A Voxel-Based Morphometric Study of Gray Matter in Specific Phobia
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Instruments
2.3. Design
2.4. Procedure
2.5. MRI Data Acquisition
2.6. MRI Processing
2.7. Statistical Analysis
2.7.1. Demographic and Clinical Data Analyses
2.7.2. VBM Analyses
3. Results
3.1. Demographic, Normalization, and Clinical Characteristics
3.2. Groups Differences in GMV
4. Discussion
4.1. Limitations and Future Directions
4.2. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santomauro, D.F.; Herrera, A.M.M.; Shadid, J.; Zheng, P.; Ashbaugh, C.; Pigott, D.M.; Hay, S.I.; Vos, T.; Murray, C.J.L.; Whiteford, H.A.; et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 2021, 398, 1700–1712. [Google Scholar] [CrossRef]
- Craske, M.G.; Rauch, S.L.; Ursano, R.; Prenoveau, J.; Pine, D.S.; Zinbarg, R.E. What is an anxiety disorder? Focus 2011, 9, 369–388. [Google Scholar] [CrossRef]
- Olatunji, B. The Cambridge Handbook of Anxiety and Related Disorders; Cambridge University Press: Cambridge, UK, 2019. [Google Scholar]
- Mah, L.; Szabuniewicz, C.; Fiocco, A.J. Can anxiety damage the brain? Curr. Opin. Psychiatry 2016, 29, 56–63. [Google Scholar] [CrossRef]
- Mufford, M.S.; van der Meer, D.; Andreassen, O.A.; Ramesar, R.; Stein, D.J.; Dalvie, S. A review of systems biology research of anxiety disorders. Braz. J. Psychiatry. 2020, 43, 414–423. [Google Scholar] [CrossRef]
- Tanaka, M.; Bohár, Z.; Martos, D.; Telegdy, G.; Vécsei, L. Antidepressant-like effects of kynurenic acid in a modified forced swim test. Pharmacol. Rep. 2020, 72, 449–455. [Google Scholar] [CrossRef]
- Vargas, T.G.; Mittal, V.A. Brain morphometry points to emerging patterns of psychosis, depression, and anxiety vulnerability over a 2-year period in childhood. Psychol. Med. 2022, 1–13. [Google Scholar] [CrossRef]
- Zhou, F.; Zhao, W.; Qi, Z.; Geng, Y.; Yao, S.; Kendrick, K.M.; Wager, T.D.; Becker, B. A distributed fMRI-based signature for the subjective experience of fear. Nat. Commun. 2021, 12, 1–16. [Google Scholar] [CrossRef]
- Holzschneider, K.; Mulert, C. Neuroimaging in anxiety disorders. Dialogues Clin. Neurosci. 2022, 13, 453–461. [Google Scholar] [CrossRef]
- Madonna, D.; Delvecchio, G.; Soares, J.C.; Brambilla, P. Structural and functional neuroimaging studies in generalized anxiety disorder: A systematic review. Braz. J. Psychiatry 2019, 41, 336–362. [Google Scholar] [CrossRef]
- Peñate, W.; Fumero, A.; Viña, C.; Herrero, M.; Marrero, R.J.; Rivero, F. A meta-analytic review of neuroimaging studies of specific phobia to small animals. Eur. J. Psychiatry 2017, 31, 23–36. [Google Scholar] [CrossRef]
- Ipser, J.C.; Singh, L.; Stein, D.J. Meta-analysis of functional brain imaging in specific phobia. Psychiatry Clin. Neurosci. 2013, 67, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Wittfoth, D.; Beise, J.; Manuel, J.; Bohne, M.; Wittfoth, M. Bifocal emotion regulation through acupoint tapping in fear of flying. Neuroimage Clin. 2022, 34, 102996. [Google Scholar] [CrossRef] [PubMed]
- Borgomaneri, S.; Battaglia, S.; Avenanti, A.; di Pellegrino, G. Don’t Hurt Me No More: State-dependent Transcranial Magnetic Stimulation for the treatment of specific phobia. J. Affect. Disord. 2021, 286, 78–79. [Google Scholar] [CrossRef] [PubMed]
- Fumero, A.; Marrero, R.J.; Rivero, F.; Alvarez-Pérez, Y.; Bethencourt, J.M.; González, M.; Peñate, W. Neuronal Correlates of Small Animal Phobia in Human Subjects through fMRI: The Role of the Number and Proximity of Stimuli. Life 2021, 11, 275. [Google Scholar] [CrossRef]
- Ashburner, J.; Friston, K.J. Why voxel-based morphometry should be used. Neuroimage 2001, 14, 1238–1243. [Google Scholar] [CrossRef]
- Brandl, F.; Weise, B.; Mulej Bratec, S.; Jassim, N.; Hoffmann-Ayala, D.; Bertram, T.; Ploner, M.; Sorg, C. Common and specific large-scale brain changes in major depressive disorder, anxiety disorders, and chronic pain: A transdiagnostic multimodal meta-analysis of structural and functional MRI studies. Neuropsychopharmacology 2022, 47, 1071–1080. [Google Scholar] [CrossRef]
- Kolesar, T.A.; Bilevicius, E.; Wilson, A.D.; Kornelsen, J. Systematic review and meta-analyses of neural structural and functional differences in generalized anxiety disorder and healthy controls using magnetic resonance imaging. Neuroimage Clin. 2019, 24, 102016. [Google Scholar] [CrossRef]
- Liu, X.; Klugah-Brown, B.; Zhang, R.; Chen, H.; Zhang, J.; Becker, B. Pathological fear, anxiety and negative affect exhibit distinct neurostructural signatures: Evidence from psychiatric neuroimaging meta-analysis. Transl. Psychiatry 2022, 12, 1–19. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, B.; Wang, S.; Lu, F.; Luo, Y.; Long, X.; Kong, D. Distinct grey matter volume alterations in adult patients with panic disorder and social anxiety disorder: A systematic review and voxel-based morphometry meta-analysis. J. Affect. Disord. 2021, 281, 805–823. [Google Scholar] [CrossRef]
- Tükel, R.; Aydın, K.; Yüksel, Ç.; Ertekin, E.; Koyuncu, A.; Taş, C. Gray matter abnormalities in patients with social anxiety disorder: A voxel-based morphometry study. Psychiatry Res. Neuroimaging 2015, 234, 106–112. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, B.; Luo, Q.; Qiu, L.; Wang, S. Gray matter structural alterations in social anxiety disorder: A voxel-based meta-analysis. Front. Psychiatry 2018, 9, 449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Suo, X.; Yang, X.; Lai, H.; Pan, N.; He, M.; Li, Q.; Kuang, W.; Wang, S.; Gong, Q. Structural and functional deficits and couplings in the cortico-striato-thalamo-cerebellar circuitry in social anxiety disorder. Transl. Psychiatry 2022, 12, 1–11. [Google Scholar] [CrossRef]
- Shang, J.; Fu, Y.; Ren, Z.; Zhang, T.; Du, M.; Gong, Q.; Lui, S.; Zhang, W. The common traits of the ACC and PFC in anxiety disorders in the DSM-5: Meta-analysis of voxel-based morphometry studies. PLoS ONE 2014, 9, e93432. [Google Scholar] [CrossRef]
- Battaglia, S.; Harrison, B.J.; Fullana, M.A. Does the human ventromedial prefrontal cortex support fear learning, fear extinction or both? A commentary on subregional contributions. Mol. Psychiatry. 2022, 27, 784–786. [Google Scholar] [CrossRef] [PubMed]
- Serra-Blasco, M.; Radua, J.; Soriano-Mas, C.; Gómez-Benlloch, A.; Porta-Casteràs, D.; Carulla-Roig, M.; Albajes-Eizagirre, A.; Arnone, D.; Klauser, P.; Canales-Rodríguez, E.J.; et al. Structural brain correlates in major depression, anxiety disorders and post-traumatic stress disorder: A voxel-based morphometry meta-analysis. Neurosci. Biobehav. Rev. 2021, 129, 269–281. [Google Scholar] [CrossRef]
- Bas-Hoogendam, J.M.; van Steenbergen, H.; Pannekoek, J.N.; Fouche, J.P.; Lochner, C.; Hattingh, C.J.; Henk, R.; Cremers, H.R.; Furmark, T.; Månsson, K.N.T.; et al. Voxel-based morphometry multi-center mega-analysis of brain structure in social anxiety disorder. Neuroimage Clin. 2017, 16, 678–688. [Google Scholar] [CrossRef]
- Fisler, M.S.; Federspiel, A.; Horn, H.; Dierks, T.; Schmitt, W.; Wiest, R.; Quervain, D.; Soravia, L.M. Spider phobia is associated with decreased left amygdala volume: A cross-sectional study. BMC Psychiatry 2013, 13, 1–7. [Google Scholar] [CrossRef]
- Wabnegger, A.; Scharmüller, W.; Schienle, A. Sex-specific associations between grey matter volume and phobic symptoms in dental phobia. Neurosci. Lett. 2014, 580, 83–87. [Google Scholar] [CrossRef]
- Hilbert, K.; Evens, R.; Maslowski, N.I.; Wittchen, H.U.; Lueken, U. Neurostructural correlates of two subtypes of specific phobia: A voxel-based morphometry study. Psychiatry Res. Neuroimaging 2015, 231, 168–175. [Google Scholar] [CrossRef]
- Schienle, A.; Scharmüller, W.; Leutgeb, V.; Schäfer, A.; Stark, R. Sex differences in the functional and structural neuroanatomy of dental phobia. Brain Struct. Funct. 2013, 218, 779–787. [Google Scholar] [CrossRef]
- Kessler, R.C.; Üstün, T.B. The world mental health (WMH) survey initiative version of the world health organization (WHO) composite international diagnostic interview (CIDI). Int. J. Meth. Psych. Res. 2004, 13, 93–121. [Google Scholar] [CrossRef] [PubMed]
- Endler, N.S.; Hunt, J.M.; Rosenstein, A.J. An S-R Inventory of Anxiousness. Psychol. Monogr. 1962, 76, 143–146. [Google Scholar] [CrossRef]
- Kameoka, V.A.; Tanaka-Matsumi, J. The appropriateness of using the S-R Inventory of Anxiousness to measure sources ofbehavioral variability. Appl. Psych. Meas. 1981, 5, 229–235. [Google Scholar] [CrossRef]
- Oldfield, R. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Ashburner, J.; Friston, K.J. Unified segmentation. Neuroimage 2005, 26, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, J. A fast diffeomorphic image registration algorithm. Neuroimage 2007, 38, 95–113. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, J.; Friston, K.J. Voxel-based morphometry—The methods. Neuroimage 2000, 11, 805–821. [Google Scholar] [CrossRef]
- Friston, K.J.; Worsley, K.J.; Frackowiak, R.S.; Mazziotta, J.C.; Evans, A.C. Assessing the significance of focal activations using their spatial extent. Hum. Brain Mapp. 1994, 1, 210–220. [Google Scholar] [CrossRef]
- Nichols, T.; Hayasaka, S. Controlling the familywise error rate in functional neuroimaging: A comparative review. Stat. Methods Med. Res. 2003, 12, 419–446. [Google Scholar] [CrossRef]
- Worsley, K.J.; Marrett, S.; Neelin, P.; Vandal, A.C.; Friston, K.; Evans, A.C. A unified statistical approach for determining significant signals in images of cerebral activation. Hum. Brain Mapp. 1996, 4, 58–73. [Google Scholar] [CrossRef]
- Cohen, M.X.; Heller, A.S.; Ranganath, C. Functional connectivity with anterior cingulate and orbitofrontal cortices during decision-making. Cog. Brain Res. 2005, 23, 61–70. [Google Scholar] [CrossRef]
- Petrovic, P.; Ekman, C.J.; Klahr, J.; Tigerström, L.; Rydén, G.; Johansson, A.G.; Sellgren, C.; Golkar, A.; Olsson, A.; Öhman, A.; et al. Significant grey matter changes in a region of the orbitofrontal cortex in healthy participants predicts emotional dysregulation. Soc. Cogn. Affect. Neurosci. 2016, 11, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, A.; Lerner, T.N.; Finkelstein, J.; Pak, S.; Jennings, J.H.; Davidson, T.J.; Ferenczi, E.; Gunaydin, L.A.; Mirzabekov, J.J.; Ye, L.; et al. Basomedial amygdala mediates top-down control of anxiety and fear. Nature 2015, 527, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Blackmon, K.; Barr, W.B.; Carlson, C.; Devinsky, O.; DuBois, J.; Pogash, D.; Quinn, B.T.; Kuzniecky, R.; Halgren, E.; Thesen, T. Structural evidence for involvement of a left amygdala-orbitofrontal network in subclinical anxiety. Psychiatry Res. Neuroimaging 2011, 194, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Hasler, G.; Haynes, M.; Müller, S.T.; Tuura, R.; Ritter, C.; Buchmann, A. The association between adolescent residential mobility and adult social anxiety, BDNF and amygdala-orbitofrontal functional connectivity in young adults with higher education. Front. Psychiatry. 2020, 11, 561464. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.H. Fear network model in panic disorder: The past and the future. Psychiatry Investig. 2019, 16, 16–26. [Google Scholar] [CrossRef]
- Xiong, H.; Guo, R.J.; Shi, H.W. Altered default mode network and salience network functional connectivity in patients with generalized anxiety disorders: An ICA-based resting-state fMRI study. Evid. Based Complement. Altern. Med. 2020, 2020, 4048916. [Google Scholar] [CrossRef] [PubMed]
- Fumero, A.; Marrero, R.J.; Olivares, T.; Rivero, F.; Alvarez-Pérez, Y.; Pitti, C.; Peñate, W. Neuronal Activity during Exposure to Specific Phobia through fMRI: Comparing Therapeutic Components of Cognitive Behavioral Therapy. Life 2022, 12, 1132. [Google Scholar] [CrossRef]
- Chen, Y.; Cui, Q.; Tang, Q.; Sheng, W.; Yang, Y.; Chen, H. Amygdala-Based Structural Covariance and Functional Connectivity Alterations in Patients with Generalized Anxiety Disorder. In Proceedings of the Fourth International Symposium on Image Computing and Digital Medicine, Shenyang, China, 5–7 December 2020; pp. 192–196. [Google Scholar] [CrossRef]
- Steiger, V.R.; Brühl, A.B.; Weidt, S.; Delsignore, A.; Rufer, M.; Jäncke, L.; Herwig, U.; Hänggi, J. Pattern of structural brain changes in social anxiety disorder after cognitive behavioral group therapy: A longitudinal multimodal MRI study. Mol. Psychiatry 2017, 22, 1164–1171. [Google Scholar] [CrossRef]
- Álvarez-Pérez, Y.; Rivero, F.; Herrero, M.; Viña, C.; Fumero, A.; Betancort, M.; Peñate, W. Changes in brain activation through cognitive-behavioral therapy with exposure to virtual reality: A neuroimaging study of specific phobia. J. Clin. Med. 2021, 10, 3505. [Google Scholar] [CrossRef]
- Howells, F.M.; Hattingh, C.J.; Syal, S.; Breet, E.; Stein, D.J.; Lochner, C. 1H-magnetic resonance spectroscopy in social anxiety disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2015, 58, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Yang, F.; Zhang, Y.; He, Z.; Su, L.; Li, L. Lack of gender effects on gray matter volumes in adolescent generalized anxiety disorder. J. Affect. Disord. 2014, 155, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Günther, V.; Ihme, K.; Kersting, A.; Hoffmann, K.T.; Lobsien, D.; Suslow, T. Volumetric associations between amygdala, nucleus accumbens, and socially anxious tendencies in healthy women. Neuroscience 2018, 374, 25–32. [Google Scholar] [CrossRef]
- Kanai, R.; Rees, G. The structural basis of inter-individual differences in human behaviour and cognition. Nat. Rev. Neurosci. 2011, 12, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Hormigo, S.; Vega-Flores, G.; Castro-Alamancos, M.A. Basal ganglia output controls active avoidance behavior. J. Neurosci. 2016, 36, 10274–10284. [Google Scholar] [CrossRef]
- Fredrikson, M.; Annas, P.; Fischer, H.; Wik, G. Gender and age differences in the prevalence of specific fears and phobias. Behav. Res. Ther. 1996, 34, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Pfefferbaum, A.; Mathalon, D.H.; Sullivan, E.V.; Rawles, J.M.; Zipursky, R.B.; Lim, K.O. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch. Neurol. 1994, 51, 874–887. [Google Scholar] [CrossRef] [PubMed]
- Lebel, C.; Walker, L.; Leemans, A.; Phillips, L.; Beaulieu, C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage 2008, 40, 1044–1055. [Google Scholar] [CrossRef]
- Narvacan, K.; Treit, S.; Camicioli, R.; Martin, W.; Beaulieu, C. Evolution of deep gray matter volume across the human lifespan. Hum. Brain Mapp. 2017, 38, 3771–3790. [Google Scholar] [CrossRef] [PubMed]
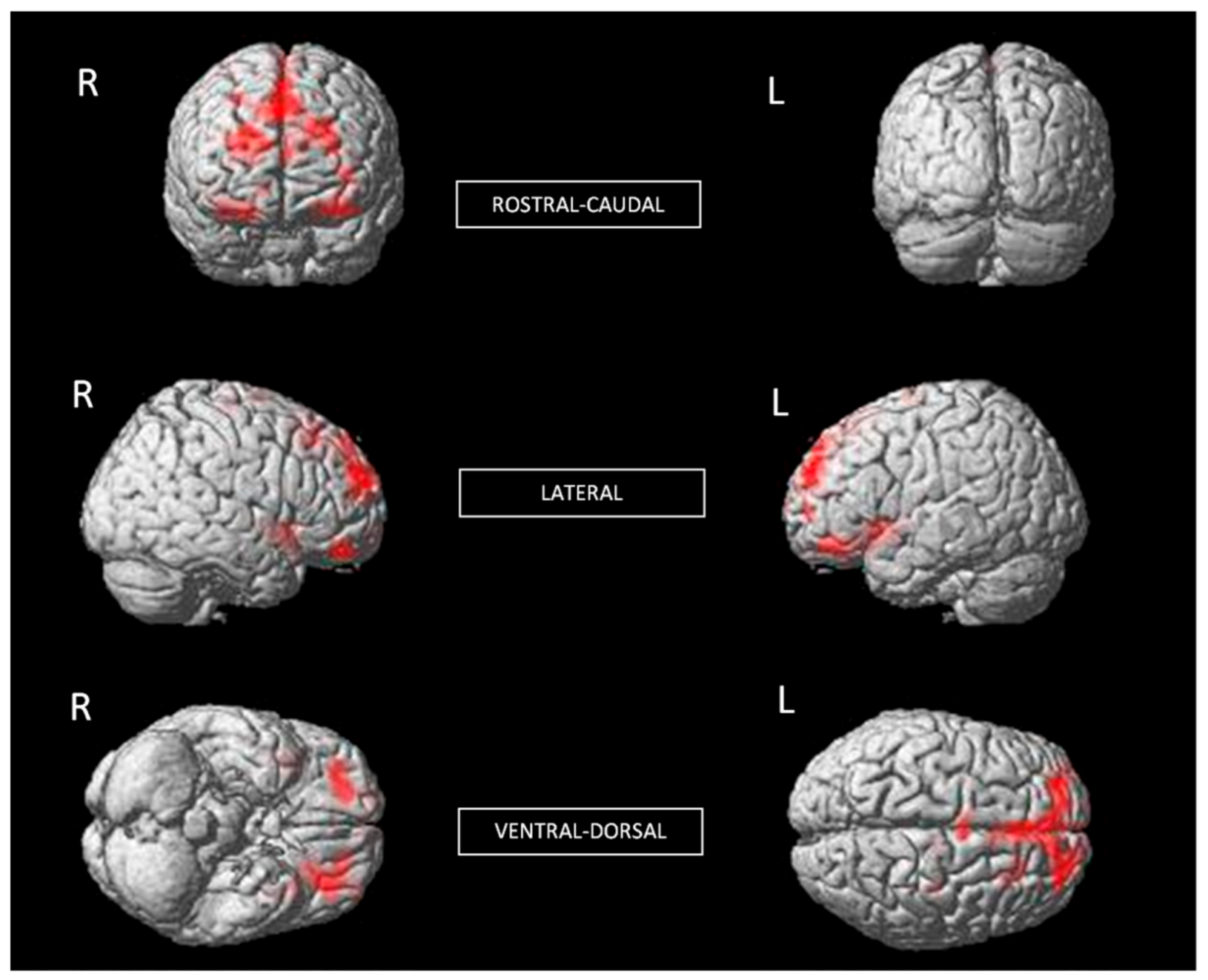
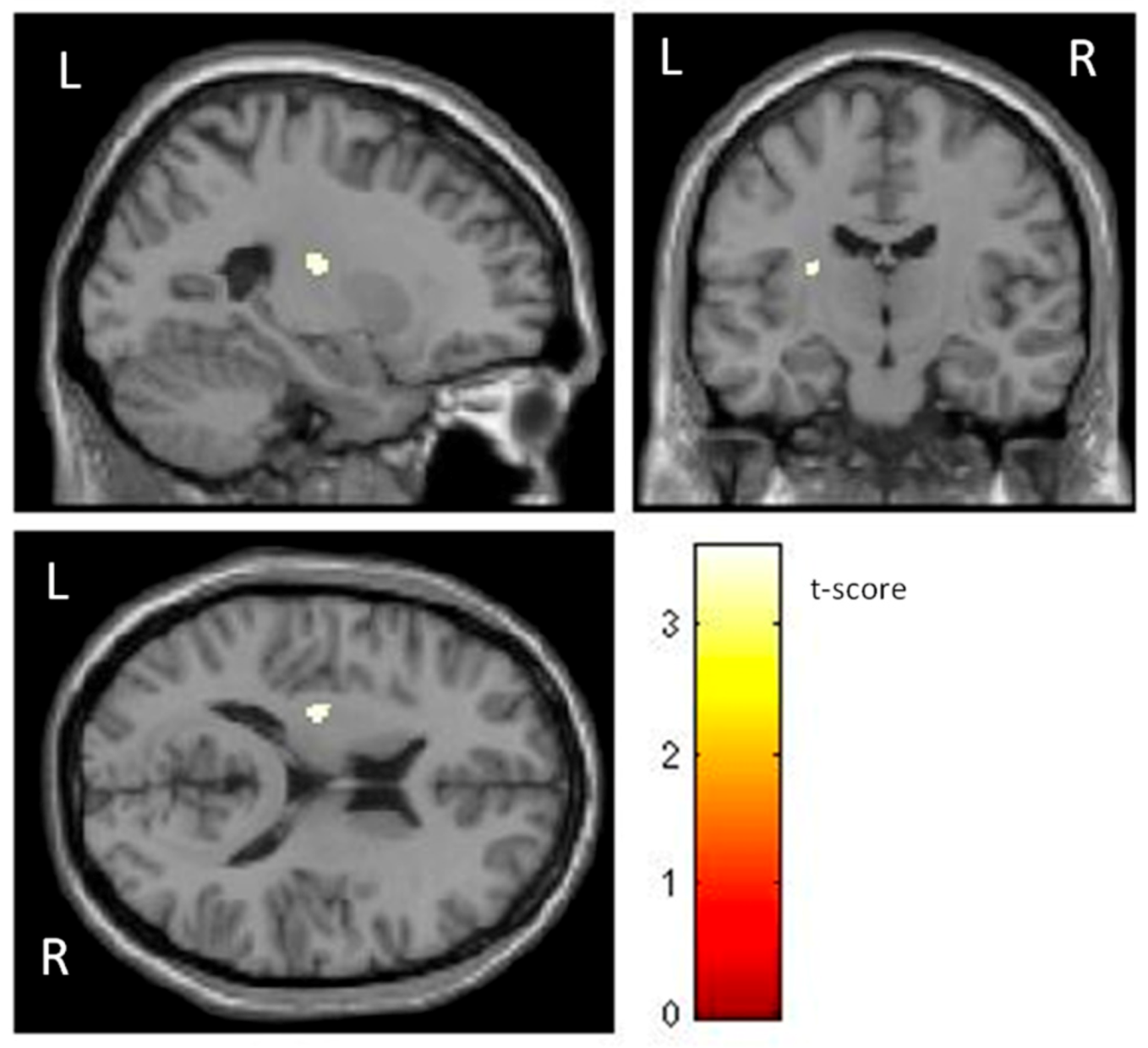
| Brain Structures | MNI Coordinates | k | t | p | r2 |
|---|---|---|---|---|---|
| x y z | |||||
| Non-fearful controls > Individuals with phobia | |||||
| R Insula | 45 9 −9 | 961 | 5.18 | <0.001 | 0.31 |
| R Lateral OFC R Anterior OFC R Inferior OFC | 24 48 −21 | 948 | 4.98 | <0.001 | 0.29 |
| L Posterior OFC L Middle OFC L Lateral OFC L Insula | −19 27 −13 | 2924 | 4.86 | <0.001 | 0.28 |
| L Superior Medial Frontal R ACC R Superior Frontal | −25 51 28 | 7104 | 4.48 | <0.001 | 0.25 |
| Individuals with phobia > Non-fearful controls | |||||
| L Putamen | −26 −15 15 | 60 | 3.58 | <0.001 | 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivero, F.; Marrero, R.J.; Olivares, T.; Peñate, W.; Álvarez-Pérez, Y.; Bethencourt, J.M.; Fumero, A. A Voxel-Based Morphometric Study of Gray Matter in Specific Phobia. Life 2023, 13, 119. https://doi.org/10.3390/life13010119
Rivero F, Marrero RJ, Olivares T, Peñate W, Álvarez-Pérez Y, Bethencourt JM, Fumero A. A Voxel-Based Morphometric Study of Gray Matter in Specific Phobia. Life. 2023; 13(1):119. https://doi.org/10.3390/life13010119
Chicago/Turabian StyleRivero, Francisco, Rosario J. Marrero, Teresa Olivares, Wenceslao Peñate, Yolanda Álvarez-Pérez, Juan Manuel Bethencourt, and Ascensión Fumero. 2023. "A Voxel-Based Morphometric Study of Gray Matter in Specific Phobia" Life 13, no. 1: 119. https://doi.org/10.3390/life13010119
APA StyleRivero, F., Marrero, R. J., Olivares, T., Peñate, W., Álvarez-Pérez, Y., Bethencourt, J. M., & Fumero, A. (2023). A Voxel-Based Morphometric Study of Gray Matter in Specific Phobia. Life, 13(1), 119. https://doi.org/10.3390/life13010119







