Microbial Inoculants as Plant Biostimulants: A Review on Risk Status
Abstract
:1. Introduction
2. History of Classification of Biostimulants
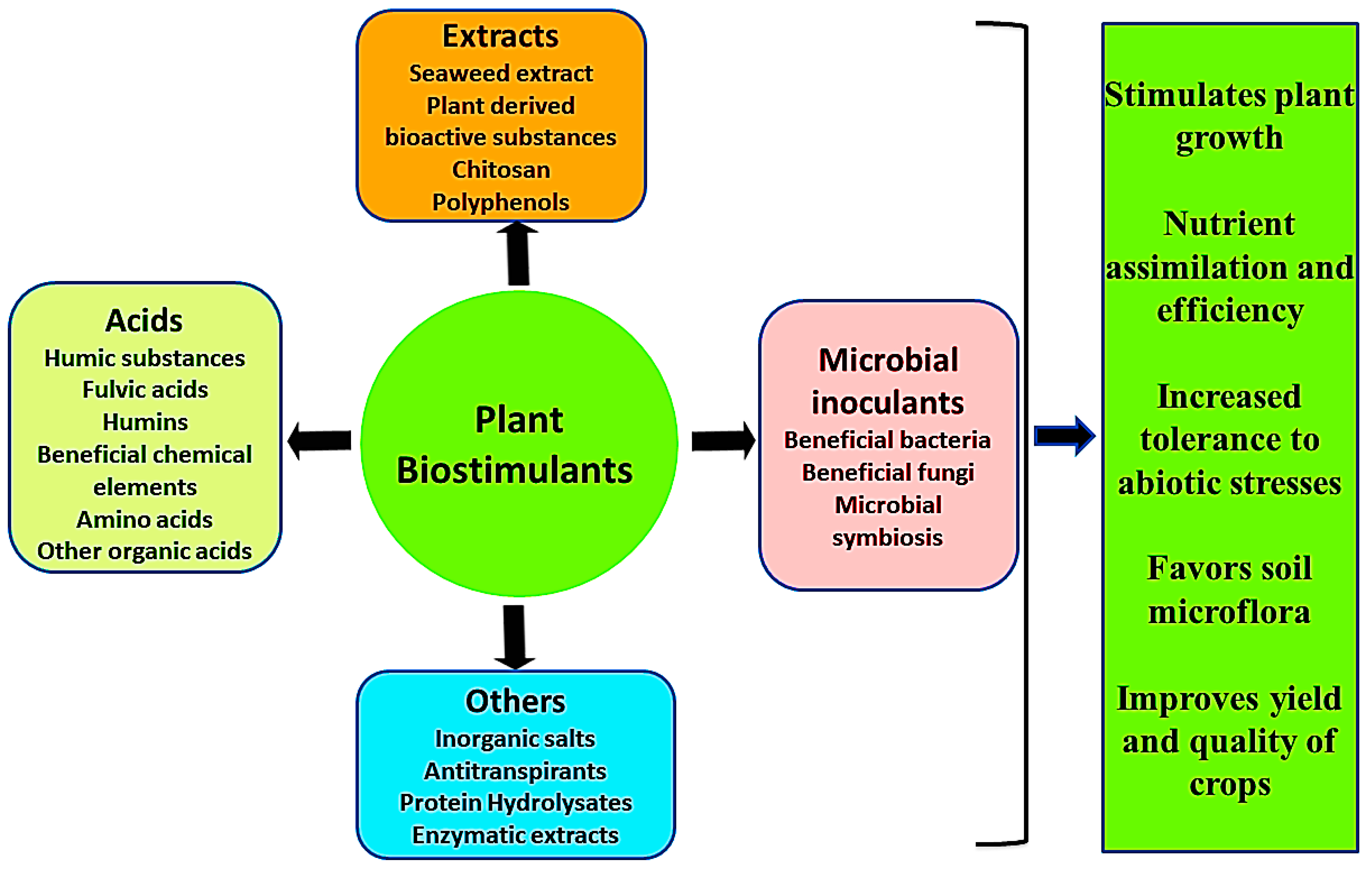
3. Properties of Plant Biostimulants
- Improving plant metabolism which induces crop yield and increases the quality of crops [18].
- Plant biostimulants protect plants against environmental stresses such as water deficiency, exposure to sub-optimal growth temperatures, and soil salinization [1].
- They are also known to promote plant growth through better nutrient uptake.
- Increasing soil enzymatic as well as microbial activities [16].
- Enhancing fertility of the soil, predominantly by nurturing the development of complementary soil microbes [18].
4. Plant Biostimulants and Their Mechanism
4.1. Humic Substances (HS)
4.2. Protein Hydrolysates and Amino Acids
4.3. Seaweed Extracts and Botanicals
4.4. Chitin and Chitosan Derivatives
4.5. Antitranspirants
4.6. Microbial Inoculants
4.6.1. Plant Growth Promoting Bacteria
Biological Nitrogen Fixation
Solubilisation of Phosphate
Production of an Iron Chelating Compound
Phytohormone Production
4.6.2. Arbuscular Mycorrhizal Fungi (AMF)
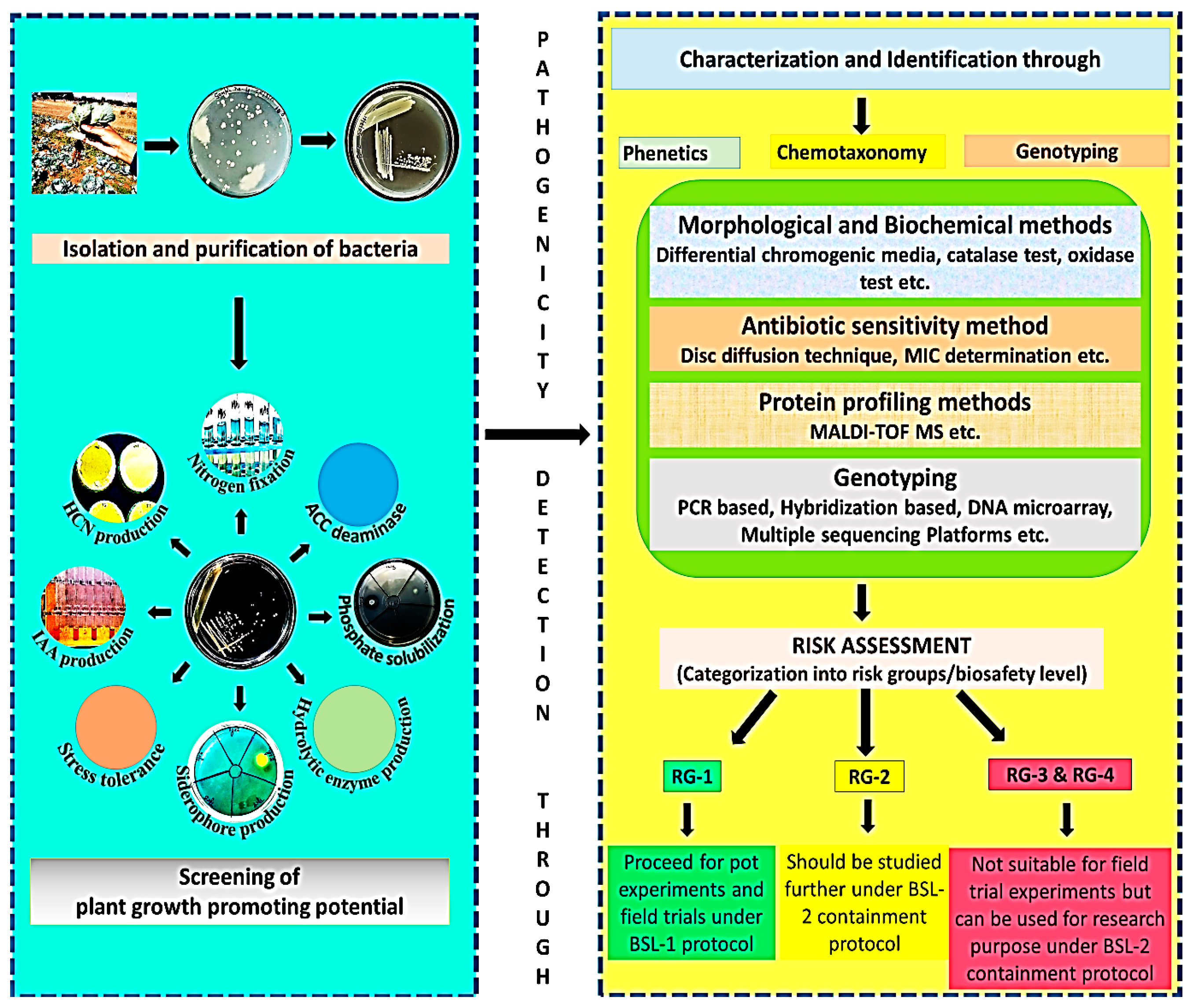
5. Risk Status of Microbial Inoculants (Plant Growth Promoting Bacteria)
| Microbial Inoculants in Research and Commercial Biofertilizers (Risk Group by TRBA/ATCC/ZKBS) | Commercial Status/Formulation (Brand Name and Manufacturer) | Plants | Effects on Plants |
|---|---|---|---|
| Pseudomonas putida [107] (RG2G/BSL1/RG2) [156,157,158] #BSL 1- P. putida (Trevisan) migula | Yes/Powder (Pseudomonas putida, Organoponix private Limited, Orissa [159] | Rice | Increased iron uptake |
| Pseudomonas fluorescens [160,161,162] (RG1/BSL1/- [156,158,163] #BSL-1- P. fluorescens migula | Yes/Powder and Liquid (PSEUDOMONAS FLUORESCENS Bacterial biocontrol agent, Manidharma Biotech Private Limited, Tamil Nadu, India) [164] | Rapeseed, sweet potato, rice | Increased plant height, biomass, grain yield |
| Streptomyces strain [165,166] (RG1/BSL 1/RG1) [156,158,167] #BSL 1- Streptomyces azureus Kelley et al. | No/- | Tomato and rice | Plant growth |
| Azospirillum brasilense Sp245 [115] (RG 1/BSL 1/RG1) [115,156,158,168] #BSL1- A. brasilense | Yes/Liquid (Sardar Liquid Biofertilizers- Azospirillum culture, Gujrat State Fertilizers, and Chemicals, India) species and strain not specified) [169] | Spring wheat | The increased dry weight of the shoot and leaf length |
| Aeromonas spp [170] (RG 1/BSL 2/RG2) [156,158,171] #BSL 2- Aeromonas hydrophila (Chester) Stanier | No/- | Rice | Increased root area |
| Comamonas acidovorans [172] (RG1G/BSL1/RG2) [156,158,173] #BSL1- Comamonas sp. | No/- | Lettuce | Plant growth promotion such as IAA production |
| Bacillus subtilis [174] (RG1/BSL1/RG1) [156,158,175] #BSL1- B. subtilis (Ehrenberg) Cohn | Yes/Aqueous suspension and wettable powder (Biosubtilin, Biotech International Limited, New Delhi, India) [176] | Lettuce | Increased cytokinin content in roots and shoots |
| Bacillus licheniformis [177] (RG1/BSL1/RG1) [156,158,178] #BSL1- B. licheniformis (Weigmann) Chester | No/- | Cucumber | Increased fresh weight, higher chlorophyll content, and enhanced cell division |
| Azospirillum lipoferum [179] (RG1/BSL1/RG1) [156,158,180] #BSL1- A. lipoferum (Beijerinck) | Yes/Carrier powder, soluble powder, and soluble liquid (Nitrofix, Agri Life, Andhra Pradesh, India) [181] | Maize seedlings | Increased root hair density |
| Azospirillum lipoferum [182] (RG1/BSL1/RG1) [156,158,180] #BSL1- A. lipoferum (Beijerinck) Tarrand et al. | Yes/Carrier powder, soluble powder, and soluble liquid (Nitrofix, Agri Life, Andhra Pradesh, India) [181] | Wheat | Increased tolerance to salinity conditions |
| P. putida [183] (RG2G/BSL1/RG2) [156,157,158] #BSL1- P. putida (Trevisan) migula | Yes/Powder (Pseudomonas putida, Organoponix private Limited, Orissa) [159] | White clover | Increased root and shoot biomass and water content |
| B. megaterium [183] (RG1/BSL1/RG1) [156,158,184] #BSL1- B. megaterium de Bary | Yes/Carrier powder, soluble powder, and soluble liquid (P Sol B®, Agri Life, Andhra Pradesh, India) [185] | White clover | Increased root and shoot biomass and water content |
| Alternaria sp. [186,187] (-/BSL1/RG1/2) [158,188] | No/- | Wheat | Stimulate drought tolerance |
| Trichoderma sp. [40,140,186,187] (-/ BSL1/RG1) [158,189] #BSL1- T. harzianum Rifai | Yes/Wettable powder and Aqueous suspension (Bioderma, Biotech International Limited, New Delhi) [190]; Ecosom®- TV, [191]; Ecosom®-TH [192] (Agri Life, Andhra Pradesh, India) | Barley | Increased drought tolerance |
| Azoarcus sp. [193] (RG1/BSL1/RG1) [156,158,194] #BSL1- A. oleivorans | No/- | Wheat | Enhanced plant nitrogen nutrition and root growth and alleviate the nutrient deficiency |
| Azorhizobium sp. [195] (RG1/BSL1/-) [156,196] #BSL1- A. caulinodans Dreyfus et al. | No/- | Wheat | Enhanced plant nitrogen nutrition and root growth and alleviate the nutrient deficiency |
| Azospirillum sp. [193] (RG1/BSL1/RG1) [156,158,168,180] #BSL1- A. lipoferum (Beijerinck) Tarrand et al., A. brasilense Tarrand et al. | Yes/Liquid (Sardar Liquid Biofertilizers- Azospirillum culture, Gujrat State Fertilizers, and Chemicals, India) [169] | Wheat | Enhanced plant nitrogen nutrition and root growth and alleviate the nutrient deficiency |
| Bradyrhizobium sp. (RG1/BSL1/RG1) [156,158,197,198,199] #BSL1- Bradyrhizobium sp. | No/- | Mungbeans | Increases growth parameters and seed yield |
| Rhizobium meliloti [200,201] (RG1/BSL1/RG1) [156,158,202] #BSL1- Rhizobium sp. | Yes/Aqueous suspension and wettable powder (Biobium Biofertilizers, Biotech International Limited, New Delhi), Species not specified [203] | Peanuts | Increases plant growth, quality of pods enhanced, and efficiency in the use of nitrogen |
| R. leguminosarum [204] (RG1/BSL1/RG1) [156,158,205] #BSL1- R. leguminosarum jordan | Yes/Aqueous suspension and wettable powder (Biobium) Species not specified [203] | Soybean | Increases growth and yield performance under drought stress |
| Bacillus spp. [206,207] (RG1/BSL 1/ RG1/2/3) [156,158,208] | Yes/Carrier powder, soluble powder, and soluble liquid (Si-Sol B TM, Agri Life, Andhra Pradesh, India) [209] | Strawberry | Increases fresh and dry weight parameters, increases yield |
| Azotobacter chroococcum [210] (RG1/BSL1/RG1) [156,158,211] #BSL1- A. chroococcum Beijerinck | Yes/Liquid (Reap®-N1, NCS Green Earth Private Limited, Maharashtra) [212] | Maize | Increased shoot and root length, leaf and root number, chlorophyll content |
| Azotobacter vinelandii [210] (RG1/BSL1/RG1) [156,158,213] #BSL1- A. vinelandii Lipman | Yes/Carrier-based powder (Nitrofix ®, Agri Life, Andhra Pradesh, India) [181] | Maize | Increased shoot and root length, leaf and root number, chlorophyll content |
| Bacillus halotolerans [204,214] (RG1/-/-) [156] | No/- | Wheat and soybean | Improved germination, growth, and yield, better draught resistance, improved nitrogen, potassium, and Zn uptake |
| Enterobacter hormaechei [204,214] (RG2/BSL2/-) [156,215] #BSL 2- E. cloacae (Jordan) Hormaeche and Edwards | No/- | Wheat and soybean | Improved germination, growth, and yield, better draught resistance, improved nitrogen, potassium, and Zn uptake |
| Pseudomonas frederiksbergensis RG2G * [204,214] (RG1/BSL1/-) [156,216] #BSL1- P. frederiksbergensis Andersen et al. | No/- | Wheat and soybean | Improved germination, growth, and yield, better draught resistance, improved nitrogen, potassium, and Zn uptake |
6. Safety Determination of Microbial Inoculants
6.1. Morphological and Biochemical Methods
6.2. Antibiotic Sensitivity Method
6.3. Protein Profiling Method
6.4. Molecular Level Detection Techniques
| Technological Approach | Major Targets for Pathogens Detection | Advantage/Limitations | References |
|---|---|---|---|
| Phenotypic methods | |||
| (i) Morphological and biochemical methods | Metabolic potential and specific enzymes such as catalase, oxidase, phosphatase, hydrolase enzymes, etc. | Traditional low-cost, easy-to-operate, standardized methods cannot differentiate between target and non-target endogenous microorganisms, time and labor-consuming procedures, and also unable to detect viable unculturable organisms | [227] |
| (ii) Antibiotic-sensitivity testing | Resistant markers transmission | ||
| Protein profiling method (Proteomics) MALDI-TOF MS | Specific proteins of particular bacteria to identify specific genera and species. | Qualitative and quantitative determination of proteins in most clinical laboratories. Low concentration of proteins leads to errors in the data interpretation (resistant mechanisms). Unable to differentiate taxonomically related bacteria | [227,234,235] |
| Molecular methods (genomics) | |||
| (i) Amplification methods: Quantitative real-time polymerase chain reaction (qPCR), reverse transcriptase real-time PCR (RT-qPCR), and Loop-Mediated Isothermal Amplification (LAMP) | hybridization between the target nucleic acid and the pathogen-specific probe | More sensitive methods for the identification of pathogens at the molecular level suffer in case of low concentrations of pathogens. | [228,231,236,237] |
| (ii) Hybridization-based methods | |||
| (iii) DNA microarrays (gene chip technology) | hybridization between the target nucleic acid and the pathogen-specific marker gene panels. | ||
| (iv) Whole-genome sequencing | whole genome sequence | Identification of pathogens, profiling of resistant genes, recognition of outbreaks, and immediate design of PCR probes based on the generated genetic data in the outbreaks. | |
| (v) Next-generation sequencing | |||
| Microfluidics based methods | It is a multidisciplinary strategy and utilizes pathogen markers | extraction and identification of pathogens from clinical/environmental samples. | [238,239] |
7. Legal Framework of Biofertilizer Implementation in Different Countries
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- EBIC. European Biostimulants Industry Council (EBIC) and Biostimulants in Brief. 2012. Available online: https://www.biostimulants.eu/ (accessed on 17 October 2022).
- Filatov, V.P. Tissue treatment.(Doctrine on biogenic stimulators). I. Background, methods and the clinical tissue treatment. Priroda 1951, 11, 39–46. [Google Scholar]
- Russo, R.O.; Berlyn, G.P. The use of organic biostimulants to help low-input sustainable agriculture. J. Sustain. Agric. 1991, 1, 19–42. [Google Scholar] [CrossRef]
- Torre, L.A.; Battaglia, V.; Caradonia, F. Legal aspects of the use of plant strengtheners (biostimulants) in Europe. Bulg. J. Agric. Sci. 2013, 19, 1183–1189. [Google Scholar]
- Martínez-Viveros, O.; Jorquera, M.A.; Crowley, D.E.; Gajardo, G.; Mora, M.L. Mechanisms and practical considerations involved in plant growth promotion by rhizobacteria. J. Soil Sci. Plant Nutr. 2010, 10, 293–319. [Google Scholar] [CrossRef] [Green Version]
- EBIC. 2011. Available online: http://www.biostimulants.eu/2011/10/biostimulants-definition-agreed/ (accessed on 29 September 2021).
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef] [Green Version]
- EBIC. European Biostimulants Industry Council, What Are Biostimulants? 2012. Available online: http://www.biostimulants.eu/about/what-arebiostimulants (accessed on 1 September 2022).
- Kauffman, G.L.; Kneivel, D.P.; Watschke, T.L. Effects of a biostimulant on the heat tolerance associated with photosynthetic capacity, membrane thermostability, and polyphenol production of perennial ryegrass. Crop Sci. 2007, 47, 261–267. [Google Scholar] [CrossRef]
- Gu, D.; Wang, X.-F.; Ding, F.-J. Plant biostimulants: A review on categories, effects and application. In Proceedings of the Chinese Society of Plant Nutrition and Fertilizer Science 2014 Academic Annual Conference, Harbin, China, 1–9 August 2014. [Google Scholar]
- Parrado, J.; Bautista, J.; Romero, E.J.; García-Martínez, A.M.; Friaza, V.; Tejada, M. Production of a carob enzymatic extract: Potential use as a biofertilizer. Bioresour. Technol. 2008, 99, 2312–2318. [Google Scholar] [CrossRef]
- du Jardin, P. The Science of Plant Biostimulants–A bibliographic Analysis. In Ad Hoc Study Report; European Commission: Brussels, Belgium, 2012. [Google Scholar]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The use of biostimulants for enhancing nutrient uptake. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2015; Volume 130, pp. 141–174. [Google Scholar]
- AHDB. Agriculture and Horticulture Development Board, Stoneleigh Park, Kenilworth, Warwickshire, CV8 2TL, Plant Biostimulants: Function and Efficacy. Available online: https://ahdb.org.uk/biostimulants (accessed on 27 October 2022).
- De Pascale, S.; Rouphael, Y.; Colla, G. Plant biostimulants: Innovative tool for enhancing plant nutrition in organic farming. Eur. J. Hortic. Sci. 2017, 82, 277–285. [Google Scholar] [CrossRef]
- Sible, C.N.; Seebauer, J.R.; Below, F.E. Plant Biostimulants: A Categorical Review, Their Implications for Row Crop Production, and Relation to Soil Health Indicators. Agronomy 2021, 11, 1297. [Google Scholar] [CrossRef]
- Baltazar, M.; Correia, S.; Guinan, K.J.; Sujeeth, N.; Bragança, R.; Gonçalves, B. Recent advances in the molecular effects of biostimulants in plants: An overview. Biomolecules 2021, 11, 1096. [Google Scholar] [CrossRef] [PubMed]
- Ertani, A.; Cavani, L.; Pizzeghello, D.; Brandellero, E.; Altissimo, A.; Ciavatta, C.; Nardi, S. Biostimulant activity of two protein hydrolyzates in the growth and nitrogen metabolism of maize seedlings. J. Plant Nutr. Soil Sci. 2009, 172, 237–244. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Canaguier, R.; Svecova, E.; Cardarelli, M. Biostimulant action of a plant-derived protein hydrolysate produced through enzymatic hydrolysis. Front. Plant Sci. 2014, 5, 448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Nardi, S.; Muscolo, A.; Vaccaro, S.; Baiano, S.; Spaccini, R.; Piccolo, A. Relationship between molecular characteristics of soil humic fractions and glycolytic pathway and krebs cycle in maize seedlings. Soil Biol. Biochem. 2007, 39, 3138–3146. [Google Scholar] [CrossRef]
- Sutton, R.; Sposito, G. Molecular structure in soil humic substances: The new view. Environ. Sci. Technol. 2005, 39, 9009–9015. [Google Scholar] [CrossRef]
- Muscolo, A.; Sidari, M.; Attinà, E.; Francioso, O.; Tugnoli, V.; Nardi, S. Biological activity of humic substances is related to their chemical structure. Soil Sci. Soc. Am. J. 2007, 71, 75–85. [Google Scholar] [CrossRef] [Green Version]
- Adey, W.H.; Loveland, K. CHAPTER 22—Estuaries: Ecosystem modeling and restoration. Dyn. Aquar. 2007, 3, 405–441. [Google Scholar]
- Piccolo, A. The supramolecular structure of humic substances: A novel understanding of humus chemistry and implications in soil science. Adv. Agron. 2002, 75, 57–134. [Google Scholar]
- Simpson, A.J.; Kingery, W.L.; Spraul, M.; Humpfer, E.; Dvortsak, P.; Kerssebaum, R. Separation of structural components in soil organic matter by diffusion ordered spectroscopy. Environ. Sci. Technol. 2001, 35, 4421–4425. [Google Scholar] [CrossRef]
- Aiken, G.R.; McKnight, D.M.; Wershaw, R.L.; Maccarthy, P. Humic substances in soil, sediment, and water. Soil Sci. 1986, 142, 323. [Google Scholar] [CrossRef]
- Berbara, R.L.; García, A.C. Humic substances and plant defense metabolism. In Physiological Mechanisms and Adaptation Strategies in Plants under Changing Environment; Springer: New York, NY, USA, 2014; pp. 297–319. [Google Scholar]
- Piccolo, A.; Spiteller, M. Electrospray ionization mass spectrometry of terrestrial humic substances and their size fractions. Anal. Bioanal. Chem. 2003, 377, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Varanini, Z.; Pinton, R. Plant-soil relationship: Role of humic substances in iron nutrition. In Iron Nutrition in Plants and Rhizospheric Microorganisms; Springer: Dordrecht, The Netherlands, 2006; pp. 153–168. [Google Scholar]
- Chen, Y.; De Nobili, M.; Aviad, T. Stimulatory effects of humic substances on plant growth. In Soil Organic Matter in Sustainable Agriculture; CRC Press: Boca Raton, FL, USA, 2004; pp. 103–129. [Google Scholar]
- Garcia-Mina, J.M.; Antolin, M.C.; Sanchez-Diaz, M. Metal-humic complexes and plant micronutrient uptake: A study based on different plant species cultivated in diverse soil types. Plant Soil 2004, 258, 57–68. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus chemistry: Genesis, composition, reactions. In Humus Chemitry; John Wiley & Sons: Hoboken, NJ, USA, 1994; pp. 1–512. [Google Scholar]
- Nardi, S.; Carletti, P.; Pizzeghello, D.; Muscolo, A. Biological activities of humic substances. In Biophysico-Chemical Processes Involving Natural Nonliving Organic Matter in Environmental Systems; John Wiley & Sons: Hoboken, NJ, USA, 2009; Volume 2, pp. 305–339. [Google Scholar]
- Rice, J.A.; MacCarthy, P. A model of humin. Environ. Sci. Technol. 1990, 24, 1875–1877. [Google Scholar] [CrossRef]
- Zeng, K.; Hwang, H.M.; Yuzuri, H. Effect of dissolved humic substances on the photochemical degradation rate of 1-aminopyrene and atrazine. Int. J. Mol. Sci. 2002, 3, 1048–1057. [Google Scholar] [CrossRef] [Green Version]
- Shahid, M.; Dumat, C.; Silvestre, J.; Pinelli, E. Effect of fulvic acids on lead-induced oxidative stress to metal sensitive Vicia faba L. plant. Biol. Fertil. Soils 2012, 48, 689–697. [Google Scholar] [CrossRef] [Green Version]
- Santos, N.M.D.; Accioly, A.M.D.A.; Nascimento, C.W.A.D.; Santos, J.A.G.; Silva, I.R. Humic acids and activated charcoal as soil amendments to reduce toxicity in soil contaminated by lead. Rev. Bras. De Ciência Do Solo 2014, 38, 345–351. [Google Scholar] [CrossRef] [Green Version]
- Colla, G.; Rouphael, Y.; Di Mattia, E.; El-Nakhel, C.; Cardarelli, M. Co-inoculation of Glomus intraradices and Trichoderma atroviride acts as abiostimulant to promote growth, yield and nutrient uptake of vegetable crops. J. Sci. Food Agric. 2015, 95, 1706–1715. [Google Scholar] [CrossRef]
- Chen, T.H.; Murata, N. Glycinebetaine protects plants against abiotic stress: Mechanisms and biotechnological applications. Plant Cell Environ. 2011, 34, 1–20. [Google Scholar] [CrossRef]
- Farrell, M.; Prendergast-Miller, M.; Jones, D.L.; Hill, P.W.; Condron, L.M. Soil microbial organic nitrogen uptake is regulated by carbon availability. Soil Biol. Biochem. 2014, 77, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, M.F.M.R.; Foolad, M. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Chen, T.H.; Murata, N. Glycinebetaine: An effective protectant against abiotic stress in plants. Trends Plant Sci. 2008, 13, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, M.; De Diego, N.; Ugena, L.; Spíchal, L.; Lucini, L.; Miras-Moreno, B.; Zhang, L.; Rouphael, Y.; Colla, G.; Panzarová, K. Seed priming with protein hydrolysates improves arabidopsis growth and stress tolerance to abiotic stresses. Front. Plant Sci. 2021, 837. [Google Scholar] [CrossRef] [PubMed]
- Apone, F.; Tito, A.; Carola, A.; Arciello, S.; Tortora, A.; Filippini, L.; Colucci, G. A mixture of peptides and sugars derived from plant cell walls increases plant defense responses to stress and attenuates ageing-associated molecular changes in cultured skin cells. J. Biotechnol. 2010, 145, 367–376. [Google Scholar] [CrossRef]
- Ertani, A.; Pizzeghello, D.; Altissimo, A.; Nardi, S. Use of meat hydrolyzate derived from tanning residues as plant biostimulant for hydroponically grown maize. J. Plant Nutr. Soil Sci. 2013, 176, 287–295. [Google Scholar] [CrossRef]
- dos Reis, S.P.; Lima, A.M.; de Souza, C.R.B. Recent molecular advances on downstream plant responses to abiotic stress. Int. J. Mol. Sci. 2012, 13, 8628–8647. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Lim, C.J.; Kwon, S.Y. Glycine betaine: A versatile compound with great potential for gene pyramiding to improve crop plant performance against environmental stresses. Plant Biotechnol. Rep. 2013, 7, 49–57. [Google Scholar] [CrossRef]
- Corte, L.; Dell’Abate, M.T.; Magini, A.; Migliore, M.; Felici, B.; Roscini, L.; Benedetti, A. Assessment of safety and efficiency of nitrogen organic fertilizers from animal-based protein hydrolysates—A laboratory multidisciplinary approach. J. Sci. Food Agric. 2014, 94, 235–245. [Google Scholar] [CrossRef]
- Kandale, A.; Meena, A.K.; Rao, M.M.; Panda, P.; Mangal, A.K.; Reddy, G.; Babu, R. Marine algae: An introduction, food value and medicinal uses. J. Pharm. Res. 2011, 4, 219–221. [Google Scholar]
- Federation, W.E.; American Public Health Association. Standard Methods for the Examination of Water and Wastewater; American Public Health Association (APHA): Washington, DC, USA, 2005. [Google Scholar]
- Godlewska, K.; Michalak, I.; Tuhy, Ł.; Chojnacka, K. Plant growth biostimulants based on different methods of seaweed extraction with water. BioMed Res. Int. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Milton, R.F. Liquid seaweed as a fertilizer. In Proceedings of International Seaweed Symposium; University of Chicago: Chicago, IL, USA, 1964; Volume 4, pp. 428–431. [Google Scholar]
- Hong, D.D.; Hien, H.M.; Son, P.N. Seaweeds from Vietnam used for functional food, medicine and biofertilizer. J. Appl. Phycol. 2007, 19, 817–826. [Google Scholar] [CrossRef]
- Mukherjee, A.; Patel, J.S. Seaweed extract: Biostimulator of plant defense and plant productivi-ty. Int. J. Environ. Sci. Technol. 2020, 17, 553–558. [Google Scholar] [CrossRef]
- Sharma, S.H.S.; Lyons, G.; McRoberts, C.; McCall, D.; Carmichael, E.; Andrews, F.; Swan, R.; McCormack, R.; Mellon, R. Biostimulant activity of brown seaweed species from Strangford Lough: Compositional analyses of polysaccharides and bioassay of extracts using mung bean (Vigno mungo L.) and pak choi (Brassica rapa chinensis L.). J. Appl. Phycol. 2012, 24, 1081–1091. [Google Scholar] [CrossRef]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant properties of seaweed extracts in plants: Implications towards sustainable crop production. Plants 2021, 10, 531. [Google Scholar] [CrossRef] [PubMed]
- Seiber, J.N.; Coats, J.; Duke, S.O.; Gross, A.D. Biopesticides: State of the art and future opportunities. J. Agric. Food Chem. 2014, 62, 11613–11619. [Google Scholar] [CrossRef] [Green Version]
- Godlewska, K.; Pacyga, P.; Michalak, I.; Biesiada, A.; Szumny, A.; Pachura, N.; Piszcz, U. Systematic Investigation of the Effects of Seven Plant Extracts on the Physiological Parameters, Yield, and Nutritional Quality of Radish (Raphanus sativus var. sativus). Front. Plant Sci. 2021, 12, 651152. [Google Scholar] [CrossRef]
- Godlewska, K.; Pacyga, P.; Michalak, I.; Biesiada, A.; Szumny, A.; Pachura, N.; Piszcz, U. Effect of Botanical Extracts on the Growth and Nutritional Quality of Field-Grown White Head Cabbage (Brassica oleracea var. capitata). Molecules 2021, 26, 1992. [Google Scholar] [CrossRef]
- Dutta, P.K.; Ravikumar, M.N.V.; Dutta, J. Chitin and chitosan for versatile applications. J. Macromol. Sci. Part C Polym. Rev. 2002, 42, 307–354. [Google Scholar] [CrossRef]
- Ramírez, M.A.; Rodríguez, A.T.; Alfonso, L.; Peniche, C. Chitin and its derivatives as biopolymers with potential agricultural applications. Biotecnol. Apl. 2010, 27, 270–276. [Google Scholar]
- Prashanth, K.H.; Tharanathan, R.N. Chitin/chitosan: Modifications and their unlimited application potential—An overview. Trends Food Sci. Technol. 2007, 18, 117–131. [Google Scholar] [CrossRef]
- Feng, J.; Zhao, L.; Yu, Q. Receptor-mediated stimulatory effect of oligochitosan in macrophages. Biochem. Biophys. Res. Commun. 2004, 317, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Sharp, R.G. A review of the applications of chitin and its derivatives in agriculture to modify plant-microbial interactions and improve crop yields. Agronomy 2013, 3, 757–793. [Google Scholar] [CrossRef] [Green Version]
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Tzortzakis, N.; Petropoulos, S.A. Sustainable agriculture systems in vegetable production using chitin and chitosan as plant biostimulants. Biomolecules 2021, 11, 819. [Google Scholar] [CrossRef]
- Pandey, P.P.; Sharma, R.; Neelkanthe, S.S. Climate change: Combating drought with antitranspirants and super absorbent. Plant Arch. 2017, 17, 1146–1156. [Google Scholar]
- Shinohara, T.; Leskovar, D.I. Effects of ABA, antitranspirants, heat and drought stress on plant growth, physiology and water status of artichoke transplants. Sci. Hortic. 2014, 165, 225–234. [Google Scholar] [CrossRef]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Alori, E.T.; Babalola, O.O. Microbial inoculants for improve crop quality and human health. Front. Microbiol. 2018, 9, 2213. [Google Scholar] [CrossRef] [Green Version]
- Babalola, O.O.; Glick, B.R. The use of microbial inoculants in African agriculture: Current practice and future prospects. J. Food Agric. Environ. 2012, 10, 540–549. [Google Scholar]
- Bashan, Y.; Holguin, G. Proposal for division of plant growthpromoting rhizobacteria into two classifications: Biocontrol- PGPB (Plant growth-promoting bacteria) and PGPB. Soil Biol. Biochem. 1998, 30, 1225–1228. [Google Scholar] [CrossRef]
- Vessey, J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Berg, G. Plant–microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 2009, 84, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Dodd, I.C.; Ruiz-Lozano, J.M. Microbial enhancement of crop resource use efficiency. Curr. Opin. Biotechnol. 2012, 23, 236–242. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Lifshitz, R.; Zablotowicz, R.M. Free-living bacterial inocula for enhancing crop productivity. Trends Biotechnol. 1989, 7, 39–44. [Google Scholar] [CrossRef]
- Dalmastri, C.; Chiarini, L.; Cantale, C.; Bevivino, A.; Tabacchioni, S. Soil type and maize cultivar affect the genetic diversity of maize root–associated Burkholderia cepacia populations. Microb. Ecol. 1999, 38, 273–284. [Google Scholar] [CrossRef]
- Remans, R.; Beebe, S.; Blair, M.; Manrique, G.; Tovar, E.; Rao, I.; Vanderleyden, J. Physiological and genetic analysis of root responsiveness to auxin-producing plant growth-promoting bacteria in common bean (Phaseolus vulgaris L.). Plant Soil 2008, 302, 149–161. [Google Scholar] [CrossRef]
- Khalid, A.; Arshad, M.; Zahir, Z.A. Screening plant growth-promoting rhizobacteria for improving growth and yield of wheat. J. Appl. Microbiol. 2004, 96, 473–480. [Google Scholar] [CrossRef]
- Shridhar, B.S. RNitrogen fixing microorganisms. Int. J. Microbiol. Res. 2012, 3, 46–52. [Google Scholar]
- Alavi, P.; Starcher, M.; Zachow, C.; Müller, H.; Berg, G. Root-microbe systems: The effect and mode of interaction of stress protecting agent (SPA) Stenotrophomonas rhizophila DSM14405T. Front. Plant Sci. 2013, 4, 141. [Google Scholar] [CrossRef] [Green Version]
- Lucy, M.; Reed, E.; Glick, B.R. Applications of free living plant growth-promoting rhizobacteria. Antonie Van Leeuwenhoek 2004, 86, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Bashan, Y.; De-Bashan, L.E. Plant growth-promoting. Encycl. Soils Environ. 2005, 1, 103–115. [Google Scholar]
- Alam, M.; Khaliq, A.; Sattar, A.; Shukla, R.S.; Anwar, M.; Dharni, S. Synergistic effect of arbuscular mycorrhizal fungi and Bacillus subtilis on the biomass and essential oil yield of rose-scented geranium (Pelargonium graveolens). Arch. Agron. Soil Sci. 2011, 57, 889–898. [Google Scholar] [CrossRef]
- Smith, R.S. Legume inoculant formulation and application. Can. J. Microbiol. 1992, 38, 485–492. [Google Scholar] [CrossRef]
- van Veen, J.A.; van Overbeek, L.S.; van Elsas, J.D. Fate and activity of microorganisms introduced into soil. Microbiol. Mol. Biol. Rev. 1997, 61, 121–135. [Google Scholar] [PubMed]
- Boddey, R.M.; Dobereiner, J. Nitrogen fixation associated with grasses and cereals: Recent progress and perspectives for the future. In Nitrogen Economy in Tropical Soils; Springer: Dordrecht, The Netherlands, 1995; pp. 241–250. [Google Scholar]
- Döbereiner, J. Biological nitrogen fixation in the tropics: Social and economic contributions. Soil Biol. Biochem. 1997, 29, 771–774. [Google Scholar] [CrossRef]
- De Freitas, J.R.; Banerjee, M.R.; Germida, J.J. Phosphate-solubilizing rhizobacteria enhance the growth and yield but not phosphorus uptake of canola (Brassica napus L.). Biol. Fertil. Soils 1997, 24, 358–364. [Google Scholar] [CrossRef]
- Ahemad, M.; Kibret, M. Mechanisms and applications of plant growth promoting rhizobacteria: Current perspective. J. King Saud Univ. Sci. 2014, 26, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Mrkovacki, N.; Milic, V. Use of Azotobacter chroococcum as potentially useful in agricultural application. Ann. Microbiol. 2001, 51, 145–158. [Google Scholar]
- Omar, M.N.A.; Hamouda, A.M.; Mahrous, N.M. Evaluating the efficiency of inoculating some diazotrophs on yield and protein content of 3 wheat cultivars under graded levels of nitrogen fertilization. Ann. Agric. Sci. Ain Shams Univ. 1996, 41, 579–590. [Google Scholar]
- Boddey, R.M.; Polidoro, J.C.; Resende, A.S.; Alves, B.J.; Urquiaga, S. Use of the15N natural abundance technique for the quantification of the contribution of N2 fixation to sugar cane and other grasses. Funct. Plant Biol. 2001, 28, 889–895. [Google Scholar] [CrossRef]
- Baldani, V.D.; Baldani, J.I.; Döbereiner, J. Inoculation of rice plants with the endophytic diazotrophs Herbaspirillum seropedicae and Burkholderia spp. Biol. Fertil. Soils 2000, 30, 485–491. [Google Scholar] [CrossRef]
- Oliveira, A.L.M.; Stoffels, M.; Schmid, M.; Reis, V.M.; Baldani, J.I.; Hartmann, A. Colonization of sugarcane plantlets by mixed inoculations with diazotrophic bacteria. Eur. J. Soil Biol. 2009, 45, 106–113. [Google Scholar] [CrossRef]
- Richardson, A.E. Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Funct. Plant Biol. 2001, 28, 897–906. [Google Scholar] [CrossRef]
- Goldstein, A.H. Recent progress in understanding the molecular genetics and biochemistry of calcium phosphate solubilization by gram negative bacteria. Biol. Agric. Hortic. 1995, 12, 185–193. [Google Scholar] [CrossRef]
- Rodríguez, H.; Fraga, R.; Gonzalez, T.; Bashan, Y. Genetics of phosphate solubilization and its potential applications for improving plant growth-promoting bacteria. Plant Soil 2006, 287, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Kpomblekou, K.; Tabatabai, M.A. Effect of organic acids on release of phosphorus from phosphate rocks. Soil Sci. Soc. Am. J. 1994, 158, 442–453. [Google Scholar] [CrossRef]
- Rodriguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef]
- Richardson, A.E.; Barea, J.M.; McNeill, A.M.; Prigent-Combaret, C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 2009, 321, 305–339. [Google Scholar] [CrossRef]
- Gupta, G.; Parihar, S.S.; Ahirwar, N.K.; Snehi, S.K.; Singh, V. Plant growth promoting rhizobacteria (PGPR): Current and future prospects for development of sustainable agriculture. J. Microb. Biochem. Technol. 2015, 7, 096–102. [Google Scholar]
- Masalha, J.; Kosegarten, H.; Elmaci, Ö.; Mengel, K. The central role of microbial activity for iron acquisition in maize and sunflower. Biol. Fert. Soils 2000, 30, 433–439. [Google Scholar] [CrossRef]
- Sharma, A.; Shankhdar, D.; Shankhdhar, S.C. Enhancing grain iron content of rice by the application of plant growth promoting rhizobacteria. Plant Soil Env. 2013, 59, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, P.N.; Jha, D.K. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef] [PubMed]
- Dodd, I.C.; Zinovkina, N.Y.; Safronova, V.I.; Belimov, A.A. Rhizobacterial mediation of plant hormone status. Ann. Appl. Biol. 2010, 157, 361–379. [Google Scholar] [CrossRef]
- Idris, E.E.; Iglesias, E.J.; Talon, M.; Borriss, R. Tryptophan dependent production of indole-3-acetic acid (IAA) affects level of plant growth promotion by Bacillus amyloliquefaciens FZB42. Mol. Plant Microbe Interact. 2007, 20, 619–626. [Google Scholar] [CrossRef] [Green Version]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Ali, B.; Sabri, A.N.; Ljung, K.; Hasnain, S. Auxin production by plant associated bacteria: Impact on endogenous IAA content and growth of Triticum aestivum L. Lett. Appl. Microbiol. 2009, 48, 542–547. [Google Scholar] [CrossRef]
- Métraux, J.P. Gibberellins and plant cell elongation. In Plant Hormones and Their Role in Plant Growth and Development; Davies, P.J., Ed.; Martinus Nijhoff Publishers: Dordrecht, The Netherlands, 1987; pp. 296–317. [Google Scholar]
- Miyakawa, T.; Fujita, Y.; Yamaguchi-Shinozaki, K.; Tanokura, M. Structure and function of abscisic acid receptors. Trends Plant Sci. 2013, 18, 259–266. [Google Scholar] [CrossRef]
- Spaepen, S.; Vanderleyden, J.; Okon, Y. Plant growth-promoting actions of rhizobacteria. Adv. Bot. Res. 2009, 51, 283–320. [Google Scholar]
- Ortíz-Castro, R.; Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; López-Bucio, J. The role of microbial signals in plant growth and development. Plant Signal. Behav. 2009, 4, 701–712. [Google Scholar] [CrossRef] [Green Version]
- Kai, M.; Haustein, M.; Molina, F.; Petri, A.; Scholz, B.; Piechulla, B. Bacterial volatiles and their action potential. Appl. Microbiol. Biotechnol. 2009, 81, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Fernando, W.G.D.; Ramarathnam, R.; Krishnamoorthy, A.S.; Savchuk, S.C. Identification and use of potential bacterial organic antifungal volatiles in biocontrol. Soil Biol. Biochem. 2005, 37, 955–964. [Google Scholar] [CrossRef]
- Vespermann, A.; Kai, M.; Piechulla, B. Rhizobacterial volatiles affect the growth of fungi and Arabidopsis thaliana. Appl. Environ. Microbiol. 2007, 73, 5639–5641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, C.-M.; Farag, M.A.; Hu, C.-H.; Reddy, M.S.; Wei, H.-X.; Paré, P.W.; Kloepper, J.W. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927–4932. [Google Scholar] [CrossRef] [PubMed]
- Remy, W.; Taylor, T.N.; Hass, H.; Kerp, H. Four hundred million-year-old vesicular arbuscular mycorrhizae. Proc. Natl. Acad. Sci. USA 1994, 91, 11841–11843. [Google Scholar] [CrossRef] [Green Version]
- Schüßler, A.; Schwarzott, D.; Walker, C. A new fungal phylum, the Glomeromycota: Phylogeny and evolution. Mycol. Res. 2001, 105, 1413–1421. [Google Scholar] [CrossRef] [Green Version]
- Bonfante, P.; Genre, A. Interactions in mycorrhizal symbiosis. Nat. Commun. 2010, 1, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Behie, S.W.; Bidochka, M.J. Nutrient transfer in plant-fungal symbioses. Trends Plant Sci. 2014, 19, 734–740. [Google Scholar] [CrossRef]
- Augé, R.M. Water relations, drought and vesicular arbuscular mycorrhizal symbiosis. Mycorrhiza 2001, 11, 3–42. [Google Scholar] [CrossRef]
- Gianinazzi, S.; Gollotte, A.; Binet, M.-N.; van Tuinen, D.; Redecker, D.; Wipf, D. Agroecology: The key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 2010, 20, 519–530. [Google Scholar] [CrossRef]
- Hamel, C.; Plenchette, C. Mycorrhizae in Crop Production; The Haworth Press Inc.: New York, NY, USA, 2007. [Google Scholar]
- Harrier, L.A.; Watson, C.A. The potential role of arbuscular mycorrhizal (AM)fungi in the bioprotection of plants against soil-borne pathogens in organicand/or other sustainable farming systems. Pest Manag. Sci. 2004, 60, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, Z.A.; Akhtar, M.S.; Futai, K. Mycorrhizae: Sustainable Agricultureand Forestry; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- van der Heijden, M.G.A.; Van Der Streitwolf-engel, R.; Riedl, R.; Siegrist, S.; Neudecker, A.; Boller, T.; Wiemken, A.; Sanders, I.R. The mycorrhizalcontribution to plant productivity, plant nutrition and soil structure inexperimental grassland. New Phytol. 2004, 172, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.; Gilbert, L. Interplant signalling through hyphal networks. New Phytol. 2015, 205, 1448–1453. [Google Scholar] [CrossRef] [PubMed]
- Simard, S.W.; Beiler, K.J.; Bingham, M.A.; Deslippe, J.R.; Philip, L.J.; Teste, F.P. Mycorrhizal networks: Mechanisms, ecology and modelling. Fungal Biol. Rev. 2012, 26, 39–60. [Google Scholar] [CrossRef]
- Querejeta, J.I.; Barea, J.M.; Allen, M.F.; Caravaca, F.; Roldan, A. Differential response of δ13C and water use efficiency to arbuscular mycorrhizal infection in two arid land woody plant species. Oecologia 2003, 135, 510–515. [Google Scholar] [CrossRef]
- Gamalero, E.; Berta, G.; Glick, B. The use of microorganisms to facilitate the growth of plants in saline soils. In Microbial Strategies for Crop Improvement; Khan, M.S., Zaidi, A., Musarrat, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–22. [Google Scholar]
- Brundrett, M.C. Mycorrhizas in natural ecosystems. In Advances in Ecological Research; Macfayden, A., Begoon, M., Fitter, A.H., Eds.; Academic Press: London, UK, 1991; pp. 376–391. [Google Scholar]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress toler-ance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef] [Green Version]
- Leyval, C.; Turnau, K.; Haselwandter, K. Effect of heavy metal pollution on mycorrhizal colonization and function: Physiological, ecological and applied aspects. Mycorrhiza 1997, 7, 139–153. [Google Scholar] [CrossRef]
- Vivas, A.; Marulanda, A.; Ruiz-Lozano, J.; Barea, J.; Azcón, R. Influence of a Bacillus sp. on physiological activities of two arbuscular mycorrhizal fungi and on plant responses to PEG induced drought stress. Mycorrhiza 2003, 13, 249–256. [Google Scholar] [CrossRef]
- Dalpé, Y.; Monreal, M. Arbuscular mycorrhiza inoculum to support sustainable cropping systems. Crop Manag. Netw. 2004, 3, 1–11. [Google Scholar]
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef] [Green Version]
- Berg, G.; Alavi, M.; Schmid, M.; Hartmann, A. The rhizosphere as a reservoir for opportunistic human pathogenic bacteria. In Molecular Microbial Ecology of the Rhizosphere; De Bruijn, F.J., Ed.; Wiley: New York, NY, USA, 2013; pp. 1209–1212. [Google Scholar]
- Selvakumar, G.; Panneerselvam, P.; Ganeshamurthy, A.N. Biosafety of novel biofertilizers. J. Biofertil. Biopestici. 2014, 5, 145. [Google Scholar]
- Banerjee, D.; Stableforth, D. The treatment of respiratory Pseudomonas infection in cystic fibrosis. Drugs 2000, 60, 1053–1064. [Google Scholar] [CrossRef]
- de Blackburn, C.W.; McClure, P.J. Pathogenic Bacillus species. In Foodborne Pathogens; Woodhead Publishing: Sorston, UK, 2009; pp. 844–888. [Google Scholar]
- Janda, J.M.; Abbott, S.L. The genus Aeromonas: Taxonomy, pathogenicity, and infection. Clin. Microbiol. Rev. 2010, 23, 35–73. [Google Scholar] [CrossRef] [Green Version]
- Ryan, M.P.; Sevjahova, L.; Gorman, R.; White, S. The Emergence of the Genus Comamonas as Important Opportunistic Pathogens. Pathogens 2022, 11, 1032. [Google Scholar] [CrossRef]
- Herbrík, A.; Corretto, E.; Chroňáková, A.; Langhansová, H.; Petrásková, P.; Hrdý, J.; Čihák, M.; Krištůfek, V.; Bobek, J.; Petříček, M.; et al. A human lung-associated streptomyces sp. TR1341 produces various secondary metabolites responsible for virulence, cytotoxicity and modulation of immune response. Front. Microbiol. 2020, 10, 3028. [Google Scholar] [CrossRef] [Green Version]
- Hatvani, L.; Manczinger, L.; Vágvölgyi, C.; Kredics, L. Trichoderma as a human pathogen. Trichoderma Biol. Appl. 2013, 17, 292–313. [Google Scholar]
- Salimiyanrizi, K.; Ghazvini, K.; Farsiani, H. Clinical and pathogenesis overview of Enterobacter infections. Rev. Clin. Med. 2020, 6, 146–154. [Google Scholar]
- Keswani, C.; Prakash, O.; Bharti, N.; Vílchez, J.I.; Sansinenea, E.; Lally, R.D.; Borriss, R.; Singh, S.P.; Gupta, V.K.; Fraceto, L.F.; et al. Re-addressing the biosafety issues of plant growth promoting rhizobacteria. Sci. Total Environ. 2019, 690, 841–852. [Google Scholar] [CrossRef]
- Berg, G.; Eberl, L.; Hartmann, A. The rhizosphere as a reservoir for opportunistic human pathogenic bacteria. Environ. Microbiol. 2005, 7, 1673–1685. [Google Scholar] [CrossRef]
- Stuart, T.L.; Sandhu, J.; Stirling, R.; Corder, J.; Ellis, A.; Misa, P.; Goh, S.; Wong, B.; Martiquet, P.; Hoang, L.; et al. Campylobacteriosis outbreak associated with ingestion of mud during a mountain bike race. Epidemiol. Infect. 2010, 138, 1695–1703. [Google Scholar] [CrossRef] [Green Version]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States–major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Tindall, B.J.; Rossello-Mo’ra, R.; Busse, H.J.; Ludwig, W.; Kämpfer, P. Notes on the characterization of prokaryote strains for taxonomic purposes. Int. J. Syst. Evol. Microbiol. 2010, 60, 249–266. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Oh, H.S.; Park, S.C.; Chun, J. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int. J. Syst. Evol. Microbiol. 2014, 64, 346–351. [Google Scholar] [CrossRef] [PubMed]
- BAuA-TRB-466, German Federal Institute for Occupational Safety and Health Technical Rule for biological agents (TRBA) # 466: Classification of Prokaryotes (Bacteria and Archaea) into Risk Groups. 2015. Available online: https://www.baua.de/DE/Angebote/Rechtstexte-und-Technische/Regeln/Regelwerk/TRBA/TRBA-466.html (accessed on 27 October 2022).
- Pseudomonas putida (Trevisan) Migula (ATCC 12633). Available online: https://www.atcc.org/products/12633- (accessed on 27 October 2022).
- ZKBS, The Central Committee on Biological Safety: Database of Safety-Assessed Microorganisms; Federal Office of Consumer Protection and Food Safety. Available online: https://zag.bvl.bund.de/organismen/index.jsf (accessed on 17 September 2022).
- Pseudomonas Putida. Available online: https://dir.indiamart.com/search.mp?ss=pseudomonas+putida&prdsrc=1 (accessed on 27 October 2022).
- Lally, R.D.; Galbally, P.; Moreira, A.S.; Spink, J.; Ryan, D.; Germaine, K.J.; Dowling, D.N. Application of endophytic Pseudomonas fluorescens and a bacterial consortium to Brassica napus can increase plant height and biomass under greenhouse and field conditions. Front. Plant Sci. 2017, 8, 2193. [Google Scholar] [CrossRef] [Green Version]
- Santana-Fernández, A.; Beovides-García, Y.; Simó-González, J.E.; Pérez-Peñaranda, M.C.; López-Torres, J.; Rayas-Cabrera, A.; Santos-Pino, A.; Basail-Pérez, M. Effect of a Pseudomonas fluorescens-based Biofertilizer on Sweet Potato Yield Components. Asian J. Appl. Sci. 2021, 9, 105–113. [Google Scholar] [CrossRef]
- Hasani, H.; Aminpanah, H. Effect of Pseudomonas fluorescens Inoculation on Yield and Yield Components of Rice (Oryza sativa L.) under Different Levels of Phosphorus Fertilizer. J. Agric. Sci. 2015, 48, 157–163. [Google Scholar]
- Pseudomonas fluorescens Migula (ATCC 13525). Available online: https://www.atcc.org/products/13525 (accessed on 27 October 2022).
- Pseudomonas Fluorescens Biocontrol Agents. Available online: https://www.manidharmabiotech.com/bio-control-agents.html (accessed on 27 October 2022).
- Rungin, S.; Indananda, C.; Suttiviriya, P.; Kruasuwan, W.; Jaemsaeng, R.; Thamchaipenet, A. Plant growth enhancing effects by a siderophore-producing endophytic streptomycete isolated from a Thai jasmine rice plant (Oryza sativa L. cv. KDML105). Antonie Van Leeuwenhoek 2012, 102, 463–472. [Google Scholar] [CrossRef]
- Verma, V.C.; Singh, S.K.; Prakash, S. Bio-control and plant growth promotion potential of siderophore producing endophytic Streptomyces from Azadirachta indica A. Juss J. Basic Microbiol. 2011, 51, 550–556. [Google Scholar] [CrossRef]
- Streptomyces azureus Kelley et al. (ATCC 14921). Available online: https://www.atcc.org/atcc/productsheetpdf/generatehtmlpdf/14921 (accessed on 27 October 2022).
- Azospirillum brasilense Tarrand et al. (ATCC 29145). Available online: https://www.atcc.org/products/29145 (accessed on 27 October 2022).
- Sardar Liquid Biofertilizers. Available online: https://www.gsfcagrotech.com/sardar-liquid-biofertilizers/ (accessed on 27 October 2022).
- Mehnaz, S.; Mirza, M.S.; Haurat, J.; Bally, R.; Normand, P.; Bano, A.; Malik, K.A. Isolation and 16S rRNA sequence analysis of the beneficial bacteria from the rhizosphere of rice. Can. J. Microbiol. 2001, 472, 110–117. [Google Scholar] [CrossRef]
- Aeromonas hydrophila (Chester) Stanier (ATCC 7966). Available online: https://www.atcc.org/products/7966 (accessed on 27 October 2022).
- Barazani, O.; Friedman, J. Is IAA the major root growth factor secreted from plant-growth-mediating bacteria? J. Chem. Ecol. 1999, 25, 2397–2406. [Google Scholar] [CrossRef]
- Comamonas sp. (ATCC 700038). Available online: https://www.atcc.org/products/700038 (accessed on 27 October 2022).
- Arkhipova, T.N.; Veselov, S.U.; Melentiev, A.I.; Martynenko, E.V.; Kudoyarova, G.R. Ability of bacterium Bacillus subtilis to produce cytokinins and to influence the growth and endogenous hormone content of lettuce plants. Plant Soil 2005, 272, 201–209. [Google Scholar] [CrossRef]
- Bacillus subtilis (Ehrenberg) Cohn (ATCC 6051). Available online: https://www.atcc.org/products/6051 (accessed on 27 October 2022).
- BIOSUBTILIN. Available online: https://www.biotech-int.com/biotech/detail_sheet1d95.html?page=biosubtilin_rograkshak (accessed on 27 October 2022).
- Hussain, A.; Hasnain, S. Cytokinin production by some bacteria: Its impact on cell division in cucumber cotyledons. Afr. J. Microbiol. Res. 2009, 3, 704–712. [Google Scholar]
- Bacillus licheniformis (Weigmann) Chester (ATCC 14580). Available online: https://www.atcc.org/products/14580 (accessed on 27 October 2022).
- Fulchieri, M.; Lucangeli, C.; Bottini, R. Inoculation with Azospirillum lipoferum affects growth and gibberellin status of corn seedling roots. Plant Cell Physiol. 1993, 34, 1305–1309. [Google Scholar]
- Azospirillum lipoferum (Beijerinck) Tarrand et al. (ATCC 29707). Available online: https://www.atcc.org/products/29707 (accessed on 27 October 2022).
- AgriLife Nitrofix®—RJ. Available online: https://www.agrilife.in/products/agrilife_nitrofix_rj.php (accessed on 27 October 2022).
- Bacilio, M.; Rodriguez, H.; Moreno, M.; Hernandez, J.P.; Bashan, Y. Mitigation of salt stress in wheat seedlings by a gfp-tagged Azospirillum lipoferum. Biol. Fert. Soils 2004, 40, 188–193. [Google Scholar] [CrossRef]
- Marulanda, A.; Barea, J.-M.; Azcón, R. Stimulation of plant growth and drought tolerance by native microorganisms (AM fungi and bacteria) from dry environments:mechanisms related to bacterial effectiveness. J. Plant Growth Regul. 2009, 28, 115–124. [Google Scholar] [CrossRef]
- Bacillus megaterium de Bary (ATCC 14581). Available online: https://www.atcc.org/products/14581 (accessed on 27 October 2022).
- P Sol B®. Available online: https://www.agrilife.in/products/psolb-bm.php (accessed on 27 October 2022).
- Bae, H.; Sicher, R.C.; Kim, M.S.; Kim, S.-H.; Strem, M.D.; Melnick, R.L.; Bailey, B.A. The beneficial endophyte Trichoderma hamatum isolate DIS 219b promotes growth and delays the onset of the drought response in Theobroma cacao. J. Exp. Bot. 2009, 60, 3279–3295. [Google Scholar] [CrossRef]
- Singh, L.P.; Gill, S.S.; Tuteja, N. Unraveling the role of fungal symbionts in plant abiotic stress tolerance. Plant Signal. Behav. 2011, 6, 175–191. [Google Scholar] [CrossRef] [Green Version]
- Alternaria sp. (ATCC 20831). Available online: https://www.atcc.org/products/20831 (accessed on 27 October 2022).
- Trichoderma harzianum Rifai (ATCC 60850). Available online: https://www.atcc.org/products/60850 (accessed on 27 October 2022).
- BIODERMA-H. Available online: https://www.biotech-int.com/biofungicides.html (accessed on 27 October 2022).
- Ecosom®-, TV. Available online: https://www.agrilife.in/products/microbial_ecosom_tv2.php (accessed on 27 October 2022).
- Ecosom®-, TH. Available online: http://agrilife.in/products/microbial_ecosom_th1.php (accessed on 27 October 2022).
- Dal Cortivo, C.; Ferrari, M.; Visioli, G.; Lauro, M.; Fornasier, F.; Barion, G.; Panozzo, A.; Vamerali, T. Effects of seed-applied biofertilizers on rhizosphere biodiversity and growth of common wheat (Triticum aestivum L.) in the field. Front. Plant Sci. 2020, 11, 72. [Google Scholar] [CrossRef] [Green Version]
- Azoarcus oleivorans (ATCC BAA2411). Available online: https://www.atcc.org/products/baa-2411 (accessed on 27 October 2022).
- Dal Cortivo, C.; Barion, G.; Visioli, G.; Mattarozzi, M.; Mosca, G.; Vamerali, T. Increased root growth and nitrogen accumulation in common wheat following PGPR inoculation: Assessment of plant-microbe interactions by ESEM. Agric. Ecosyst. Environ. 2017, 247, 396–408. [Google Scholar] [CrossRef]
- Azorhizobium caulinodans Dreyfus et al. (ATCC 43989). Available online: https://www.atcc.org/products/43989 (accessed on 27 October 2022).
- Favero, V.O.; Carvalho, R.H.; Motta, V.M.; Leite, A.B.C.; Coelho, M.R.R.; Xavier, G.R.; Rumjanek, N.G.; Urquiaga, S. Bradyrhizobium as the only rhizobial inhabitant of mung bean (Vigna radiata) nodules in tropical soils: A strategy based on microbiome for improving biological nitrogen fixation using bio-products. Front. Plant Sci. 2021, 11, 602645. [Google Scholar] [CrossRef]
- Alkurtany, A.; Ali, S.; Mahdi, W. The efficiency of prepared biofertilizer from local isolate of Bradyrhizobium sp on growth and yield of mungbean plant. Iraqi J. Agric. Sci. 2018, 49, 722–730. [Google Scholar] [CrossRef]
- Bradyrhizobium sp. (ATCC 10319). Available online: https://www.atcc.org/products/10319 (accessed on 27 October 2022).
- Das, K.; Prasanna, R.; Saxena, A.K. Rhizobia: A potential biocontrol agent for soilborne fungal pathogens. Folia Microbiol. 2017, 62, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Mondal, M.; Skalicky, M.; Garai, S.; Hossain, A.; Sarkar, S.; Banerjee, H.; Kundu, R.; Brestic, M.; Barutcular, C.; Erman, M. Supplementing nitrogen in combination with rhizobium inoculation and soil mulch in peanut (Arachis hypogaea L.) production system: Part II. Effect on phenology, growth, yield attributes, pod quality, profitability and nitrogen use efficiency. Agronomy 2020, 10, 1513. [Google Scholar] [CrossRef]
- Rhizobium sp. (ATCC BAA-868). Available online: https://www.atcc.org/products/baa-868 (accessed on 27 October 2022).
- Biobium. Available online: https://www.biotech-int.com/biofertilizers.html (accessed on 27 October 2022).
- Igiehon, N.O.; Babalola, O.O.; Aremu, B.R. Genomic insights into plant growth promoting rhizobia capable of enhancing soybean germination under drought stress. BMC Microbiol. 2019, 19, 159. [Google Scholar] [CrossRef] [Green Version]
- Rhizobium leguminosarum Jordan (ATCC 10004). Available online: https://www.atcc.org/products/10004 (accessed on 27 October 2022).
- Ali, M.A.; Ilyas, F.; Arshad, M.; Hussain, S.; Iqbal, M.; Ahmad, S.; Saboor, A.; Mustafa, G.; Ahmed, N. Microbial inoculation of seeds for better plant growth and productivity. In Priming and Pretreatment of Seeds and Seedlings; Springer: Berlin/Heidelberg, Germany, 2019; pp. 523–550. [Google Scholar]
- Rahman, M.; Sabir, A.A.; Mukta, J.A.; Khan, M.M.A.; Mohi-Ud-Din, M.; Miah, M.G.; Rahman, M.; Islam, M.T. Plant probiotic bacteria Bacillus and Paraburkholderia improve growth, yield and content of antioxidants in strawberry fruit. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Bacillus sp. (ATCC 19659). Available online: https://www.atcc.org/products/19659 (accessed on 27 October 2022).
- Si Sol B®, Silica Solubilizing Bacteria. Available online: https://www.agrilife.in/products/sisolb.php (accessed on 27 October 2022).
- Jain, D.; Sharma, J.; Kaur, G.; Bhojiya, A.A.; Chauhan, S.; Sharma, V.; Suman, A.; Mohanty, S.R.; Maharjan, E. Phenetic and molecular diversity of nitrogen fixating plant growth promoting Azotobacter isolated from semiarid regions of India. BioMed Res. Int. 2021, 2021, 1–9. [Google Scholar] [CrossRef]
- Azotobacter chroococcum Beijerinck (ATCC 9043). Available online: https://www.atcc.org/products/9043 (accessed on 27 October 2022).
- Green® Earth, REAP ®. Available online: https://www.indiamart.com/ncsgreenearthprivatelimited/bio-fertilizer.html#17855591033 (accessed on 27 October 2022).
- Azotobacter vinelandii Lipman (ATCC 478). Available online: https://www.atcc.org/products/478 (accessed on 27 October 2022).
- Fahsi, N.; Mahdi, I.; Mesfioui, A.; Biskri, L.; Allaoui, A. Plant Growth-Promoting Rhizobacteria isolated from the Jujube (Ziziphus lotus) plant enhance wheat growth, Zn uptake, and heavy metal tolerance. Agriculture 2021, 11, 316. [Google Scholar] [CrossRef]
- Enterobacter cloacae (Jordan) Hormaeche and Edwards (ATCC BAA-2341). Available online: https://www.atcc.org/products/baa-2341 (accessed on 27 October 2022).
- Pseudomonas frederiksbergensis Andersen et al. (ATCC BAA-257). Available online: https://www.atcc.org/products/baa-257 (accessed on 27 October 2022).
- Amor, D.R.; Ratzke, C.; Gore, J. Transient inoculants can induce shifts between alternative stable states of microbial communities. Sci. Adv. 2020, 6, eaay8676. [Google Scholar] [CrossRef] [Green Version]
- McNally, L.; Brown, S.P. Building the microbiome in health and disease: Niche construction and social conflict in bacteria. Philosophical Transactions of the Royal Society of London. Ser. B Biol. Sci. 2015, 370, 20140298. [Google Scholar] [CrossRef]
- Kurkjian, H.M.; Akbari, M.J.; Momeni, B. The impact of interactions on invasion and colonization resistance in microbial communities. PLoS Comput. Biol. 2021, 17, e1008643. [Google Scholar] [CrossRef]
- Walsh, U.F.; Moe¨nne-Loccoz, Y.; Tichy, H.V.; Gardner, A.; Corkery, D.M.; Lorkhe, S.; O’Gara, F. Residual impact of the biocontrol inoculant Pseudomonas fluorescens F113 on the resident population of rhizobia nodulating a red clover rotation crop. Microbiol. Ecol. 2003, 45, 145–155. [Google Scholar] [CrossRef]
- Albright, M.B.N.; Sevanto, S.; Gallegos-Graves, L.V.; Dunbar, J. Biotic interactions are more important than propagule pressure in microbial community invasions. mBio 2020, 11, e02089-20. [Google Scholar] [CrossRef]
- Lee, D.G.; Urbach, J.M.; Wu, G.; Liberati, N.T.; Feinbaum, R.L.; Miyata, S.; Diggins, L.T.; He, J.; Saucier, M.; Déziel, E.; et al. Genomic analysis reveals that Pseudomonas aeruginosa virulence is combinatorial. Genome Biol. 2006, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Baldini, R.L.; Rahme, L.G. Common mechanisms for pathogens of plants and animals. Annu. Rev. Phytopathol. 2001, 39, 259–284. [Google Scholar] [CrossRef]
- Jorgensen, J.H.; Ferraro, M.J.; McElmeel, M.L.; Spargo, J.; Swenson, J.M.; Tenover, F.C. Detection of penicillin and extended-spectrum cephalosporin resistance among Streptococcus pneumoniae clinical isolates by use of the E test. J. Clin. Microbiol. 1994, 32, 159–163. [Google Scholar] [CrossRef] [Green Version]
- Orenga, S.; James, A.L.; Manafi, M.; Perry, J.D.; Pincus, D.H. Enzymatic substrates in microbiology. J. Microbiol. Methods 2009, 79, 139–155. [Google Scholar] [CrossRef]
- Perry, J.D. A decade of development of chromogenic culture media for clinical microbiology in an era of molecular diagnostics. Clin. Microbiol. Rev. 2017, 30, 449–479. [Google Scholar] [CrossRef] [Green Version]
- Váradi, L.; Luo, J.L.; Hibbs, D.E.; Perry, J.D.; Anderson, R.J.; Orenga, S.; Groundwater, P.W. Methods for the detection and identification of pathogenic bacteria: Past, present, and future. Chem. Soc. Rev. 2017, 46, 4818–4832. [Google Scholar] [CrossRef]
- Kempf, V.A.; Trebesius, K.; Autenrieth, I.B. Fluorescent in situ hybridization allows rapid identification of microorganisms in blood cultures. J. Clin. Microbiol. 2000, 38, 830–838. [Google Scholar] [CrossRef] [Green Version]
- Harris, D.M.; Hata, D.J. Rapid identification of bacteria and Candida using PNA-FISH from blood and peritoneal fluid cultures: A retrospective clinical study. Ann. Clin. Microbiol. Antimicrob. 2013, 12, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Brown, E.W.; González-Escalona, N. Comparison of real-time PCR, reverse transcriptase real-time PCR, loop-mediated isothermal amplification, and the FDA conventional microbiological method for the detection of Salmonella spp. in produce. Appl. Environ. Microbiol. 2011, 77, 6495–6501. [Google Scholar] [CrossRef] [PubMed]
- Blaschke, A.J.; Heyrend, C.; Byington, C.L.; Fisher, M.A.; Barker, E.; Garrone, N.F.; Thatcher, S.A.; Pavia, A.T.; Barney, T.; Alger, G.D.; et al. Rapid identification of pathogens from positive blood cultures by multiplex polymerase chain reaction using the Film Array system. Diagn. Microbiol. Infect. Dis. 2012, 74, 349–355. [Google Scholar] [CrossRef] [Green Version]
- Ambardar, S.; Gupta, R.; Trakroo, D.; Lal, R.; Vakhlu, J. High throughput sequencing: An overview of sequencing chemistry. Indian J. Microbiol. 2016, 56, 394–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moorthie, S.; Mattocks, C.J.; Wright, C.F. Review of massively parallel DNA sequencing technologies. HUGO J. 2011, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Llarena, F.J.; Bou, G. Proteomics as a tool for studying bacterial virulence and antimicrobial resistance. Front. Microbiol. 2016, 7, 410. [Google Scholar] [CrossRef] [Green Version]
- Doern, C.D.; Butler-Wu, S.M. Emerging and future applications of matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry in the clinical microbiology laboratory: A report of the association for molecular pathology. J. Mol. Diagn. 2016, 18, 789–802. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.S.; Johnson, C.L.; Pritchard, L.; Hepler, R.; Ton, T.T.; Dunn, J.J. Performance of the Verigene® enteric pathogens test, Biofire FilmArray™ gastrointestinal panel and Luminex xTAG® gastrointestinal pathogen panel for detection of common enteric pathogens. Diagn. Microbiol. Infect. Dis. 2016, 86, 336–339. [Google Scholar] [CrossRef]
- Miller, R.R.; Montoya, V.; Gardy, J.L.; Patrick, D.M.; Tang, P. Metagenomics for pathogen detection in public health. Genome Med. 2013, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Bi, H.; Liu, B.; Qiao, L. Detection of pathogenic microorganisms by microfluidics based analytical methods. Anal. Chem. 2018, 90, 5512–5520. [Google Scholar] [CrossRef]
- Feng, X.; Du, W.; Luo, Q.; Liu, B.F. Microfluidic chip: Next-generation platform for systems biology. Anal. Chim. Acta 2009, 650, 83–97. [Google Scholar] [CrossRef]
- Arjjumend, H.; Koutouki, K.; Donets, O. Comparative Advantages of Using Biopesticides in Ukrainian Agroecosystems. Eur. J. Agric. Food Sci. 2020, 2, 111. [Google Scholar] [CrossRef]
- Arjjumend, H.; Koutouki, K.; Getman, A.; Donets, O. Legal Barriers in the Business of Biofertilizers and Biopesticides in Ukraine. EU Agrar. Law 2020, 9, 1–6. [Google Scholar] [CrossRef]
- Malusá, E.; Vassilev, N. A contribution to set a legal framework for biofertilizers. Appl. Microbiol. Biotechnol. 2014, 98, 6599–6607. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, A. Biopesticides for integrated crop management: Environmental and regulatory aspects. J. Fertil. Pestic. 2014, 5, 1–3. [Google Scholar]
- Suh, J.S.; Jiarong, P.; Toan, P.V. Quality control of biofertilizers. Biofertilizers Manual. Forum Nucl. Coop. Asia Jpn. 2006, 112–115. [Google Scholar]
| Year | 2012 [13] | 2014 [8] | 2015 [1] | 2017 [15] | 2018 [16] |
|---|---|---|---|---|---|
| Basis of categorization | Bibliographic analysis | A critical review of selected scientific publications | Substances and microorganisms | Type of products | Plant nutrition |
| Role of microbes | No | Yes | Yes | Yes | Yes |
| Categories | 1. HS 2. Complex organic materials 3. Beneficial chemical elements 4. Inorganic salts 5. SWE 6. Chitin and chitosan derivatives 7. Antitranspirants 8. Free amino acids and other N-containing substances | 1. Humic acids 2. Fulvic acids 3. Microbial inoculants 4. PHs and amino acids 5. SWE | 1. Humic and fulvic acids 2. Protein hydrolysates and other N-containing compounds 3. Seaweed extracts and botanicals 4. Chitosan and other biopolymers 5. Inorganic compounds 6. Beneficial fungi 7. Beneficial bacteria | 1. Non-microbial (i) SWE (ii) HS (iii) Phosphite and other inorganic salts (iv) Chitin and chitosan derivatives (v) Antitranspirants (vi) PHs and free amino acids (vii) Complex organic materials 2. Microbial (i) PGPR (ii) Non-pathogenic fungi (iii) AMF (iv) Protozoa and nematodes | 1. HS 2. PHs 3. SWE 4. PGPR 5. AMF |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumari, M.; Swarupa, P.; Kesari, K.K.; Kumar, A. Microbial Inoculants as Plant Biostimulants: A Review on Risk Status. Life 2023, 13, 12. https://doi.org/10.3390/life13010012
Kumari M, Swarupa P, Kesari KK, Kumar A. Microbial Inoculants as Plant Biostimulants: A Review on Risk Status. Life. 2023; 13(1):12. https://doi.org/10.3390/life13010012
Chicago/Turabian StyleKumari, Menka, Preeti Swarupa, Kavindra Kumar Kesari, and Anil Kumar. 2023. "Microbial Inoculants as Plant Biostimulants: A Review on Risk Status" Life 13, no. 1: 12. https://doi.org/10.3390/life13010012
APA StyleKumari, M., Swarupa, P., Kesari, K. K., & Kumar, A. (2023). Microbial Inoculants as Plant Biostimulants: A Review on Risk Status. Life, 13(1), 12. https://doi.org/10.3390/life13010012