Valorization of Brewery Waste through Polyhydroxyalkanoates Production Supported by a Metabolic Specialized Microbiome
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Feedstock Characterization
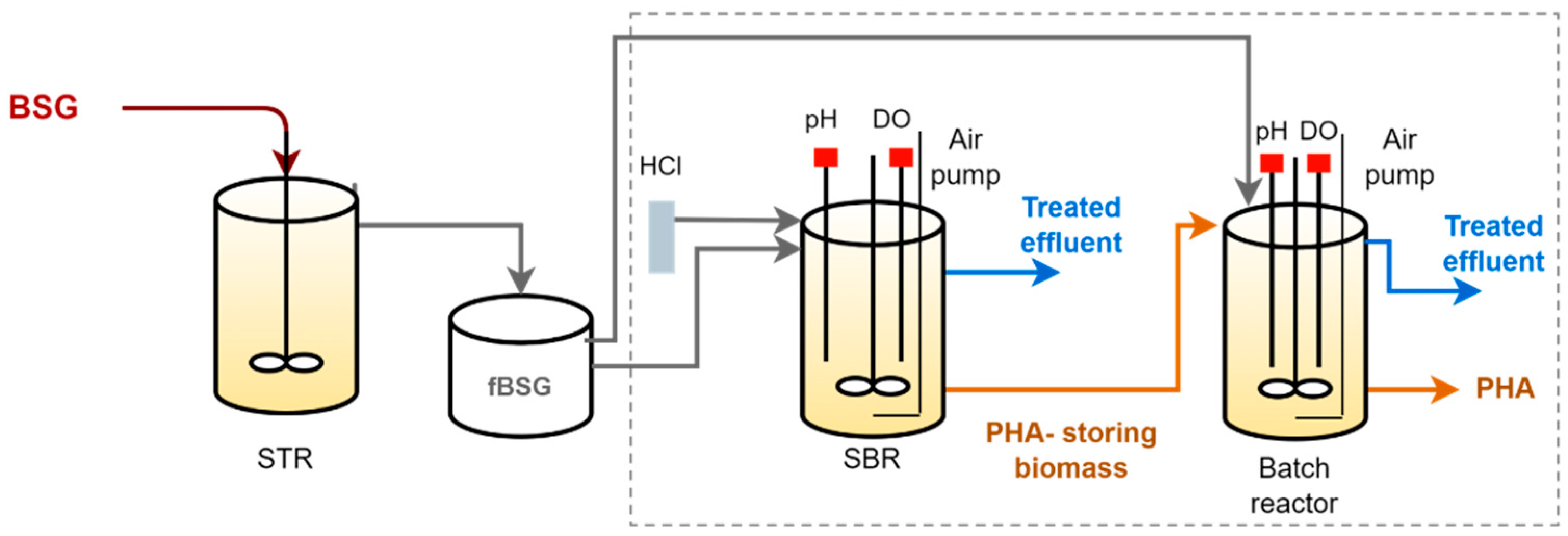
2.2. Experimental Setup
2.2.1. MMC Enrichment in PHA-Storing Organisms
2.2.2. PHA Accumulation
2.3. Analytical Methods
2.4. Calculations
3. Results and Discussion
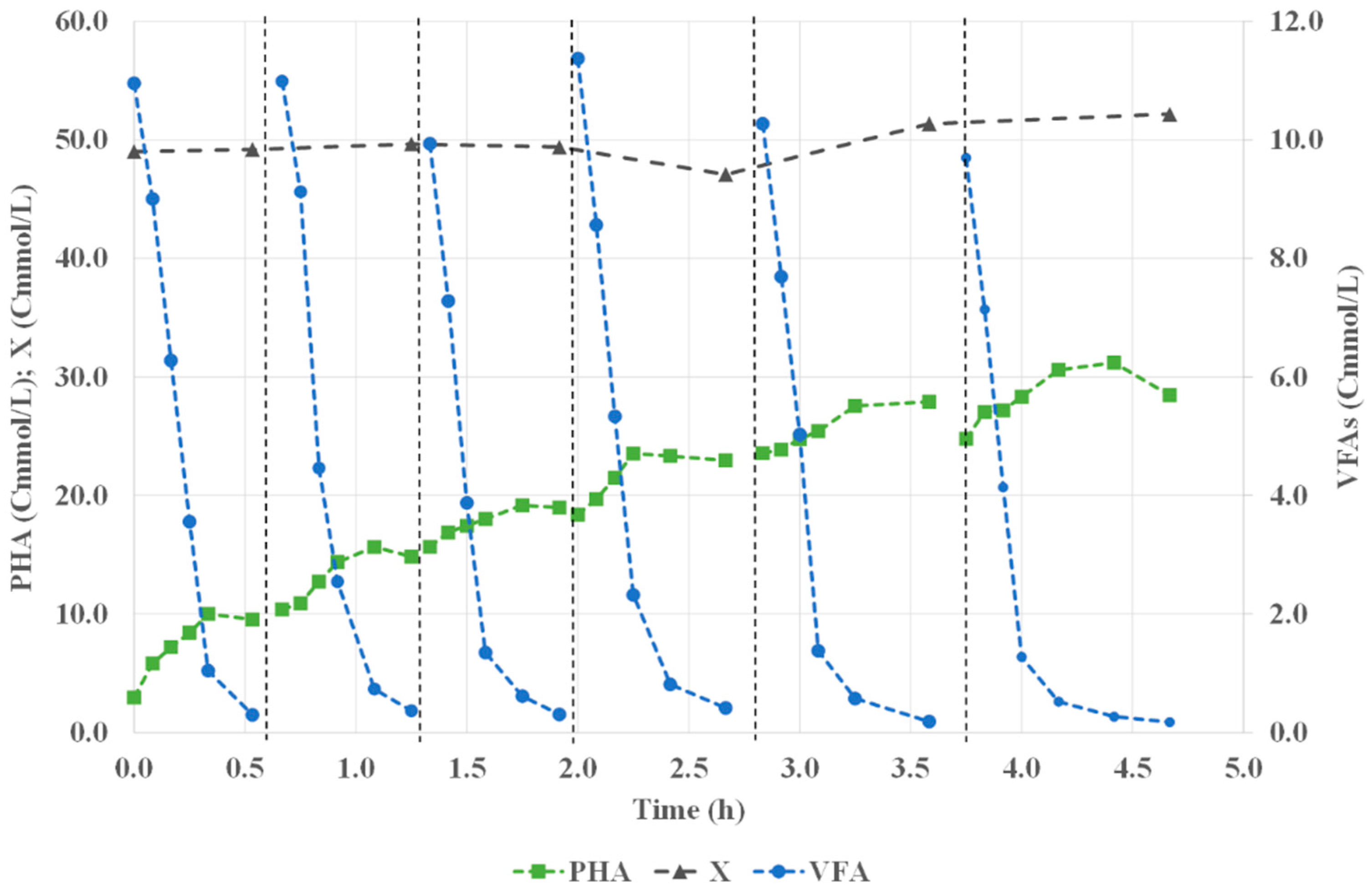
3.1. Culture Enrichment on PHA-Storing Organisms
3.2. Assessment of PHA Production
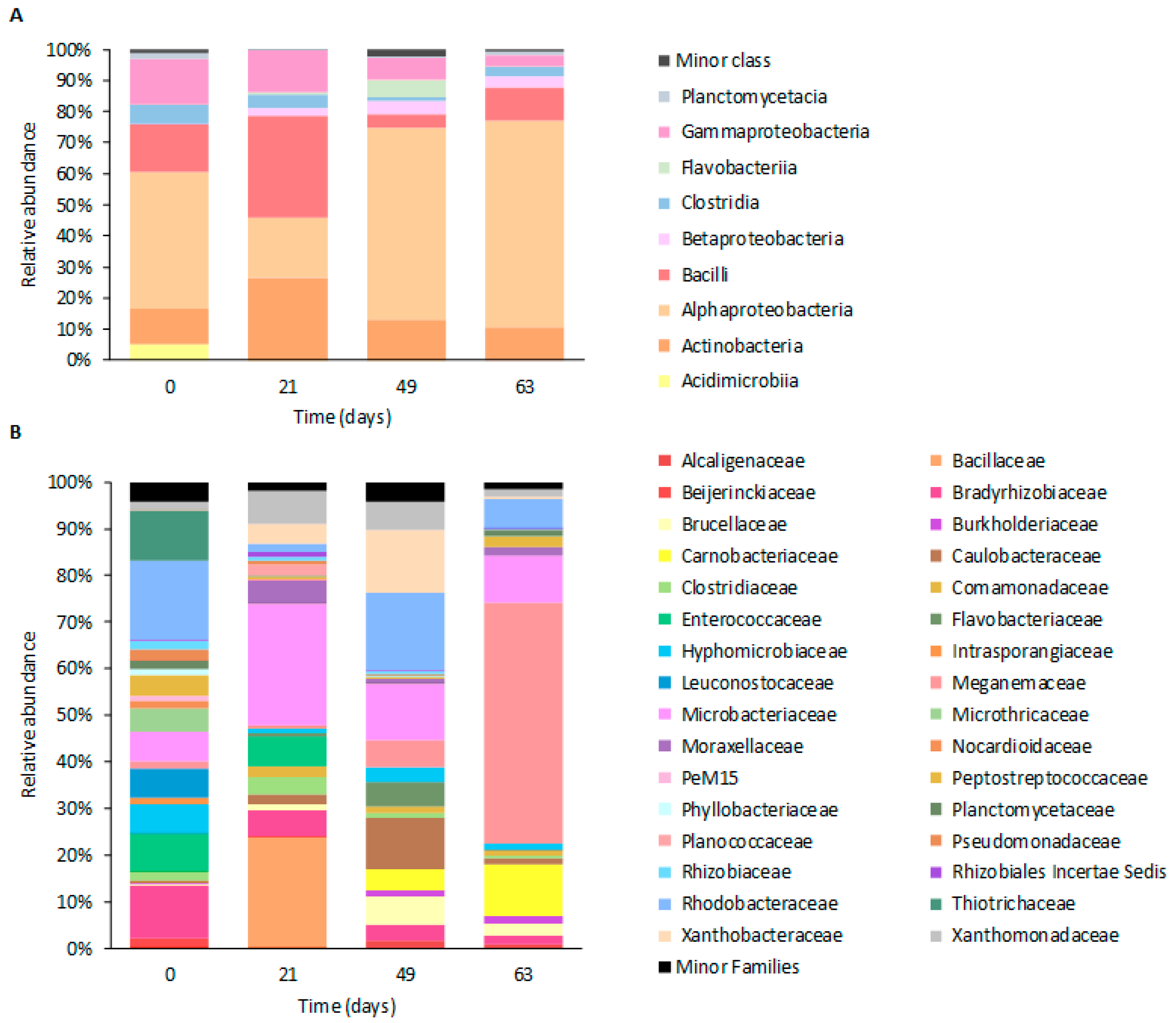
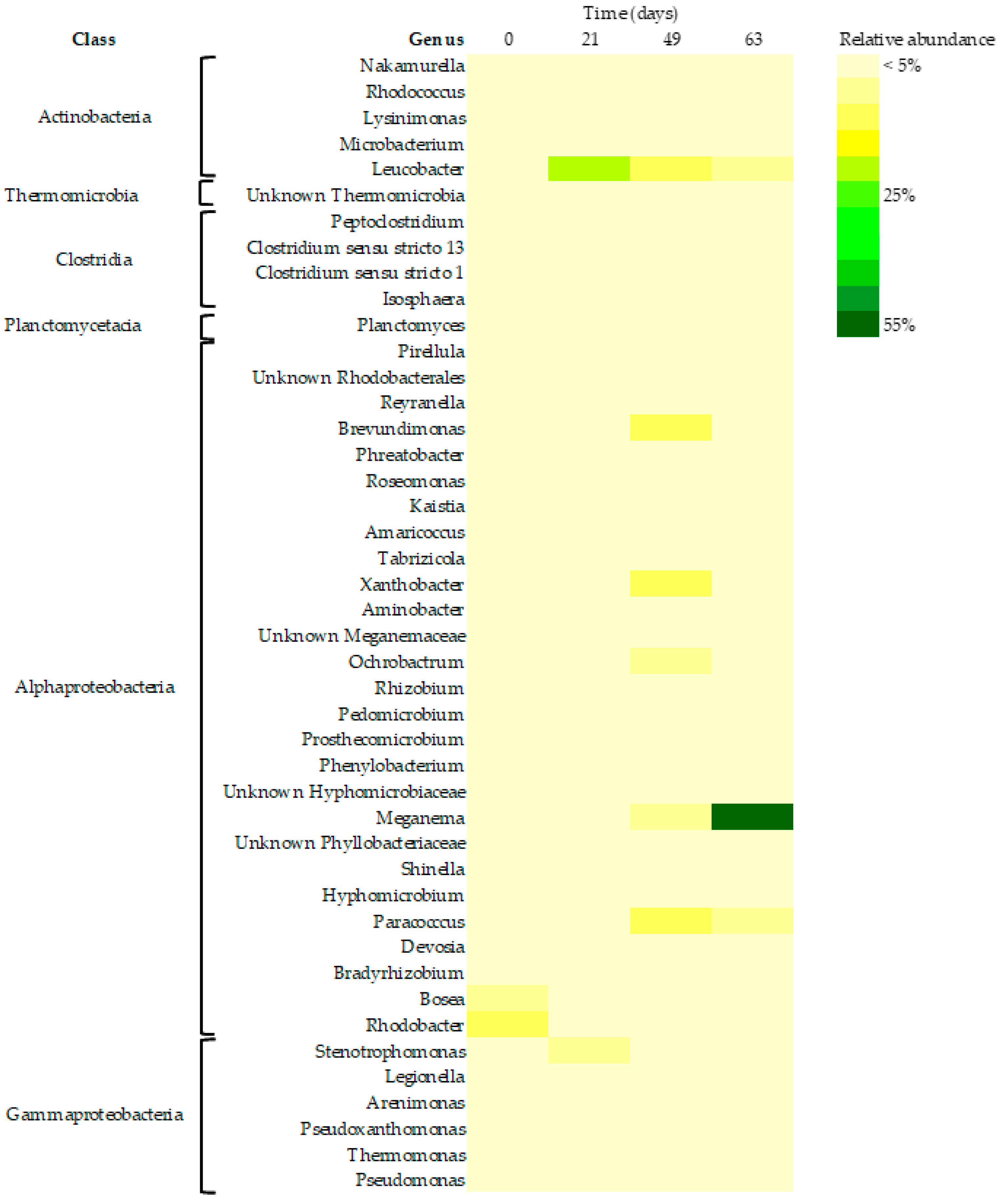
3.3. Microbiome Composition Dynamics
3.3.1. Richness and Diversity
3.3.2. Taxonomic Composition
3.3.3. Core Bacteriome
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A review of the production and applications of waste-derived volatile fatty acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Vandamme, E.J. Agro-Industrial Residue Utilization for Industrial Biotechnology Products. In Biotechnology for Agro-Industrial Residues Utilisation; Singh nee’ Nigam, P., Pandey, A., Eds.; Springer: Dordrecht, The Netherland, 2009; pp. 3–11. ISBN 978-1-4020-9942-7. [Google Scholar]
- Costa, C.F.F.A.; Amorim, C.L.; Duque, A.F.; Reis, M.A.M.; Castro, P.M.L. Valorization of wastewater from food industry: Moving to a circular bioeconomy. Rev. Environ. Sci. Bio/Technol. 2022, 21, 269–295. [Google Scholar] [CrossRef]
- Duque, A.F.; Campo, R.; Val del Rio, A.; Amorim, C.L. Wastewater Valorization: Practice around the World at Pilot- and Full-Scale. Int. J. Environ. Res. Public Health 2021, 18, 9466. [Google Scholar] [CrossRef] [PubMed]
- Reis, M.A.M.; Albuquerque, M.; Villano, M.; Majone, M. Mixed Culture Processes for Polyhydroxyalkanoate Production from Agro-Industrial Surplus/Wastes as Feedstocks. In Comprehensive Biotechnology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 669–684. ISBN 9780080885049. [Google Scholar]
- Raza, Z.A.; Abid, S.; Banat, I.M. Polyhydroxyalkanoates: Characteristics, production, recent developments and applications. Int. Biodeterior. Biodegrad. 2018, 126, 45–56. [Google Scholar] [CrossRef]
- De Donno Novelli, L.; Moreno Sayavedra, S.; Rene, E.R. Polyhydroxyalkanoate (PHA) production via resource recovery from industrial waste streams: A review of techniques and perspectives. Bioresour. Technol. 2021, 331, 124985. [Google Scholar] [CrossRef]
- Oliveira, C.S.S.; Silva, C.E.; Carvalho, G.; Reis, M.A. Strategies for efficiently selecting PHA producing mixed microbial cultures using complex feedstocks: Feast and famine regime and uncoupled carbon and nitrogen availabilities. N. Biotechnol. 2017, 37, 69–79. [Google Scholar] [CrossRef]
- Duque, A.F.; Oliveira, C.S.S.; Carmo, I.T.D.; Gouveia, A.R.; Pardelha, F.; Ramos, A.M.; Reis, M.A.M. Response of a three-stage process for PHA production by mixed microbial cultures to feedstock shift: Impact on polymer composition. N. Biotechnol. 2014, 31, 276–288. [Google Scholar] [CrossRef]
- Salehizadeh, H.; Van Loosdrecht, M.C.M. Production of polyhydroxyalkanoates by mixed culture: Recent trends and biotechnological importance. Biotechnol. Adv. 2004, 22, 261–279. [Google Scholar] [CrossRef]
- Kourmentza, C.; Plácido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A.M. Recent advances and challenges towards sustainable polyhydroxyalkanoate (PHA) production. Bioengineering 2017, 4, 55. [Google Scholar] [CrossRef]
- Teixeira, M.R.; Guarda, E.C.; Freitas, E.B.; Galinha, C.F.; Duque, A.F.; Reis, M.A.M. Valorization of raw brewers’ spent grain through the production of volatile fatty acids. N. Biotechnol. 2020, 57, 4–10. [Google Scholar] [CrossRef]
- Guarda, E.C.; Oliveira, A.C.; Antunes, S.; Freitas, F.; Castro, P.M.L.; Duque, A.F.; Reis, M.A.M. A Two-Stage Process for Conversion of Brewer’s Spent Grain into Volatile Fatty Acids through Acidogenic Fermentation. Appl. Sci. 2021, 11, 3222. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ spent grain: Generation, characteristics and potential applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Mussatto, S.I. Brewer’s spent grain: A valuable feedstock for industrial applications. J. Sci. Food Agric. 2014, 94, 1264–1275. [Google Scholar] [CrossRef] [PubMed]
- Chetrariu, A.; Dabija, A. Brewer’s Spent Grains: Possibilities of Valorization, a Review. Appl. Sci. 2020, 10, 5619. [Google Scholar] [CrossRef]
- Corchado-Lopo, C.; Martínez-Avila, O.; Marti, E.; Llimós, J.; Busquets, A.M.; Kucera, D.; Obruca, S.; Llenas, L.; Ponsá, S. Brewer’s spent grain as a no-cost substrate for polyhydroxyalkanoates production: Assessment of pretreatment strategies and different bacterial strains. N. Biotechnol. 2021, 62, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Llimós, J.; Martínez-Avila, O.; Marti, E.; Corchado-Lopo, C.; Llenas, L.; Gea, T.; Ponsá, S. Brewer’s spent grain biotransformation to produce lignocellulolytic enzymes and polyhydroxyalkanoates in a two-stage valorization scheme. Biomass Convers. Biorefinery 2020, 12, 3921–3932. [Google Scholar] [CrossRef]
- Martínez-Avila, O.; Llimós, J.; Ponsá, S. Integrated solid-state enzymatic hydrolysis and solid-state fermentation for producing sustainable polyhydroxyalkanoates from low-cost agro-industrial residues. Food Bioprod. Process. 2021, 126, 334–344. [Google Scholar] [CrossRef]
- Martínez-Avila, O.; Llenas, L.; Ponsá, S. Sustainable polyhydroxyalkanoates production via solid-state fermentation: Influence of the operational parameters and scaling up of the process. Food Bioprod. Process. 2022, 132, 13–22. [Google Scholar] [CrossRef]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater, 21st ed.; APHA—American Public Health Association: Washington, DC, USA; AWWA—American Water Works Association: Washington, DC, USA; WEF—Water Environment Federation: Washington, DC, USA, 2005. [Google Scholar]
- Bengtsson, S.; Werker, A.; Christensson, M.; Welander, T. Production of polyhydroxyalkanoates by activated sludge treating a paper mill wastewater. Bioresour. Technol. 2008, 99, 509–516. [Google Scholar] [CrossRef]
- Matos, M.; Cruz, R.A.P.; Cardoso, P.; Silva, F.; Freitas, E.B.; Carvalho, G.; Reis, M.A.M. Combined Strategies to Boost Polyhydroxyalkanoate Production from Fruit Waste in a Three-Stage Pilot Plant. ACS Sustain. Chem. Eng. 2021, 9, 8270–8279. [Google Scholar] [CrossRef]
- Albuquerque, M.G.E.; Torres, C.A.V.; Reis, M.A.M. Polyhydroxyalkanoate (PHA) production by a mixed microbial culture using sugar molasses: Effect of the influent substrate concentration on culture selection. Water Res. 2010, 44, 3419–3433. [Google Scholar] [CrossRef] [PubMed]
- Dionisi, D.; Majone, M.; Vallini, G.; Di Gregorio, S.; Beccari, M. Effect of the applied organic load rate on biodegradable polymer production by mixed microbial cultures in a sequencing batch reactor. Biotechnol. Bioeng. 2006, 93, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Cruz, R.A.P.; Oehmen, A.; Reis, M.A.M. The impact of biomass withdrawal strategy on the biomass selection and polyhydroxyalkanoates accumulation of mixed microbial cultures. N. Biotechnol. 2022, 66, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Colombo, B.; Sciarria, T.P.; Reis, M.; Scaglia, B.; Adani, F. Polyhydroxyalkanoates (PHAs) production from fermented cheese whey by using a mixed microbial culture. Bioresour. Technol. 2016, 218, 692–699. [Google Scholar] [CrossRef]
- Wang, X.; Oehmen, A.; Freitas, E.B.; Carvalho, G.; Reis, M.A.M. The link of feast-phase dissolved oxygen (DO) with substrate competition and microbial selection in PHA production. Water Res. 2017, 112, 269–278. [Google Scholar] [CrossRef]
- Marang, L.; Jiang, Y.; van Loosdrecht, M.C.M.; Kleerebezem, R. Butyrate as preferred substrate for polyhydroxybutyrate production. Bioresour. Technol. 2013, 142, 232–239. [Google Scholar] [CrossRef]
- Campanari, S.; e Silva, F.A.; Bertin, L.; Villano, M.; Majone, M. Effect of the organic loading rate on the production of polyhydroxyalkanoates in a multi-stage process aimed at the valorization of olive oil mill wastewater. Int. J. Biol. Macromol. 2014, 71, 34–41. [Google Scholar] [CrossRef]
- Ben, M.; Kennes, C.; Veiga, M.C. Optimization of polyhydroxyalkanoate storage using mixed cultures and brewery wastewater. J. Chem. Technol. Biotechnol. 2016, 91, 2817–2826. [Google Scholar] [CrossRef]
- Lorini, L.; di Re, F.; Majone, M.; Valentino, F. High rate selection of PHA accumulating mixed cultures in sequencing batch reactors with uncoupled carbon and nitrogen feeding. N. Biotechnol. 2020, 56, 140–148. [Google Scholar] [CrossRef]
- Albuquerque, M.G.E.; Concas, S.; Bengtsson, S.; Reis, M.A.M. Mixed culture polyhydroxyalkanoates production from sugar molasses: The use of a 2-stage CSTR system for culture selection. Bioresour. Technol. 2010, 101, 7112–7122. [Google Scholar] [CrossRef]
- Xu, R.; Yang, Z.-H.; Zheng, Y.; Liu, J.-B.; Xiong, W.-P.; Zhang, Y.-R.; Lu, Y.; Xue, W.-J.; Fan, C.-Z. Organic loading rate and hydraulic retention time shape distinct ecological networks of anaerobic digestion related microbiome. Bioresour. Technol. 2018, 262, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhao, L.; Wang, Z.; Chen, Z.; Jia, S.; Song, Y. Ecological insight into incompatibility between polymer storage and floc settling in polyhydroxyalkanoate producer selection using complex carbon sources. Bioresour. Technol. 2022, 347, 126378. [Google Scholar] [CrossRef] [PubMed]
- Dionisi, D.; Beccari, M.; Di Gregorio, S.; Majone, M.; Papini, M.P.; Vallini, G. Storage of biodegradable polymers by an enriched microbial community in a sequencing batch reactor operated at high organic load rate. J. Chem. Technol. Biotechnol. 2005, 80, 1306–1318. [Google Scholar] [CrossRef]
- Crognale, S.; Tonanzi, B.; Valentino, F.; Majone, M.; Rossetti, S. Microbiome dynamics and phaC synthase genes selected in a pilot plant producing polyhydroxyalkanoate from the organic fraction of urban waste. Sci. Total Environ. 2019, 689, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Gliniewicz, K.; Settles, M.L.; Coats, E.R.; McDonald, A.G. Influence of organic loading rate and solid retention time on polyhydroxybutyrate production from hybrid poplar hydrolysates using mixed microbial cultures. Bioresour. Technol. 2015, 175, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ma, X.; Li, J.; Sun, B. Insights into enhanced polyhydroxyalkanoate production by the synergistic use of waste wood hydrolysate and volatile fatty acids by mixed microbial cultures. Bioresour. Technol. 2021, 337, 125488. [Google Scholar] [CrossRef]
- Albuquerque, M.G.E.; Carvalho, G.; Kragelund, C.; Silva, A.F.; Barreto Crespo, M.T.; Reis, M.A.M.; Nielsen, P.H. Link between microbial composition and carbon substrate-uptake preferences in a PHA-storing community. ISME J. 2013, 7, 1–12. [Google Scholar] [CrossRef]
- Carvalho, G.; Oehmen, A.; Albuquerque, M.G.E.; Reis, M.A.M. The relationship between mixed microbial culture composition and PHA production performance from fermented molasses. N. Biotechnol. 2014, 31, 257–263. [Google Scholar] [CrossRef]
- Argiz, L.; Fra-Vázquez, A.; del Río, Á.V.; Mosquera-Corral, A. Optimization of an enriched mixed culture to increase PHA accumulation using industrial saline complex wastewater as a substrate. Chemosphere 2020, 247, 125873. [Google Scholar] [CrossRef]
- Radhakrishnan, M.; Vijayalakshmi, G.; Balagurunathan, R. Optimization of polyhydroxyalkanoates (PHA) production from marine Carnobacterium sp E3. J. Chem. Pharm. Res. 2014, 6, 390–393. [Google Scholar]
- Sali, S.; Mackey, H.R. The application of purple non-sulfur bacteria for microbial mixed culture polyhydroxyalkanoates production. Rev. Environ. Sci. Bio/Technol. 2021, 20, 959–983. [Google Scholar] [CrossRef]
- Mizuno, S.; Enda, Y.; Saika, A.; Hiroe, A.; Tsuge, T. Biosynthesis of polyhydroxyalkanoates containing 2-hydroxy-4-methylvalerate and 2-hydroxy-3-phenylpropionate units from a related or unrelated carbon source. J. Biosci. Bioeng. 2018, 125, 295–300. [Google Scholar] [CrossRef] [PubMed]



| Operational Days | 0–21 | 22–49 | 50–63 |
|---|---|---|---|
| OLR | |||
| (Cmmol/(L·d)) | 19.2 ± 3.1 | 36.8 ± 2.8 | 48.6 ± 7.0 |
| (gCOD/(L·d)) | 0.7 ± 0.1 | 1.3 ± 0.1 | 1.8 ± 0.3 |
| Biomass productivity (gX/(L·d)) | 0.20 ± 0.03 | 0.35 ± 0.06 | 0.56 ± 0.02 |
| -qS (CmmolVFA/(CmmolX·h)) | 0.43 ± 0.11 | 0.42 ± 0.12 | 0.43 ± 0.05 |
| (gCODVFA/(gCODX·h)) | 0.51 ± 0.12 | 0.49 ± 0.13 | 0.51 ± 0.07 |
| qPHA (CmmolPHA/(CmmolX·h)) | 0.33 ± 0.07 | 0.33 ± 0.10 | 0.34 ± 0.04 |
| (gCODPHA/(gCODX·h)) | 0.40 ± 0.08 | 0.39 ± 0.11 | 0.39 ± 0.04 |
| PHAmax_feast (wt.% VSS basis) | 21.6 ± 1.9 | 22.4 ± 5.0 | 24.2 ± 3.8 |
| YPHA/VFA (CmmolPHA/CmmolVFA) | 0.81 ± 0.01 | 0.77 ± 0.03 | 0.79 ± 0.04 |
| (gCODPHA/gCODVFA) | 0.78 ± 0.06 | 0.78 ± 0.04 | 0.76 ± 0.04 |
| YX/VFA (CmmolX/CmmolVFA) | 0.08 ± 0.01 | 0.05 ± 0.01 | 0.04 ± 0.01 |
| (gCODX/gCODVFA) | 0.07 ± 0.01 | 0.05 ± 0.01 | 0.03 ± 0.01 |
| YX/PHA (CmmolX/CmmolPHA) | 0.31 ± 0.06 | 0.27 ± 0.05 | 0.24 ± 0.05 |
| (gCODX/gCODPHA) | 0.25 ± 0.05 | 0.23 ± 0.04 | 0.21 ± 0.06 |
| HB:HV (wt.%) | 55:45 | 57:43 | 46:54 |
| Index | Day-0 | Day-21 | Day-49 | Day-63 |
|---|---|---|---|---|
| Simpson_1-D | 0.9 | 0.9 | 0.9 | 0.7 |
| Shannon_H | 3.4 | 2.6 | 3.0 | 2.0 |
| Fisher_alpha | 249.4 | 111.0 | 113.5 | 112.5 |
| Chao-1 | 82.0 | 71.0 | 71.0 | 71.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalheira, M.; Amorim, C.L.; Oliveira, A.C.; Guarda, E.C.; Costa, E.; Ribau Teixeira, M.; Castro, P.M.L.; Duque, A.F.; Reis, M.A.M. Valorization of Brewery Waste through Polyhydroxyalkanoates Production Supported by a Metabolic Specialized Microbiome. Life 2022, 12, 1347. https://doi.org/10.3390/life12091347
Carvalheira M, Amorim CL, Oliveira AC, Guarda EC, Costa E, Ribau Teixeira M, Castro PML, Duque AF, Reis MAM. Valorization of Brewery Waste through Polyhydroxyalkanoates Production Supported by a Metabolic Specialized Microbiome. Life. 2022; 12(9):1347. https://doi.org/10.3390/life12091347
Chicago/Turabian StyleCarvalheira, Mónica, Catarina L. Amorim, Ana Catarina Oliveira, Eliana C. Guarda, Eunice Costa, Margarida Ribau Teixeira, Paula M. L. Castro, Anouk F. Duque, and Maria A. M. Reis. 2022. "Valorization of Brewery Waste through Polyhydroxyalkanoates Production Supported by a Metabolic Specialized Microbiome" Life 12, no. 9: 1347. https://doi.org/10.3390/life12091347








