulting in conformational changes of PKA tetramer R2C2. The two PKAc subunits are released, and phosphorylate target substrates, including metabolic enzymes and transcription factors [6,7,8]. As the main effector in the last step of the cAMP signaling pathway, PKAc gene deletion or mutation is helpful in identifying a series of target genes downstream of the cAMP/PKA pathway.
In fungi, cAMP/PKA signal transduction is reported to be involved in the regulation of fungal mycelial growth, morphogenesis, pathogenicity, etc. [2,5,9,10]. The PKAc gene was also performed by gene deletion or overexpression to understand its effect on fungal life. In Pleurotus ostreatus, the overexpression of PKAc could significantly improve the transcription levels of the genes encoding lignin-modifying enzymes, indicating that PKAc plays an important role in inducing wood degradation and mycelial nutritional growth [11]. In contrast, when the PKAc gene was deleted, the radial growth of mycelia was absent in Fusarium verticillioides [12], or the transition from budding to filamentous growth was disrupted in Ustilago maydis [13]. It suggests that the PKAc gene plays important regulatory roles in the vegetative growth of filamentous fungi. Whether this function of the PKAc gene is conserved in filamentous fungi needs further direct experimental verification. Moreover, PKAc gene deletion also affected the infection efficiency of the F. verticillioides [12,14], the production of melanin or virulence of Cryptococcus neoformans [15], indicating the possible role of the PKAc gene in regulating metabolism and responding to the environment.
In the life history of filamentous fungi, mycelial growth, primordia formation and fruiting body development are regulated by environmental factors such as temperature and light. Moderate environmental stress and oxidative stress are the key inducers of morphogenesis of mycelia and fruiting bodies [16]. Flammulina filiformis is a popular edible mushroom and a typical agaric fungus, which belongs to basidiomycete. Its fruiting body is suitable to grow at low temperatures and can be cultivated in industrialized style in a cold mushroom room. F. filiformis easily suffers from many environmental stresses such as heat and oxidative stresses in the production process. Therefore, exploring the function of the cAMP/PKA signal pathway in response to stress is important for the cultivation and production of F. filiformis. As another model filamentous fungus, Neurospora crassa belongs to ascomycete and is easy to operate in genetics. More than 70% of genes in the N. crassa genome have corresponding knockout mutants, and it is an ideal material for studying the function of the conserved genes in fungi. Thus, the Pkac gene was identified in F. filiformis and N. crassa first in this study. The expression of Pkac in response to abiotic stresses was analyzed in F. filiformis. Meanwhile, the Pkac gene in F. filiformis was complemented into the Pkac knockout mutant of N. crassa (ΔPkac). The possible downstream genes regulated by the Pkac gene were also identified and compared to the Pkac knockout mutant and complementary stain (CPkac) in N. crassa. The results will be helpful for understanding the conserved function of Pkac in filamentous fungi and its regulatory mechanism of the cAMP/PKA signal transduction pathway.
2. Materials and Methods
2.1. Strains and Culture Conditions
F. filiformis dikaryotic strain 1123, obtained by mating monokaryotic strains L11 and W23, was provided by the Fujian Edible Fungi Germplasm Resource Collection Center of China. N. crassa wild type strain FGSC#4200 (WT), the NcPkac (gene No. NCU00682) knockout mutant strain FGSC#11433 (ΔPkac) and plasmid pCB1532 were provided by the Institute of Microbiology, Chinese Academy of Sciences. The Escherichia coli DH5α was used for vector construction, purchased from Tiangen BioTech Co., Ltd. (Beijing, China). The F. filiformis strains and N. crassa strains were maintained on potato dextrose agar (PDA) medium and Vogel’s medium [17] at 25 °C, respectively.
Cultivation of the fruiting body of strain 1123 was performed as per the method described by Tao et al. [18]. The fruiting body was sampled at six different developmental stages after inoculation, including mycelia (MY), primordia (PR), pileus in elongation stage (ELP), stipe in elongation stage (ELS), pileus in maturation stage (MAP), and stipe in maturation stage (MAS).
For the collection of mycelia, F. filiformis strain 1123, L11 and W23 were cultured on the PDA medium with the cellophane sticking firmly. When the mycelia were fully grown, they were treated with heat stress (HS) at 37 °C or oxidative stress (H2O2) at 5, 10 and 20 mM for 1 h, respectively. The samples were collected and stored at −80 °C.
To compare the growth rate, the equipotent hypha blocks with mycelia of WT, ΔPkac and CPkac strains of N. crassa were inoculated onto a new 90 mm Vogel’s medium plate and incubated in the dark at 25 °C for 12 h. The length of mycelial growth was measured with a vernier caliper, and the growth rate of the colony was calculated. The viable monokaryotic microconidia were purified from conidiating strains of N. crassa grown on Westergaard and Mitchell synthetic crossing (SC) medium supplemented with iodoacetate (IAA) via the method of Ebbole et al. [19].
2.2. Sequence, Structure and Similarity Analysis of Pkac Gene in F. filiformis and N. crassa
According to the NcPkac gene in N. crassa (NCU00682), the orthologous gene FfPkac in the genome of F. filiformis L11 (GenBank accession number: APIA00000000.1; BioProject: PRJNA191865) was identified by local BLASTp searching. The sequence of FfPkac was then retrieved from the genome of the F. filiformis L11 strain. Open reading frame (ORF) of the Pkac gene was predicted by ORF Finder online software (http://www.ncbi.nlm.nih.gov/gorf/gorf.html, accessed on 25 June 2022). The structure of Pkac gene was visualized by Gene Structure Display Server (GSDS 2.0) (http://gsds.gao-lab.org/, accessed on 25 June 2022). The PKAC protein conserved domain was predicted by InterProScan online software (http://www.ebi.ac.uk/Tools/pfa/iprscan/, accessed on 25 June 2022). Phylogenetic tree construction was performed using the neighbor-joining method of MEGAX software [20], and the bootstrap value was set to 1000. DNAMAN software (Lynnon Corporation, Vaudreuil-Dorion, QC, Canada) was used to conduct the multiple sequence alignment with full-length sequences of six PKAC proteins from F. filiformis, N. crassa, Agaricus bisporus, Coprinopsis cinerea, Trichoderma reesei, and Cordyceps militaris.
2.3. Total DNA and RNA Extraction and RT-qPCR
Total DNA was isolated from the samples of F. filiformis and N. crassa using a modified cetyltrimethylammonium bromide (CTAB) method [21]. Total RNA was isolated using an Omega E.Z.N.A. Plant RNA Kit (Omega Bio-Tek, Norcross, GA, USA) according to the manufacturer’s protocol. Extracted RNA was quantified using a NanoND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). Only RNA samples with A260/A280 ratios between 1.9 and 2.1 were used for cDNA synthesis. The cDNA was synthesized using 1000 ng RNA for each sample, according to the instructions of a TransScript® All-in-One First-Strand cDNA Synthesis SuperMix for qPCR (One-Step gDNA Removal) kit (Transgen, Beijing, China).
Real-time quantitative PCR (RT-qPCR) was performed using a CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). RT-qPCR amplification included a denaturation step of 30 s at 94 °C, followed by 35 cycles of 5 s at 94 °C and 30 s at 60 °C, according to the instructions of TransStart® Top Green qPCR SuperMix (Transgen, Beijing, China). ACTB, Ras and GAPDH were used as internal control genes for the normalization of the RT-qPCR in this study [22]. All primers for RT-qPCR were designed by Primer Premier 5.0 software and are shown in Table S1 (Supplementary Materials). The relative expression levels of the tested genes were determined according to the 2−∆∆Ct method [23].
2.4. Construction of Complementation Vector of N. crassa
The FfPkac gene was amplified from the cDNA of F. filiformis L11 with the primers FfPkac-F and FfPkac-R. The 5′ end and 3′ end homologous arm fragments were amplified from the DNA of N. crassa WT strain with the primer pairs NcPkac5′-F-AscI and NcPkac5′-R and NcPkac3′-F-NotIHF and NcPkac3′-R-AscI, respectively. The TrpC terminator fragment was amplified with the primers TrpC-F and TrpC-R-SpeIHF. The above four fragments were introduced into the linearized plasmid PCB1532 by a seamless cloning kit (Transgen, Beijing, China) to obtain a new plasmid named as FfPkac-C. The recombinant plasmids were transferred into receptor cells DH5α, and the positive transformants were screened in LB plates containing ampicillin. The recombinant plasmid was cleaved with restriction endonuclease AscI and transferred into the ΔPkac strain via the electroporation method [24]. The positive transformants were screened using medium containing chlorimuron-ethyl. All the primers employed for plasmid construction are shown in Table S1 (Supplementary Materials).
2.5. RNA-Seq of N. crassa Transformants
RNA-Seq was performed using Hiseqx-ten, which was entrusted to Biomarker Technologies Co, LTD (Beijing, China). The clean data were analyzed with HISAT, StringTie and Ballgown software via the method of Pertea et al. [25]. The transcription levels of all genes were quantified by standardized FPKM values. The differentially expressed genes between the WT, ΔPkac and CPkac strains of N. crassa were screened out via the method of Thomas et al. [26]. The homologous genes of N. crassa were found in F. filiformis by local BLAST software. The amino acid sequences of the differentially expressed genes were uploaded to the Kyoto Encyclopedia of Genes and Genomes (KEGG) for pathway annotation to predict their functions.
3. Results
3.1. Structure and Protein Function of Pkac Gene
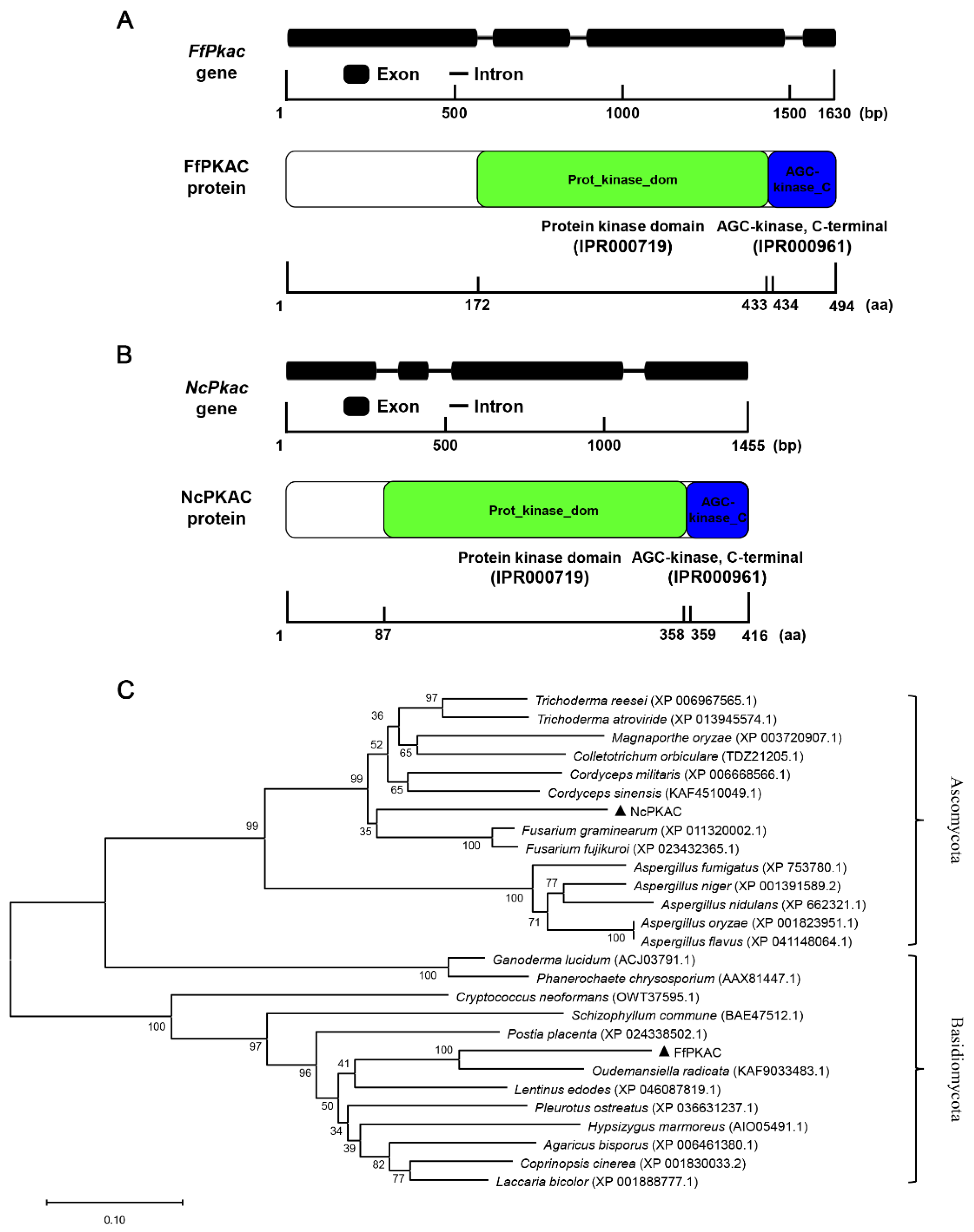
The length of the FfPkac gene is 1630 bp, and its ORF encodes 494 amino acids, containing four exons and three introns (Figure 1A). The length of NcPkac gene is 1455 bp, its ORF encodes 416 amino acids, containing four exons and three introns (Figure 1B). Both FfPKAC and NcPKAC proteins contained two conserved domains: protein kinase domain (IPR000719) in the middle and AGC-kinase (IPR000961) in the C-terminal. The protein kinase domain is a conserved catalytic core and is important for the protein kinase activity for catalyzing protein phosphorylation. The AGC (cAMP-dependent, cGMP-dependent and protein kinase C) protein kinase domain has three conserved phosphorylation sites that serve as phosphorylation-regulated switches to control both intra-molecular and inter-molecular interactions and is critical for the catalytic activity (Figure 1A,B).
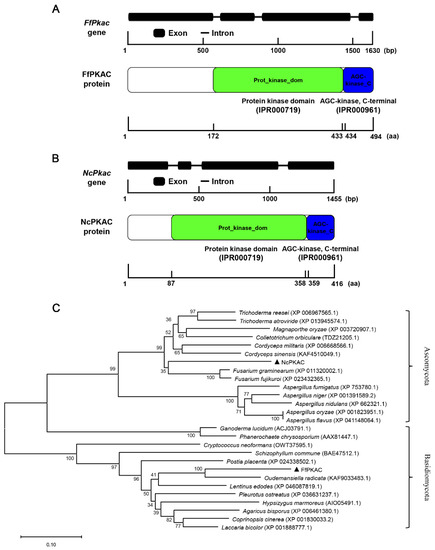
Figure 1.
Gene and protein structures and phylogeny analysis of FfPkac and NcPkac in Flammulina filiformis and Neurospora crassa. (A) Gene structure of FfPkac and protein structure of FfPKAC in F. filiformis. Thick lines represent exons, and thin lines represent introns. The green rectangle and blue rectangle represent the protein kinase domain and AGC-kinase, C-terminal, respectively. (B) Gene structure of NcPkac and protein structure of NcPKAC in N. crassa. Thick lines represent exons, and thin lines represent introns. The green rectangle and blue rectangle represent the protein kinase domain and AGC-kinase, C-terminal, respectively. (C) Phylogeny analysis of PKAC. Black triangles represent FfPKAC in F. filiformis and NcPKAC in N. crassa, respectively. Phylogenetic tree of FfPKAC and NcPKAC proteins from other species, including basidiomycetes and ascomycetes.
The evolutionary tree (Figure 1C) showed that FfPKAC and PKAC form Ganoderma lucidum, Lentinus edodes, A. bisporus, P. ostreatus and C. cinerea are clustered into basidiomycetes group; while NcPKAC and PKAC form Fusarium graminearum, C. militaris, T. reesei and Aspergillus fumigatus are clustered into the ascomycetes group. The evolution of the Pkac gene was consistent with the evolution of the species, suggesting the conserved function of the Pkac gene in fungi.
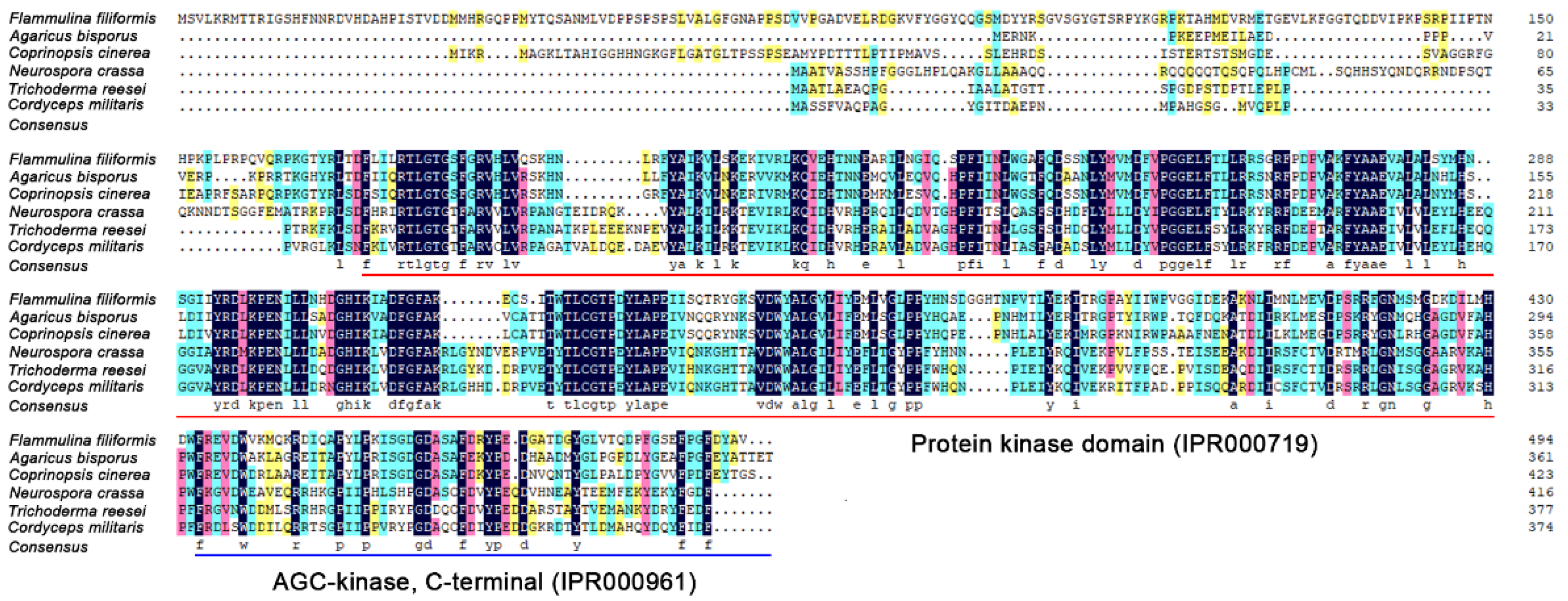
PKAC of four species from basidiomycetes (A. bisporus and C. cinerea) and ascomycetes (T. reesei and C. militaris) were selected for multiple sequence alignment with F. filiformis FfPKAC and N. crassa NcPKAC. The result showed that these six PKAC proteins shared multiple conserved sites in two conserved protein kinase and AGC-kinase domains (Figure 2). It also suggested that PKAC may have highly conserved functions in filamentous fungi.
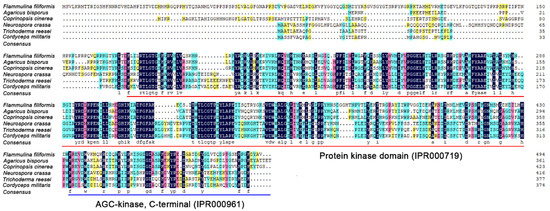
Figure 2.
Multiple sequence alignment of PKAC protein sequences from different species.
Red underline and blue underline represent protein kinase domain (IPR000719) and AGC-kinase, C-terminal (IPR000961), respectively.
3.2. Expression Pattern of Pkac Gene
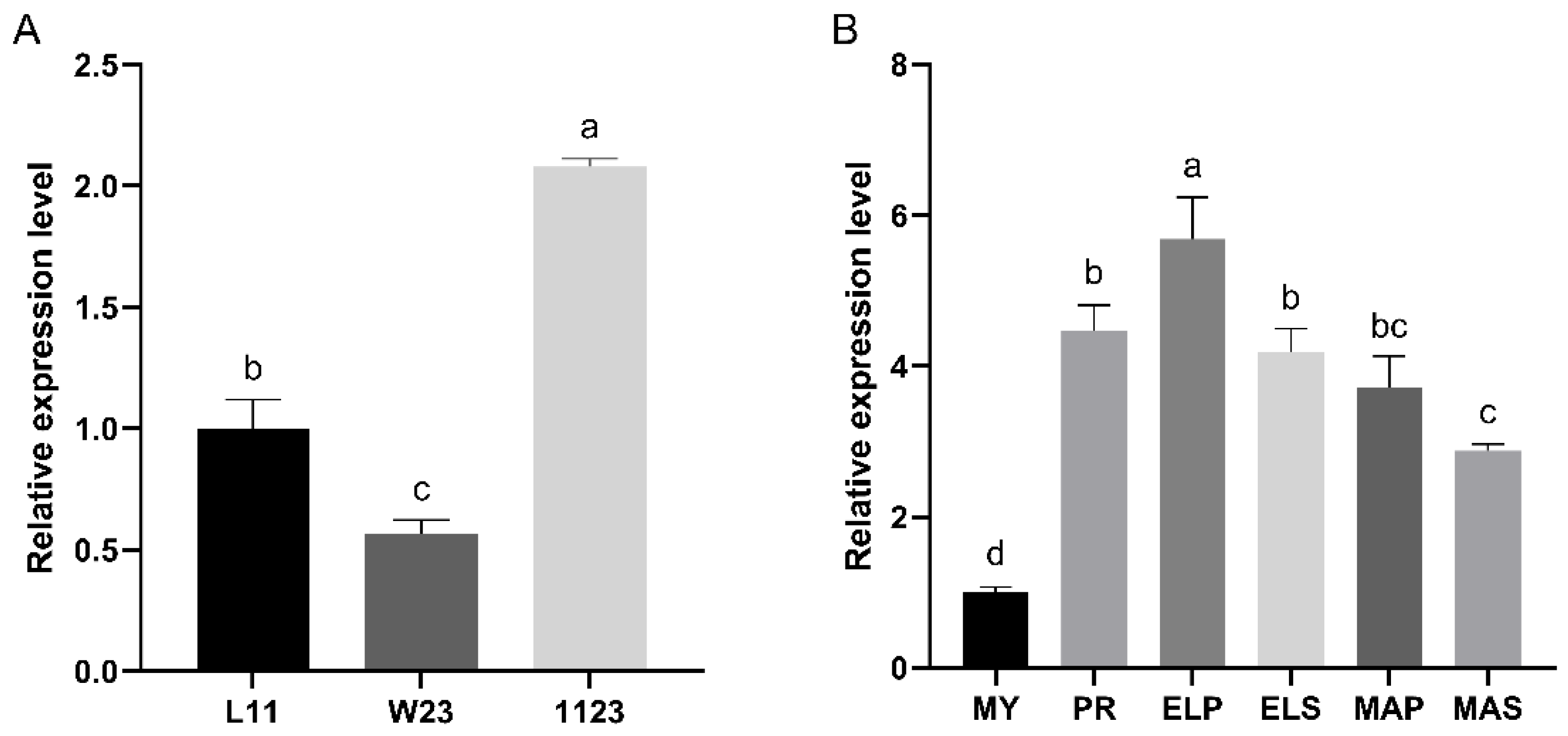
The relative expression levels of the Ffpkac gene in monokaryotic mycelia (L11 and W23), dikaryotic mycelia (1123) and fruiting bodies of F. filiformis at different developmental stages were detected by RT-qPCR (Figure 3). The relative expression level of FfPkac in dikaryotic mycelia was 2.1-fold and 3.7-fold higher than that in monokaryon L11 and W23, respectively (Figure 3A). The relative expression level of FfPkac was significantly up-regulated during the process of dikaryotic mycelia forming fruiting bodies in F. filiformis. Compared with that in MY stage, the transcription level of FfPkac was up-regulated 4.5-fold in the PR stage and maintained higher levels in ELP (5.7-fold), ELS (4.2-fold), MAP (3.7-fold) and MAS (2.9-fold) stages. It suggested that FfPkac may be related to the mycelial growth and development of the fruiting body of macro fungi.
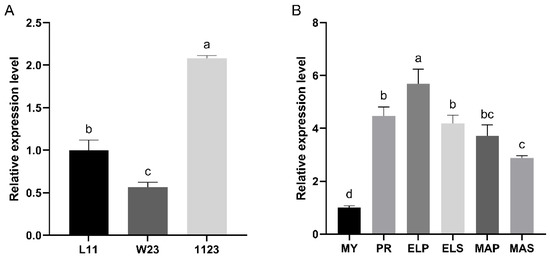
Figure 3.
Relative expression levels of FfPkac in different strains and developmental stages. (A) The relative expression levels of FfPkac in three different strains (L11, W23, 1123) of F. filiformis. L11 and W23 are the monokaryotic strains and 1123 is the dikaryotic strain. (B) The relative expression levels of FfPkac at different developmental stages of strain 1123. MY (Mycelia) is the mycelia stage, PR (Primordia), ELP (Pileus in elongation stage), ELS (Stipe in elongation stage), MAP (Pileus in maturation stage), and MAS (Stipe in maturation stage) make up the fruiting body stage. (A,B) The values are the means ± SD of three independent experiments (Tukey’s multiple comparisons test: lowercase letters represent p < 0.05).
3.3. Pkac Gene Regulates Mycelial Growth
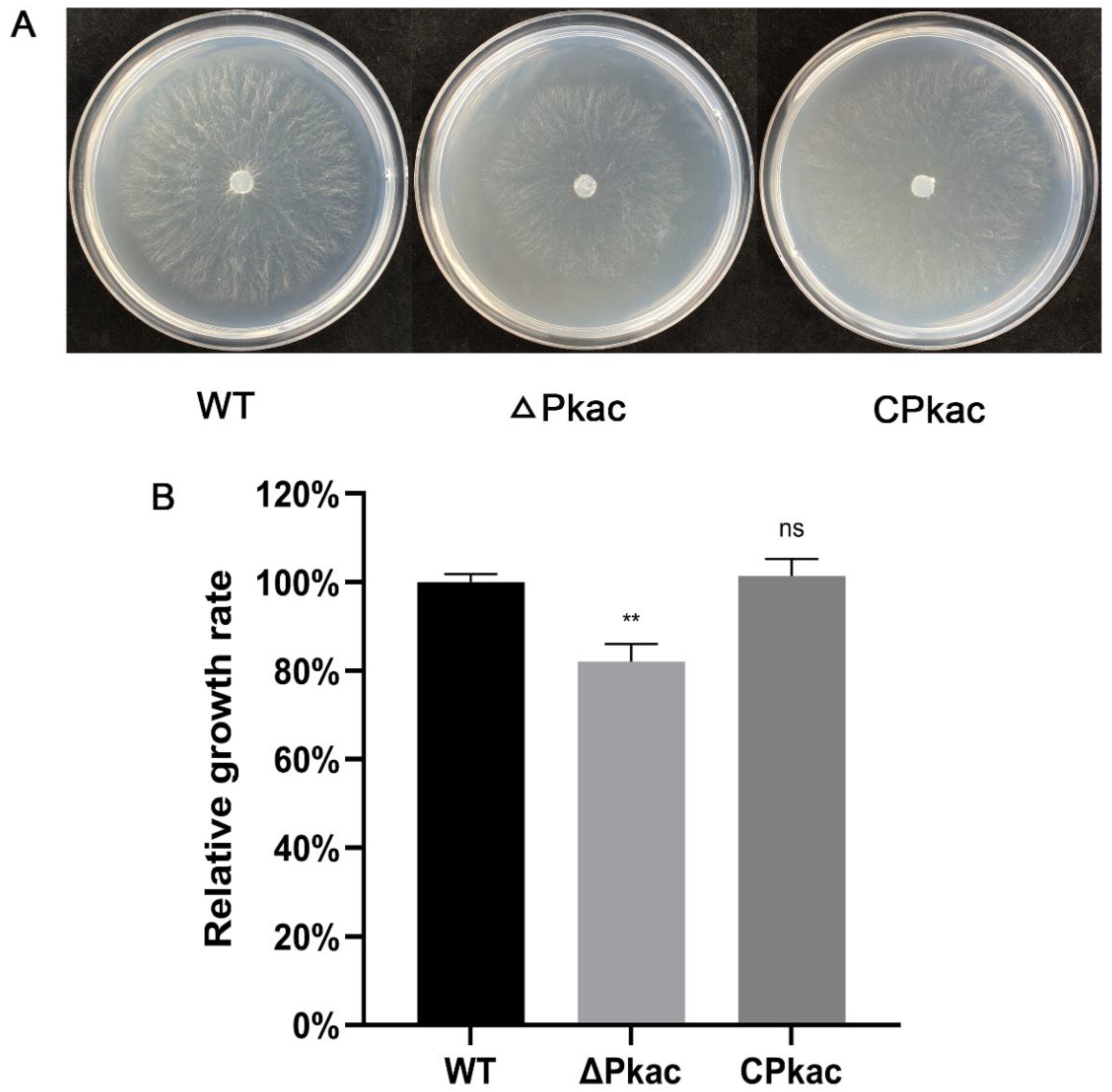
As a model fungus, N. crassa has a rich gene mutant library including the Pkac knockout mutant. We firstly investigated the phenotype of the mycelial growth of the Pkac knockout mutant strain (ΔPkac), in which the NcPkac gene was replaced by the hygromycin gene. Compared with the WT, the mycelia of ΔPkac strain grew sparsely and slowly (decreased by 17.9%) (Figure 4A). In order to further confirm the conserved role of the FfPkac gene on mycelial growth, we complemented the F. filiformis FfPkac gene into the N. crassa ΔPkac strain and obtained the complemented strain of N. crassa named CPkac. Compared with the ΔPkac strain, the mycelia of the CPkac strain grew thicker and faster (Figure 4). The growth rate of mycelia in the CPkac strain was increased by 1.2-fold compared with ΔPkac and showed no significant difference to that in WT (Figure 4B). The results suggested that the Pkac gene played an essential role in mycelial growth and shared the conserved function in both F. filiformis and N. crassa.
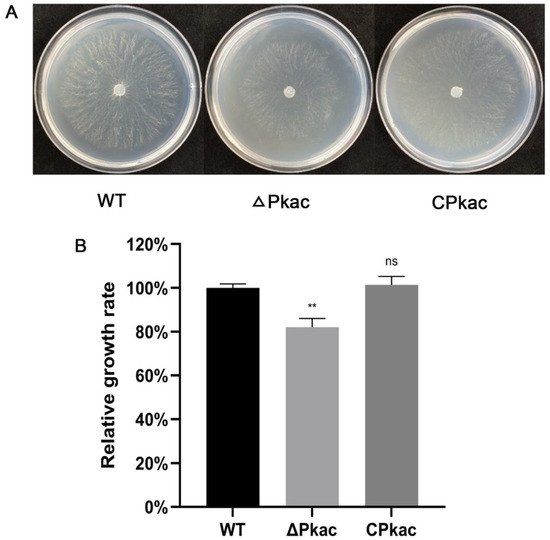
Figure 4.
Mycelial growth rates of different strains of N. crassa. (A) Mycelial phenotype of N. crassa transformants grown on Vogel’s medium. (B) Relative growth rate of mycelia of N. crassa on Vogel’s medium for 12 h. The values are the means ± SD of three independent experiments. Asterisks indicate significant differences compared to WT (Dunnett T3′s multiple comparisons test: ** p < 0.01, ns: not significant).
3.4. Pkac Gene Participates in Abiotic Stress Response
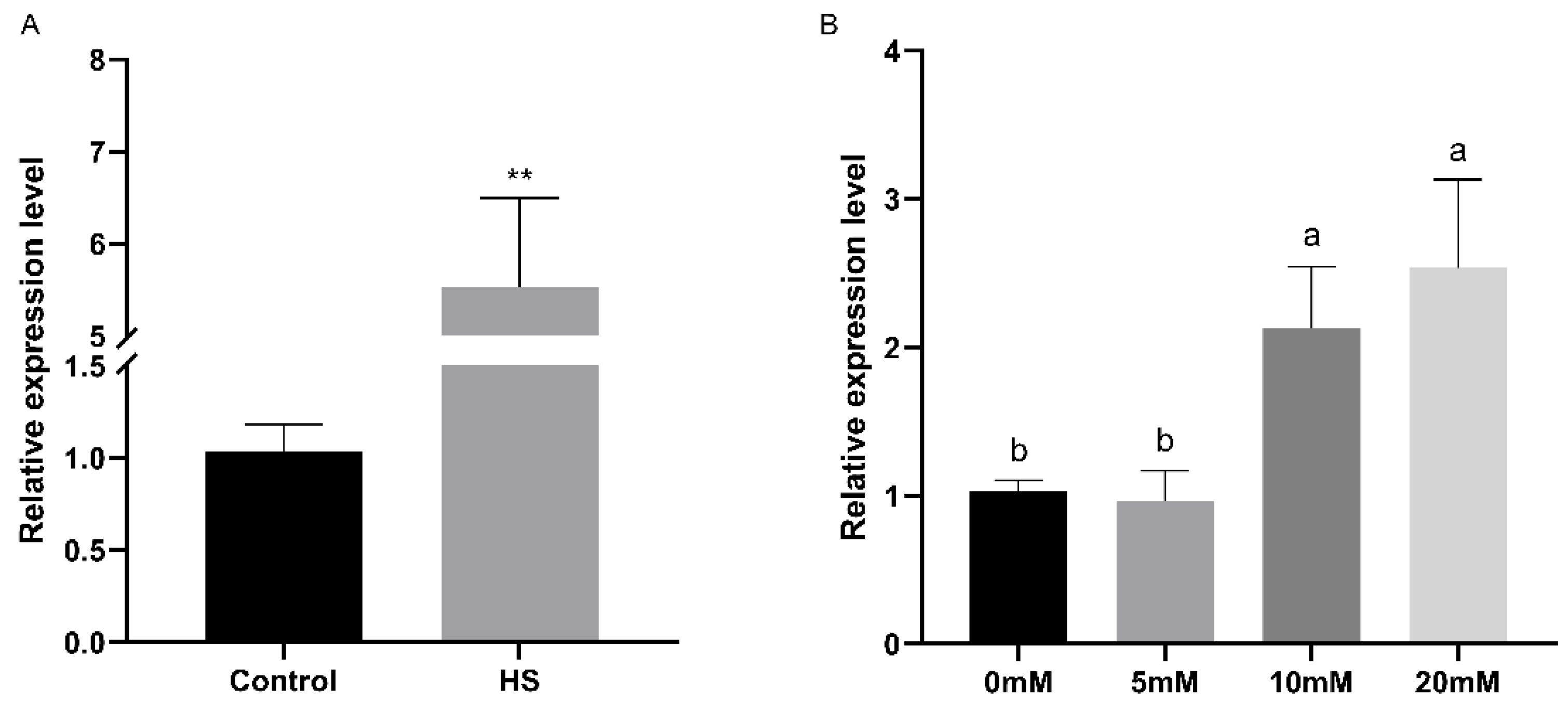
The relative expression levels of the FfPkac gene were detected under heat stress and at different concentrations of H2O2 stress in F. filiformis by RT-qPCR (Figure 5). The relative expression level of FfPkac was up-regulated 5.3-fold under heat stress (Figure 5A). Under the treatment of H2O2, the relative expression levels of the FfPkac gene showed a gradual upward trend with the increase in H2O2 concentration. Compared with that in the control (0 mM H2O2), the relative expression levels of the FfPkac gene were up-regulated 2.1-fold and 2.5-fold at 10 and 20 mM H2O2, respectively (Figure 5B). The relative expression levels of the FfPkac gene were significantly up-regulated under abiotic stress of heat and oxidative stresses, suggesting that the FfPkac gene played a role in coping with heat and oxidative stresses.
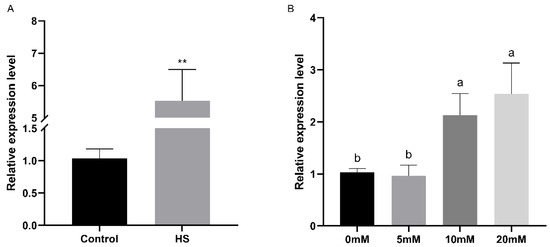
Figure 5.
Relative expression levels of the FfPkac gene in F. filiformis under heat and oxidative stresses. (A) Relative expression levels of the FfPkac gene of F. filiformis in strain 1123 under heat stress. The values are the means ± SD of three independent experiments. Asterisks indicate significant differences compared to control (Student’s t test: ** p < 0.01). (B) Relative expression levels of the FfPkac gene of F. filiformis strain 1123 under different concentrations of H2O2. The values are the means ± SD of three independent experiments (Tukey’s multiple comparisons test: lowercase letters represent p < 0.05).
3.5. Identification and Validation of Downstream Regulatory Genes of Pkac
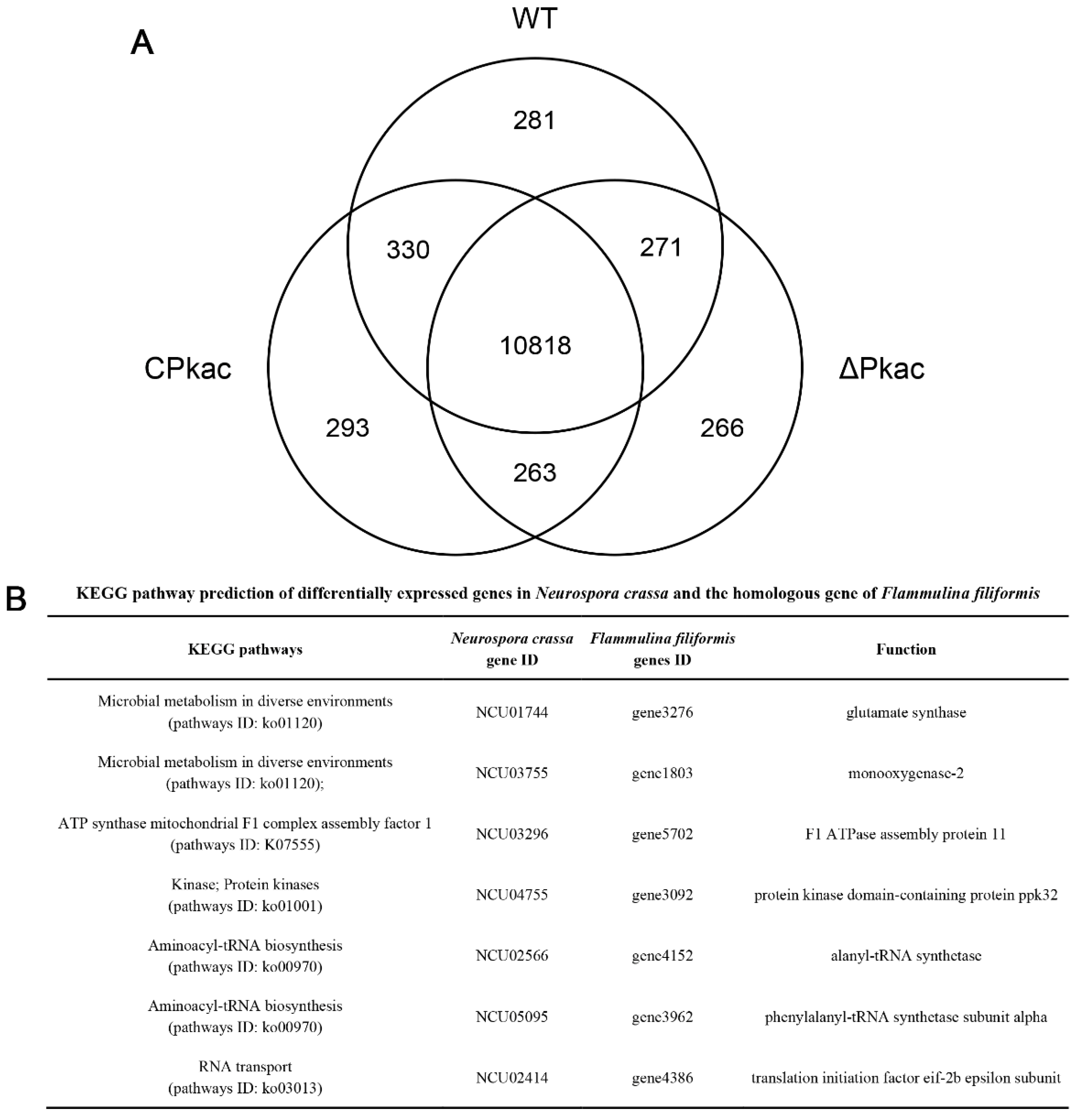
In order to investigate the downstream acting genes of the Pkac gene, RNA-Seq was performed for three strains WT, ΔPkac and CPkac. There were 11,700, 11,618 and 11,704 expressed genes detected in WT, ΔPkac and CPkac, respectively. Among them, 10,818 genes were expressed in any of the three strains, accounting for 82.7% of the total genes. There were 281 (2.1%), 266 (2.0%) and 293 (2.2%) genes specifically expressed in WT, ΔPkac and CPkac strains, respectively, suggesting that these genes could be related to Pkac gene deletion and complementation (Figure 6A).
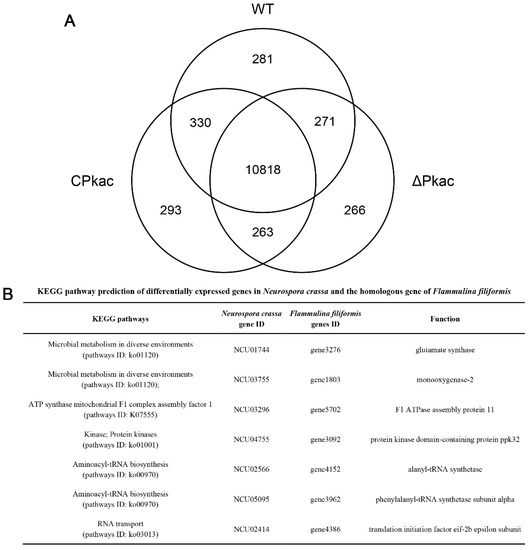
Figure 6.
Analysis of differentially expressed genes in the transcriptome of N. crassa. (A) Wayne diagram of differentially expressed genes of N. crassa. (B) KEGG pathway prediction of differentially expressed genes in N. crassa and the homologous gene of F. filiformis.
According to the FPKM values from RNA-Seq, three groups of genes, including (A) the differentially expressed genes between ΔPkac and WT, (B) the differentially expressed genes between ΔPkac and CPkac, and (C) the non-differentially expressed genes between WT and CPkac were screened, respectively. We took the intersection of A, B and C and obtained 38 genes in total after excluding the low expression genes (the average FPKM value < 20). These 38 genes underwent function annotation using the KEGG pathway and Pfam conserved domain analysis. Finally, there were seven genes with the defined functions: glutamate synthase (N. crassa: NCU01744/F. filiformis: gene3276); monooxygenase-2 (N. crassa: NCU03755/F. filiformis: gene1803); F1 ATPase assembly protein 11 (N. crassa: NCU03296/ F. filiformis: gene5702); protein kinase domain-containing protein ppk32 (N. crassa: NCU04755/F. filiformis: gene3092); alanyl-tRNA synthetase (N. crassa: NCU02566/F. filiformis: gene4152); phenylalanyl-tRNA synthetase subunit alpha (N. crassa: NCU05095/F. filiformis: gene3962); translation initiation factor eif-2b epsilon subunit (N. crassa: NCU02414/F. filiformis: gene4386). These seven genes belong to five KEGG pathways: Microbial metabolism in diverse environments (pathways ID: ko01120); ATP synthase mitochondrial F1 complex assembly factor 1 (pathways ID: K07555); Kinase; Protein kinases (pathways ID: ko01001); Aminoacyl-tRNA biosynthesis (pathways ID: ko00970); and RNA transport (pathways ID: ko03013) (Figure 6B). The functions and metabolic pathways of these seven genes were closely related to mycelial growth and response to stress, suggesting that Pkac may perform its functions by on acting these downstream genes.
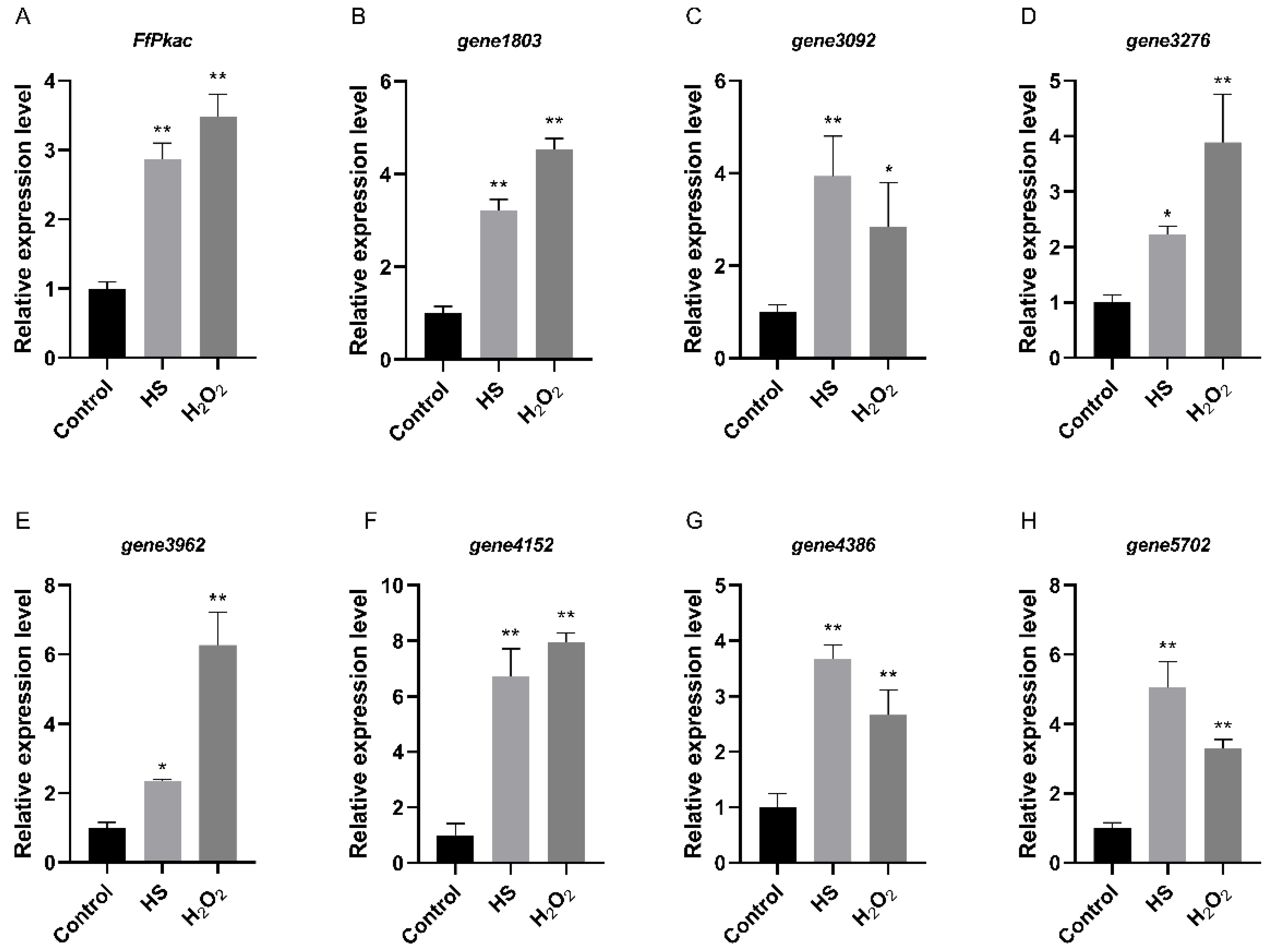
In order to verify the consistent expression patterns between Pkac and its downstream genes identified above, the expression levels of these seven genes, as well as FfPkac, were further detected by RT-qPCR under heat and oxidative stresses in F. filiformis (Figure 7). The results showed that the relative expression levels of the FfPkac gene were significantly up-regulated 2.9-fold and 3.5-fold under heat and oxidative stresses, respectively. These seven genes were up-regulated with different degrees under heat and oxidative stresses, respectively, and shared consistent expression patterns with FfPkac.
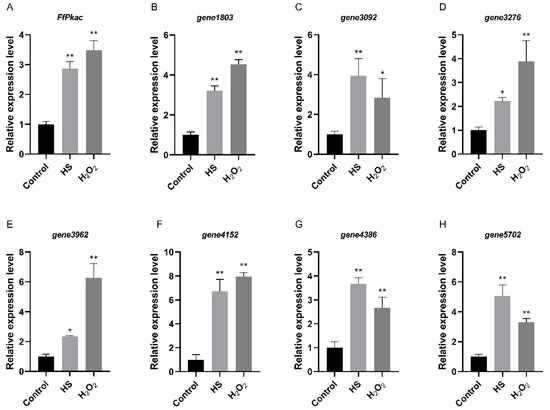
Figure 7.
Relative expression levels between FfPkac and its downstream genes in F. filiformis under heat and oxidative stresses. (A) The relative expression levels of FfPkac in F. filiformis mycelia under heat and oxidative stresses. (Dunnett T3′s multiple comparisons test: ** p < 0.01). (B–H) The relative expression levels of seven downstream genes of FfPkac in F. filiformis mycelia under heat and oxidative stresses. (Dunnett T3′s multiple comparisons test: * p < 0.05, ** p < 0.01). In (A–H), the values are the means ± SD of three independent experiments. Asterisks indicate significant differences compared to control.
4. Discussion
The filamentous fungi are composed of many morphologically and developmentally diverse species, which can be divided into basidiomycetes and ascomycetes. For functional studies of many important conserved genes, it is necessary to compare and validate them in both basidiomycetes and ascomycetes. However, the ease of genetic manipulation and the efficiency of genetic transformation vary greatly among fungi, making it impossible to perform arbitrary gene function tests in most fungi, especially in basidiomycetes. F. filiformis and N. crassa can be used as a model species of basidiomycetes and ascomycetes, respectively, due to their rich phenotypes and profound research basis. In N. crassa, the genetics of operation is easy and efficient. More than 70% of the genes in the N. crassa genome have corresponding gene knockout mutants in its mutant library, while in most basidiomycetes, including F. filiformis, the genetic transformation efficiency is low, and to date, gene knockout operation is still a challenge. Therefore, it is a rapid approach to revealing the molecular function of many conserved genes in basidiomycetes by using the existing mutant library of the ascomycete N. crassa model. In this study, the gene FfPkac was successfully complemented into N. crassa ΔPkac mutant by site-specific repair mutagenesis technique and the mycelial growth phenotypic of ΔPkac strain in N. crassa was also restored to the same level as WT after the complementation of FfPkac. This proves the feasibility of the strategy of studying gene function by using mutants of the model fungus combined with gene complementation among different species.
The cAMP/PKA signal transduction pathway is one of the conservative signaling pathways in fungi. In this study, FfPkac (on behalf of the cAMP/PKA signaling pathway) was found to be involved in response to the heat and oxidative stresses in F. filiformis. In N. crassa, the transcription level of the NcPkac gene was found to up-regulate significantly after the addition of DTT (Dithiothreitol: induce Endoplasmic Reticulum stress) in WT and down-regulate after the addition of DTT in the mutant Δrrg-2 (rrg-2 plays a role in the oxidative stress response based on the data from GSE61949 [27,28]). In addition, the cAMP/PKA signaling pathway is also found to be involved in response to heat shock and salt stress in Saccharomyces cerevisiae [29]. It suggests that the cAMP/PKA signaling pathway plays an essential role in response to a variety of environmental stresses universally. In edible fungi, the mycelial growth, the formation and development of fruiting bodies are affected and regulated by the external environment. Of course, the cAMP/PKA signaling pathway also plays a role in the growth and development of fungi. In this study, both the absence of NcPkac and the complementation of FfPkac significantly affected the mycelial growth. Based on the data GSE52153 of N. crassa [30], the transcription level of NcPkac in the Δczt-1 (CZT-1: cell death activated zinc cluster transcription factor) mutant strain (Δczt-1 was higher susceptible to the staurosporine compared with the WT) was significantly up-regulated compared to that in the WT. The roles of Pkac in the growth and development of fungi were also verified and validated in several species. In Aspergillus niger, the overexpression of pkaC could modify hyphal, colony and conidiophore growth [31]. In F. verticillioides, the fpkl gene (encoding a homolog of the PKA catalytic subunit) mutant showed reduced vegetative growth, fewer and shorter aerial mycelia, and the defects in radial growth and macroconidiation [12,14]. In Botrytis cinerea, growth and virulence were severely affected by deletion bcpka1 [32]. In Sporisorium scitamineum, PKA is also involved in the proper mating and filamentation as well as virulence [10]. In addition, the sustained high expression with varying levels of FfPkac in different developmental stages of F. filiformis indicated that the cAMP/PKA signaling pathway is also essential for the fruiting body development.
The roles of PKA or the cAMP/PKA signaling pathway have been described in detail; however, there are few studies on the identification of downstream genes in this pathway. In this study, the possible downstream genes regulated by the Pkac gene were identified by comparing ΔPkac and CPkac with WT, respectively. The downstream genes of Pkac were mainly focused on microbial metabolism in diverse environments, mitochondrial biogenesis, protein translation, nucleocytoplasmic transport, etc. RT-qPCR confirmed that the expression patterns of these downstream genes were consistent with FfPkac in response to heat and oxidative stresses. In addition, ROS (reactive oxygen species) catabolic enzymes are also found to be under the regulation of the cAMP/PKA signaling pathway in S. scitamineum [10]. In the future, increased identification of PKAC downstream regulatory genes under different conditions will provide more powerful clues to fully reveal the regulatory mechanism of PKAC as well as the cAMP/PKA signaling pathway in fungi.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/life12091336/s1, Table S1: The primers used in this study.
Author Contributions
Conceptualization, Y.T., B.X. (Baogui Xie), and S.L.; methodology, Y.Y., B.X. (Bin Xie), Z.J., and Y.L.; software, Y.C. and J.Y.; resources, Y.L., S.L., and F.L.; data curation, Y.Y., Z.J., and B.X. (Bin Xie); writing—original draft preparation, F.L., Y.C., and J.Y.; writing—review and editing, Y.T., B.X. (Baogui Xie) and Y.Y.; visualization, Y.Y. and Y.T.; supervision, Y.T. and B.X. (Baogui Xie). All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (31902088), the Natural Science Foundation of Fujian Province of China (2019J01380), the Natural Science Foundation for Distinguished Young Scholar of Fujian Agriculture and Forestry University of China (xjq201919), the National College students Innovation and Entrepreneurship Plan Training Project (202210389031 Zhuohan Jing), and the Seed Industry Innovation and Industrialization Project of Fujian Province of China (zycxny2021012).
Institutional Review Board Statement
This study did not involve humans or animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
All experimental data in this study will be made available upon reasonable request from readers.
Acknowledgments
Thanks to Shaojie Li from the Institute of Microbiology, Chinese Academy of Sciences, for providing experimental strains and plasmid support for this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fuller, K.K.; Rhodes, J.C. Protein kinase A and fungal virulence: A sinister side to a conserved nutrient sensing pathway. Virulence 2012, 3, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Li, Y.; Yue, X.; Wang, C.; Que, Y.; Kong, D.; Ma, Z.; Talbot, N.J.; Wang, Z. Two novel transcriptional regulators are essential for infection-related morphogenesis and pathogenicity of the rice blast fungus Magnaporthe oryzae. PLoS Pathog. 2011, 7, e1002385. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, C.A.; Heitman, J. Conserved cAMP signaling cascades regulate fungal development and virulence. FEMS MicroBiol. Rev. 2001, 25, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Hao, X.; Chen, L.; Akhberdi, O.; Yu, X.; Liu, Y.; Zhu, X. Gα-cAMP/PKA pathway positively regulates pigmentation, chaetoglobosin A biosynthesis and sexual development in Chaetomium globosum. PLoS ONE 2018, 13, e0195553. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wright, S.J.; Krystofova, S.; Park, G.; Borkovich, K.A. Heterotrimeric G protein signaling in filamentous fungi. Ann. Rev. Microbiol. 2007, 61, 423–452. [Google Scholar] [CrossRef]
- Hicks, J.K.; D’Souza, C.A.; Cox, G.M.; Heitman, J. Cyclic AMP-dependent protein kinase catalytic subunits have divergent roles in virulence factor production in two varieties of the fungal pathogen Cryptococcus neoformans. Eukaryot. Cell 2004, 3, 14–26. [Google Scholar] [CrossRef]
- Oliver, B.G.; Panepinto, J.C.; Fortwendel, J.R.; Smith, D.L.; Askew, D.S.; Rhodes, J.C. Cloning and expression of pkaC and pkaR, the genes encoding the cAMP-dependent protein kinase of Aspergillus fumigatus. Mycopathologia 2002, 154, 85–91. [Google Scholar] [CrossRef]
- Feliciello, A.; Gottesman, M.E.; Avvedimento, E.V. cAMP-PKA signaling to the mitochondria: Protein scaffolds, mRNA and phosphatases. Cell. Signal. 2005, 17, 279–287. [Google Scholar] [CrossRef]
- Li, L.; Borkovich, K.A. GPR-4 is a predicted G-protein-coupled receptor required for carbon source-dependent asexual growth and development in Neurospora crassa. Eukaryot. Cell 2006, 5, 1287–1300. [Google Scholar] [CrossRef]
- Chang, C.; Cai, E.; Deng, Y.Z.; Mei, D.; Qiu, S.; Chen, B.; Zhang, L.H.; Jiang, Z. cAMP/PKA signalling pathway regulates redox homeostasis essential for Sporisorium scitamineum mating/filamentation and virulence. Environ. MicroBiol. 2019, 21, 959–971. [Google Scholar] [CrossRef]
- Toyokawa, C.; Shobu, M.; Tsukamoto, R.; Okamura, S.; Honda, Y.; Kamitsuji, H.; Izumitsu, K.; Suzuki, K.; Irie, T. Effects of overexpression of PKAc genes on expressions of lignin-modifying enzymes by Pleurotus ostreatus. Biosci. Biotechnol. Biochem. 2016, 80, 1759–1767. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.E.; Xu, J.R. The cAMP signaling pathway in Fusarium verticillioides is important for conidiation, plant infection, and stress responses but not fumonisin production. Mol. Plant Microbe Interact 2010, 23, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Gold, S.; Duncan, G.; Barrett, K.; Kronstad, J. cAMP regulates morphogenesis in the fungal pathogen Ustilago maydis. Genes Dev. 1994, 8, 2805–2816. [Google Scholar] [CrossRef] [PubMed]
- Pei-Bao, Z.; Ren, A.Z.; Xu, H.J.; Li, D.C. The Gene fpk1, Encoding a cAMP-dependent Protein Kinase Catalytic Subunit Homolog, is Required for Hyphal Growth, Spore Germination, and Plant Infection in Fusarium verticillioides. J. Microbiol. Biotechnol. 2010, 20, 208–216. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, C.A.; Alspaugh, J.A.; Yue, C.; Harashima, T.; Cox, G.M.; Perfect, J.R.; Heitman, J. Cyclic AMP-dependent protein kinase controls virulence of the fungal pathogen Cryptococcus neoformans. Mol. Cell Biol. 2001, 21, 3179–3191. [Google Scholar] [CrossRef]
- Sakamoto, Y. Influences of environmental factors on fruiting body induction, development and maturation in mushroom-forming fungi. Fungal Biol. Rev. 2018, 32, 236–248. [Google Scholar] [CrossRef]
- Vogel, H.J. A convenient growth medium for Neurospora crassa. Microb. Genet. Bull. 1956, 13, 42–47. [Google Scholar]
- Tao, Y.; Chen, R.; Yan, J.; Long, Y.; Tong, Z.; Song, H.; Xie, B. A hydrophobin gene, Hyd9, plays an important role in the formation of aerial hyphae and primordia in Flammulina filiformis. Gene 2019, 706, 84–90. [Google Scholar] [CrossRef]
- Ebbole, D.; Sachs, M. A rapid and simple method for isolation of Neurospora crassa homokaryons using microconidia. Fungal Genet. Newsl. 1990, 37, 17–18. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Porebski, S.; Bailey, L.G.; Baum, B.R. Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol. Biol. Rep. 1997, 15, 8–15. [Google Scholar] [CrossRef]
- Tao, Y.; van Peer, A.F.; Huang, Q.; Shao, Y.; Zhang, L.; Xie, B.; Jiang, Y.; Zhu, J.; Xie, B. Identification of novel and robust internal control genes from Volvariella volvacea that are suitable for RT-qPCR in filamentous fungi. Sci. Rep. 2016, 6, 29236. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Margolin, B.S.; Freitag, M.; Selker, E.U. Improved plasmids for gene targeting at the his-3 locus of Neurospora crassa by electroporation. Fungal Genet. Rep. 1997, 44, 34–36. [Google Scholar] [CrossRef]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Thomas, J.G.; Olson, J.M.; Tapscott, S.J.; Zhao, L.P. An efficient and robust statistical modeling approach to discover differentially expressed genes using genomic expression profiles. Genome Res. 2001, 11, 1227–1236. [Google Scholar] [CrossRef]
- Fan, F.; Ma, G.; Li, J.; Liu, Q.; Benz, J.P.; Tian, C.; Ma, Y. Genome-wide analysis of the endoplasmic reticulum stress response during lignocellulase production in Neurospora crassa. Biotechnol. Biofuels 2015, 8, 66. [Google Scholar] [CrossRef]
- Banno, S.; Noguchi, R.; Yamashita, K.; Fukumori, F.; Kimura, M.; Yamaguchi, I.; Fujimura, M. Roles of putative His-to-Asp signaling modules HPT-1 and RRG-2, on viability and sensitivity to osmotic and oxidative stresses in Neurospora crassa. Curr. Genet. 2007, 51, 197–208. [Google Scholar] [CrossRef]
- Portela, P.; Rossi, S. cAMP-PKA signal transduction specificity in Saccharomyces cerevisiae. Curr. Genet. 2020, 66, 1093–1099. [Google Scholar] [CrossRef]
- Goncalves, A.P.; Hall, C.; Kowbel, D.J.; Glass, N.L.; Videira, A. CZT-1 is a novel transcription factor controlling cell death and natural drug resistance in Neurospora crassa. G3 2014, 4, 1091–1102. [Google Scholar] [CrossRef]
- Zheng, X.; Cairns, T.C.; Ni, X.; Zhang, L.; Zhai, H.; Meyer, V.; Zheng, P.; Sun, J. Comprehensively dissecting the hub regulation of PkaC on high-productivity and pellet macromorphology in citric acid producing Aspergillus niger. Microb. Biotechnol. 2022, 15, 1867–1882. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, J.; Kokkelink, L.; Huesmann, C.; Jimenez-Teja, D.; Collado, I.G.; Barakat, R.; Tudzynski, P.; Tudzynski, B. The cAMP-dependent signaling pathway and its role in conidial germination, growth, and virulence of the gray mold Botrytis cinerea. Mol. Plant Microbe Interact 2008, 21, 1443–1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).