Clinical Guideline on Perioperative Management of Patients with Advanced Chronic Liver Disease
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Section 1. Preoperative Evaluation and Management
3.1.1. Pathophysiology
3.1.2. Diagnosis of Advanced Chronic Liver Disease
3.1.3. Surgical Risk Prediction
3.1.4. Preoperative Management
3.1.5. Assessment of Coagulation Disorders
3.2. Section 2. Surgical Procedures
3.2.1. General Recommendations
3.2.2. Elective Surgery
3.2.3. Emergent Surgery
3.3. Section 3. Intraoperative Management
3.3.1. Anaesthetic Technique
3.3.2. Loco-Regional Anaesthesia
3.3.3. Cardiovascular Assessment and Intraoperative Monitoring
3.3.4. Fluids and Vasopressors during Surgery
3.4. Section 4. General Recomendations in Postoperative
3.4.1. Transfusion Strategy in Acute Bleeding after Surgery
3.4.2. Venous Thromboembolism Prophylaxis
3.4.3. Pain Control
| Analgesic Drug | General Considerations | Dosing Recommendation |
|---|---|---|
| Acetaminophen/ paracetamol | Safe [94,100] | Not exceed the daily dose of 2–3 g [94] |
| NSAID 1 | Avoid use [94] | Avoid use [94] |
| Metamizole/ dipyrone | Avoid use [98] | Avoid use [98] |
| Codeine | Avoid use | Avoid use [94] |
| Tramadol | Use with careful monitoring in patients taking selective serotonin reuptake inhibitors or tricyclic antidepressant. Avoid in patients with seizure history [77,94] | Start with 50 mg/day. Maximum 200 mg/d [101] |
| Fentanyl | Use with caution in patients with moderate liver disease [77,100] | Start with half of usual dose [101] |
| Morphine | Use with caution Avoid in renal disease Avoid extended release formulations [94] | Start with half of usual dose [101] |
| Oxycodone | Use with caution Avoid extended release formulations [94] | Start with a quarter of usual dose [101] |
| Meperidine | Avoid use [94] | Avoid use [94] |
3.4.4. Nutrition
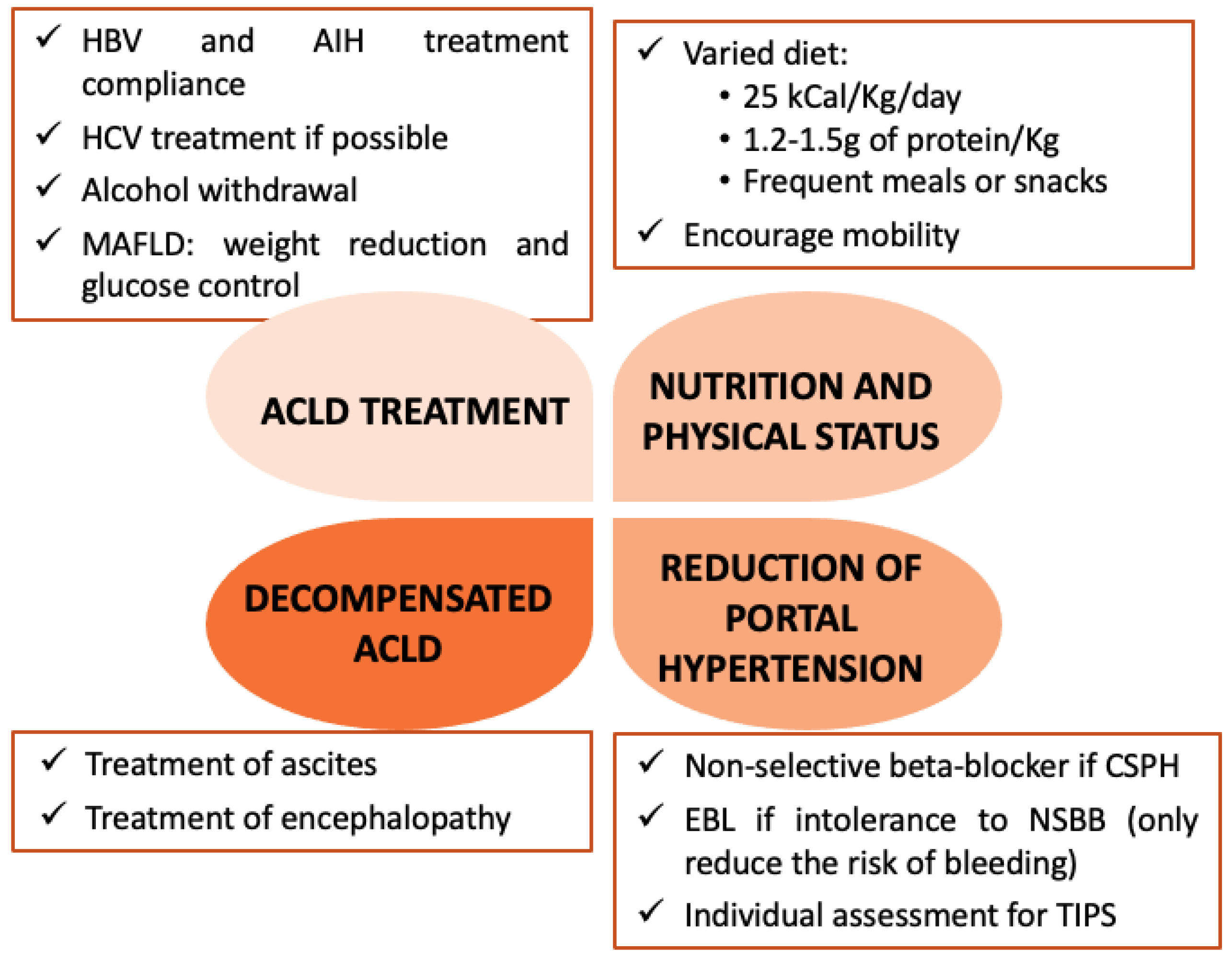
3.5. Section 5. Postoperative Management of Hepatic Decompensation and ACLD Complications
3.5.1. Acute Kidney Injury
3.5.2. Encephalopathy
3.5.3. Ascites
3.5.4. Acute-on-Chronic Liver Failure
4. Discussion
4.1. Limitations
4.2. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Aller, R.; Fernández-Rodríguez, C.; Iacono, O.L.; Bañares, R.; Abad, J.; Carrión, J.A.; García-Monzón, C.; Caballería, J.; Berenguer, M.; Rodríguez-Perálvez, M.; et al. Consensus document. Management of non-alcoholic fatty liver disease (NAFLD). Clinical practice guideline. Gastroenterol. Hepatol. 2018, 41, 328–349. [Google Scholar] [CrossRef] [PubMed]
- Collaborators GBDC. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 245–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsochatzis, E.A.; Bosch, J.; Burroughs, A.K. Liver cirrhosis. Lancet 2014, 383, 1749–1761. [Google Scholar] [CrossRef]
- Gines, P.; Krag, A.; Abraldes, J.G.; Sola, E.; Fabrellas, N.; Kamath, P.S. Liver cirrhosis. Lancet 2021, 398, 1359–1376. [Google Scholar] [CrossRef]
- Bhangui, P.; Laurent, A.; Amathieu, R.; Azoulay, D. Assessment of risk for non-hepatic surgery in cirrhotic patients. J. Hepatol. 2012, 57, 874–884. [Google Scholar] [CrossRef] [Green Version]
- Engelmann, C.; Clària, J.; Szabo, G.; Bosch, J.; Bernardi, M. Pathophysiology of decompensated cirrhosis: Portal hypertension, circulatory dysfunction, inflammation, metabolism and mitochondrial dysfunction. J. Hepatol. 2021, 75 (Suppl. 1), S49–S66. [Google Scholar] [CrossRef]
- Bernal, W.; Karvellas, C.; Saliba, F.; Saner, F.H.; Meersseman, P. Intensive care management of acute-on-chronic liver failure. J. Hepatol. 2021, 75 (Suppl. 1), S163–S177. [Google Scholar] [CrossRef]
- Northup, P.G.; Friedman, L.S.; Kamath, P.S. AGA Clinical Practice Update on Surgical Risk Assessment and Perioperative Management in Cirrhosis: Expert Review. Clin. Gastroenterol. Hepatol. 2019, 17, 595–606. [Google Scholar] [CrossRef] [Green Version]
- Newman, K.L.; Johnson, K.M.; Cornia, P.B.; Wu, P.; Itani, K.; Ioannou, G.N. Perioperative Evaluation and Management of Patients With Cirrhosis: Risk Assessment, Surgical Outcomes, and Future Directions. Clin. Gastroenterol. Hepatol. 2020, 18, 2398–2414.e2393. [Google Scholar] [CrossRef]
- Seshadri, A.; Appelbaum, R.; Carmichael, S.P., 2nd; Cuschieri, J.; Hoth, J.; Kaups, K.L.; Kodadek, L.; Kutcher, M.E.; Pathak, A.; Rappold, J.; et al. Management of Decompensated Cirrhosis in the Surgical ICU: An American Association for the Surgery of Trauma Critical Care Committee Clinical Consensus Document. Trauma Surg. Acute Care Open 2022, 7, e000936. [Google Scholar] [CrossRef] [PubMed]
- Cornberg, M.; Tacke, F.; Karlsen, T.H. European Association for the Study of the L. Clinical Practice Guidelines of the European Association for the study of the Liver—Advancing methodology but preserving practicability. J. Hepatol. 2019, 70, 5–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, J.; Guyatt, G.; Oxman, A.D.; Alderson, P.; Dahm, P.; Falck-Ytter, Y.; Nasser, M.; Meerpohl, J.; Post, P.N.; Kunz, R.; et al. GRADE guidelines: 14. Going from evidence to recommendations: The significance and presentation of recommendations. J. Clin. Epidemiol. 2013, 66, 719–725. [Google Scholar] [CrossRef]
- Patel, V.C.; Williams, R. Antimicrobial resistance in chronic liver disease. Hepatol. Int. 2020, 14, 24–34. [Google Scholar] [CrossRef] [Green Version]
- European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J. Hepatol. 2019, 70, 172–193. [Google Scholar] [CrossRef] [Green Version]
- Rees, J.R.; Rees, M.; McNair, A.G.; Odondi, L.; Metcalfe, C.; John, T.; Welsh, F.K.; Blazeby, J.M. The Prognostic Value of Patient-Reported Outcome Data in Patients With Colorectal Hepatic Metastases Who Underwent Surgery. Clin. Color. Cancer 2016, 15, 74–81.e71. [Google Scholar] [CrossRef] [PubMed]
- Goodman, Z.D. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J. Hepatol. 2007, 47, 598–607. [Google Scholar] [CrossRef]
- Bosch, J.; Abraldes, J.G.; Albillos, A.; Aracil, C.; Banares, R.; Berzigotti, A.; Calleja, J.L.; de la Peña, J.; Escorsell, A.; García-Pagán, J.C.; et al. Portal hypertension: Recommendations for evaluation and treatment: Consensus document sponsored by the Spanish Association for the Study of the Liver (AEEH) and the Biomedical Research Network Center for Liver and Digestive Diseases (CIBERehd). Gastroenterol. Hepatol. 2012, 35, 421–450. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. Electronic address eee, Clinical Practice Guideline P, Chair, representative EGB, Panel m. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef]
- Shaheen, A.A.M.; Myers, R.P. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis C-related fibrosis: A systematic review. Hepatology 2007, 46, 912–921. [Google Scholar] [CrossRef]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007, 46, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Berzigotti, A.; Ashkenazi, E.; Reverter, E.; Abraldes, J.G.; Bosch, J. Non-invasive diagnostic and prognostic evaluation of liver cirrhosis and portal hypertension. Dis. Markers 2011, 31, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Augustin, S.; Millán, L.; González, A.; Martell, M.; Gelabert, A.; Segarra, A.; Serres, X.; Esteban, R.; Genescà, J. Detection of early portal hypertension with routine data and liver stiffness in patients with asymptomatic liver disease: A prospective study. J. Hepatol. 2014, 60, 561–569. [Google Scholar] [CrossRef] [PubMed]
- de Franchis, R.; Baveno, V.I.F. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J. Hepatol. 2015, 63, 743–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pons, M.; Augustin, S.; Scheiner, B.; Guillaume, M.; Rosselli, M.; Rodrigues, S.G.; Stefanescu, H.; Ma, M.M.; Mandorfer, M.; Mergeay-Fabre, M.; et al. Noninvasive Diagnosis of Portal Hypertension in Patients With Compensated Advanced Chronic Liver Disease. Am. J. Gastroenterol. 2021, 116, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Northup, P.G.; Wanamaker, R.C.; Lee, V.D.; Adams, R.B.; Berg, C.L. Model for End-Stage Liver Disease (MELD) predicts nontransplant surgical mortality in patients with cirrhosis. Ann. Surg. 2005, 242, 244–251. [Google Scholar] [CrossRef] [PubMed]
- The, S.H.; Nagorney, D.M.; Stevens, S.R.; Offord, K.P.; Therneau, T.M.; Plevak, D.J.; Talwalkar, J.A.; Kim, W.R.; Kamath, P.S. Risk factors for mortality after surgery in patients with cirrhosis. Gastroenterology 2007, 132, 1261–1269. [Google Scholar]
- Mahmud, N.; Fricker, Z.; Hubbard, R.A.; Ioannou, G.N.; Lewis, J.D.; Taddei, T.H.; Rothstein, K.D.; Serper, M.; Goldberg, D.S.; Kaplan, D.E. Risk Prediction Models for Post-Operative Mortality in Patients With Cirrhosis. Hepatology 2021, 73, 204–218. [Google Scholar] [CrossRef]
- Mahmud, N.; Fricker, Z.; Panchal, S.; Lewis, J.D.; Goldberg, D.S.; Kaplan, D.E. External Validation of the VOCAL-Penn Cirrhosis Surgical Risk Score in 2 Large, Independent Health Systems. Liver Transplant. 2021, 27, 961–970. [Google Scholar] [CrossRef]
- Mahmud, N.; Panchal, S.; Turrentine, F.E.; Kaplan, D.E.; Zaydfudim, V.M. Performance of risk prediction models for post-operative mortality in patients undergoing liver resection. Am. J. Surg. 2022, 225, 198–205. [Google Scholar] [CrossRef]
- Mahmud, N.; Fricker, Z.; Lewis, J.D.; Taddei, T.H.; Goldberg, D.S.; Kaplan, D.E. Risk Prediction Models for Postoperative Decompensation and Infection in Patients With Cirrhosis: A Veterans Affairs Cohort Study. Clin. Gastroenterol. Hepatol. 2022, 20, e1121–e1134. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, P.; Vilstrup, H.; Lash, T.L. Development and validation of a comorbidity scoring system for patients with cirrhosis. Gastroenterology 2014, 146, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.C.; Tandon, P.; Bernal, W.; Tapper, E.B.; Ekong, U.; Dasarathy, S.; Carey, E.J. Malnutrition, Frailty, and Sarcopenia in Patients With Cirrhosis: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2021, 74, 1611–1644. [Google Scholar] [CrossRef] [PubMed]
- Tantai, X.; Liu, Y.; Yeo, Y.H.; Praktiknjo, M.; Mauro, E.; Hamaguchi, Y.; Engelmann, C.; Zhang, P.; Jeong, J.Y.; van Vugt, J.L.A.; et al. Effect of sarcopenia on survival in patients with cirrhosis: A meta-analysis. J. Hepatol. 2022, 76, 588–599. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J. Hepatol. 2018, 69, 406–460. [Google Scholar] [CrossRef] [Green Version]
- Reverter, E.; Cirera, I.; Albillos, A.; Debernardi-Venon, W.; Abraldes, J.G.; Llop, E.; Flores, A.; Martínez-Palli, G.; Blasi, A.; Martínez, J.; et al. The prognostic role of hepatic venous pressure gradient in cirrhotic patients undergoing elective extrahepatic surgery. J. Hepatol. 2019, 71, 942–950. [Google Scholar] [CrossRef]
- Rodrigues, S.G.; Mendoza, Y.P.; Bosch, J. Beta-blockers in cirrhosis: Evidence-based indications and limitations. JHEP Rep. 2020, 2, 100063. [Google Scholar] [CrossRef] [Green Version]
- de Franchis, R.; Bosch, J.; Garcia-Tsao, G.; Reiberger, T.; Ripoll, C. Baveno, VIIF. Baveno VII—Renewing consensus in portal hypertension. J. Hepatol. 2022, 76, 959–974. [Google Scholar] [CrossRef]
- Garcia-Pagan, J.C.; Saffo, S.; Mandorfer, M.; Garcia-Tsao, G. Where does TIPS fit in the management of patients with cirrhosis? JHEP Rep. 2020, 2, 100122. [Google Scholar] [CrossRef]
- Intagliata, N.M.; Argo, C.K.; Stine, J.G.; Lisman, T.; Caldwell, S.H.; Violi, F.; Faculty of the 7th International Coagulation in Liver Disease. Concepts and Controversies in Haemostasis and Thrombosis Associated with Liver Disease: Proceedings of the 7th International Coagulation in Liver Disease Conference. Thromb. Haemost. 2018, 118, 1491–1506. [Google Scholar] [CrossRef] [PubMed]
- Rout, G.; Shalimar; Gunjan, D.; Mahapatra, S.J.; Kedia, S.; Garg, P.K.; Nayak, B. Thromboelastography-guided Blood Product Transfusion in Cirrhosis Patients With Variceal Bleeding: A Randomized Controlled Trial. J. Clin. Gastroenterol. 2020, 54, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Vuyyuru, S.K.; Singh, A.D.; Gamanagatti, S.R.; Rout, G.; Gunjan, D.; Shalimar. A Randomized Control Trial of Thromboelastography-Guided Transfusion in Cirrhosis for High-Risk Invasive Liver-Related Procedures. Dig. Dis. Sci. 2020, 65, 2104–2111. [Google Scholar] [CrossRef] [PubMed]
- Janko, N.; Majeed, A.; Kemp, W.; Roberts, S.K. Viscoelastic Tests as Point-of-Care Tests in the Assessment and Management of Bleeding and Thrombosis in Liver Disease. Semin. Thromb. Hemost. 2020, 46, 704–715. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, J.G.; Greenberg, C.S.; Patton, H.M.; Caldwell, S.H. AGA Clinical Practice Update: Coagulation in Cirrhosis. Gastroenterology 2019, 157, 34–43.e31. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines on prevention and management of bleeding and thrombosis in patients with cirrhosis. J. Hepatol. 2022, 76, 1151–1184. [Google Scholar] [CrossRef]
- Lisman, T.; Caldwell, S.H.; Intagliata, N.M. Haemostatic alterations and management of haemostasis in patients with cirrhosis. J. Hepatol. 2022, 76, 1291–1305. [Google Scholar] [CrossRef]
- Nguyen, G.; Lejeune, M.; Crichi, B.; Frere, C. Hemostasis testing in patients with liver dysfunction: Advantages and caveats. World J. Gastroenterol. 2021, 27, 7285–7298. [Google Scholar] [CrossRef]
- Diaz, K.E.; Schiano, T.D. Evaluation and Management of Cirrhotic Patients Undergoing Elective Surgery. Curr. Gastroenterol. Rep. 2019, 21, 32. [Google Scholar] [CrossRef]
- de Goede, B.; Klitsie, P.J.; Lange, J.F.; Metselaar, H.J.; Kazemier, G. Morbidity and mortality related to non-hepatic surgery in patients with liver cirrhosis: A systematic review. Best Pract. Res. Clin. Gastroenterol. 2012, 26, 47–59. [Google Scholar] [CrossRef]
- Paolino, J.; Steinhagen, R.M. Colorectal surgery in cirrhotic patients. Sci. World J. 2014, 2014, 239293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Telem, D.A.; Schiano, T.; Goldstone, R.; Han, D.K.; Buch, K.E.; Chin, E.H.; Nguyen, S.Q.; Divino, C.M. Factors that predict outcome of abdominal operations in patients with advanced cirrhosis. Clin. Gastroenterol. Hepatol. 2010, 8, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-W.; Liu, F.; Li, H.-Y.; Wei, Y.-G.; Li, B. Outcomes following laparoscopic versus open major hepatectomy for hepatocellular carcinoma in patients with cirrhosis: A propensity score-matched analysis. Surg. Endosc. 2018, 32, 712–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troisi, R.I.; Pegoraro, F.; Giglio, M.C.; Rompianesi, G.; Berardi, G.; Tomassini, F.; De Simone, G.; Aprea, G.; Montalti, R.; De Palma, G.D. Robotic approach to the liver: Open surgery in a closed abdomen or laparoscopic surgery with technical constraints? Surg. Oncol. 2020, 33, 239–248. [Google Scholar] [CrossRef]
- Andraus, W.; Pinheiro, R.S.; Lai, Q.; Haddad, L.B.; Nacif, L.S.; D’Albuquerque, L.A.C.; Lerut, J. Abdominal wall hernia in cirrhotic patients: Emergency surgery results in higher morbidity and mortality. BMC Surg. 2015, 15, 65. [Google Scholar] [CrossRef]
- Forner, A.; Da Fonseca, L.G.; Díaz-González, A.; Sanduzzi-Zamparelli, M.; Reig, M.; Bruix, J. Controversies in the management of hepatocellular carcinoma. JHEP Rep. 2019, 1, 17–29. [Google Scholar] [CrossRef] [Green Version]
- Soreide, J.A.; Deshpande, R. Post hepatectomy liver failure (PHLF)—Recent advances in prevention and clinical management. Eur. J. Surg. Oncol. 2021, 47, 216–224. [Google Scholar] [CrossRef]
- Llovet, J.M.; Fuster, J.; Bruix, J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: Resection versus transplantation. Hepatology 1999, 30, 1434–1440. [Google Scholar] [CrossRef]
- Teh, S.H.; Christein, J.; Donohue, J.; Que, F.; Kendrick, M.; Farnell, M.; Cha, S.; Kamath, P.; Kim, R.; Nagorney, D.M. Hepatic resection of hepatocellular carcinoma in patients with cirrhosis: Model of End-Stage Liver Disease (MELD) score predicts perioperative mortality. J. Gastrointest. Surg. 2005, 9, 1207–1215; discussion 1215. [Google Scholar] [CrossRef]
- Rajakannu, M.; Coilly, A.; Adam, R.; Samuel, D.; Vibert, E. Prospective validation of transient elastography for staging liver fibrosis in patients undergoing hepatectomy and liver transplantation. J. Hepatol. 2017, 68, 199–200. [Google Scholar] [CrossRef] [Green Version]
- European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Memeo, R.; de’Angelis, N.; Compagnon, P.; Salloum, C.; Cherqui, D.; Laurent, A.; Azoulay, D. Laparoscopic vs. open liver resection for hepatocellular carcinoma of cirrhotic liver: A case-control study. World J. Surg. 2014, 38, 2919–2926. [Google Scholar] [CrossRef] [PubMed]
- Patton, H.; Heimbach, J.; McCullough, A. AGA Clinical Practice Update on Bariatric Surgery in Cirrhosis: Expert Review. Clin. Gastroenterol. Hepatol. 2021, 19, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Bohnen, J.D.; Ramly, E.P.; Sangji, N.F.; de Moya, M.; Yeh, D.D.; Lee, J.; Velmahos, G.C.; Chang, D.C.; Kaafarani, H.M. Perioperative risk factors impact outcomes in emergency versus nonemergency surgery differently: Time to separate our national risk-adjustment models? J. Trauma Acute Care Surg. 2016, 81, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Martin Mateos, R.; Garcia de la Filia Molina, I.; Albillos, A. Pre-surgical risk assessment in patients with cirrhosis. Acta Gastroenterol. Belg. 2020, 83, 449–453. [Google Scholar]
- Itoi, T.; Coelho-Prabhu, N.; Baron, T.H. Endoscopic gallbladder drainage for management of acute cholecystitis. Gastrointest. Endosc. 2010, 71, 1038–1045. [Google Scholar] [CrossRef]
- van der Merwe, S.W.; van Wanrooij, R.L.J.; Bronswijk, M.; Everett, S.; Lakhtakia, S.; Rimbas, M.; Hucl, T.; Kunda, R.; Badaoui, A.; Law, R.; et al. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2022, 54, 185–205. [Google Scholar] [CrossRef]
- Gelman, S. General anesthesia and hepatic circulation. Can. J. Physiol. Pharmacol. 1987, 65, 1762–1779. [Google Scholar] [CrossRef]
- Hug, B.L.; Surber, C.; Bates, D.W. Use of hepatotoxic drugs in chronic liver disease. J. Patient Saf. 2012, 8, 45–50. [Google Scholar] [CrossRef]
- Chang, J.; Meinke, J.; Geck, M.; Hebest, M.; Böhling, N.; Dolscheid-Pommerich, R.; Stoffel-Wagner, B.; Kristiansen, G.; Overhaus, M.; Peyman, L.O.; et al. Extrahepatic Surgery in Cirrhosis Significantly Increases Portal Pressure in Preclinical Animal Models. Front. Physiol. 2021, 12, 720898. [Google Scholar] [CrossRef]
- Soleimanpour, H.; Safari, S.; Rahmani, F.; Ameli, H.; Alavian, S.M. The role of inhalational anesthetic drugs in patients with hepatic dysfunction: A review article. Anesthesiol. Pain Med. 2015, 5, e23409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mcclain, R.; Ramakrishna, H.; Iii, S.; Cartwright, J.; Phar, L.; Pai, S.-L.; Rodrigues, E.S.; Martin, A.K.; Shine, T.S. Anesthetic pharmacology and perioperative considerations for the end stage liver disease patient. Curr. Clin. Pharmacol. 2015, 10, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Guacho, J.A.L.; de Moura, D.T.H.; Ribeiro, I.B.; Neto, A.M.D.P.; Singh, S.; Tucci, M.G.B.; Bernardo, W.M.; de Moura, E.G.H. Propofol vs midazolam sedation for elective endoscopy in patients with cirrhosis: A systematic review and meta-analysis of randomized controlled trials. World J. Gastrointest. Endosc. 2020, 12, 241–255. [Google Scholar] [CrossRef]
- Veroli, P.; O’Kelly, B.; Bertrand, F.; Trouvin, J.H.; Farinotti, R.; Ecoffey, C. Extrahepatic metabolism of propofol in man during the anhepatic phase of orthotopic liver transplantation. Br. J. Anaesth. 1992, 68, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Lebrault, C.; Berger, J.L.; D’Hollander, A.A.; Gomeni, R.; Henzel, D.; Duvaldestin, P. Pharmacokinetics and pharmacodynamics of vecuronium (ORG NC 45) in patients with cirrhosis. Anesthesiology 1985, 62, 601–605. [Google Scholar] [CrossRef] [PubMed]
- van Miert, M.M.; Eastwood, N.B.; Boyd, A.H.; Parker, C.J.; Hunter, J.M. The pharmacokinetics and pharmacodynamics of rocuronium in patients with hepatic cirrhosis. Br. J. Clin. Pharmacol. 1997, 44, 139–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soleimanpour, H.; Safari, S.; Nia, K.S.; Sanaie, S.; Alavian, S.M. Opioid Drugs in Patients With Liver Disease: A Systematic Review. Hepat. Mon. 2016, 16, e32636. [Google Scholar] [CrossRef] [Green Version]
- Bauer, M.E.; Arendt, K.; Beilin, Y.; Gernsheimer, T.; Perez Botero, J.; James, A.H.; Yaghmour, E.; Toledano, R.D.; Turrentine, M.; Houle, T.; et al. The Society for Obstetric Anesthesia and Perinatology Interdisciplinary Consensus Statement on Neuraxial Procedures in Obstetric Patients With Thrombocytopenia. Anesth. Analg. 2021, 132, 1531–1544. [Google Scholar] [CrossRef]
- Lopez-Delgado, J.C.; Ballus, J.; Esteve, F.; Betancur-Zambrano, N.L.; Corral-Velez, V.; Mañez, R.; Betbese, A.J.; Roncal, J.A.; Javierre, C. Outcomes of abdominal surgery in patients with liver cirrhosis. World J. Gastroenterol. 2016, 22, 2657–2667. [Google Scholar] [CrossRef]
- Kettner, S.C.; Willschke, H.; Marhofer, P. Does regional anaesthesia really improve outcome? Br. J. Anaesth. 2011, 107 (Suppl. 1), i90–i95. [Google Scholar] [CrossRef] [Green Version]
- Rahimzadeh, P.; Safari, S.; Faiz, S.H.R.; Alavian, S.M. Anesthesia for patients with liver disease. Hepat. Mon. 2014, 14, e19881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, H.; Premkumar, M. Diagnosis and Management of Cirrhotic Cardiomyopathy. J. Clin. Exp. Hepatol. 2022, 12, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.E.; Baram, M.; Vahid, B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest 2008, 134, 172–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, E.; Paugam-Burtz, C.; Jaber, S. Shock Etiologies and Fluid Management in Liver Failure. Semin. Respir. Crit. Care Med. 2018, 39, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.V.; Kumar, P.; Sharma, M.; Sowmya, T.R.; Talukdar, R.; Rao, P.N.; Reddy, D.N. Pathophysiology and Prevention of Paracentesis-induced Circulatory Dysfunction: A Concise Review. J. Clin. Transl. Hepatol. 2020, 8, 42–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jalan, R.; Schnurr, K.; Mookerjee, R.P.; Sen, S.; Cheshire, L.; Hodges, S.; Muravsky, V.; Williams, R.; Matthes, G.; Davies, N.A. Alterations in the functional capacity of albumin in patients with decompensated cirrhosis is associated with increased mortality. Hepatology 2009, 50, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Odutayo, A.; Desborough, M.J.R.; Trivella, M.; Stanley, A.J.; Dorée, C.; Collins, G.S.; Hopewell, S.; Brunskill, S.J.; Kahan, B.C.; Logan, R.F.; et al. Restrictive versus liberal blood transfusion for gastrointestinal bleeding: A systematic review and meta-analysis of randomised controlled trials. Lancet Gastroenterol. Hepatol. 2017, 2, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Gralnek, I.M.; Duboc, M.C.; Garcia-Pagan, J.C.; Fuccio, L.; Karstensen, J.G.; Hucl, T.; Jovanovic, I.; Awadie, H.; Hernandez-Gea, V.; Tantau, M.; et al. Endoscopic diagnosis and management of esophagogastric variceal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2022, 54, 1094–1120. [Google Scholar] [CrossRef]
- Mohanty, A.; Kapuria, D.; Canakis, A.; Lin, H.; Amat, M.J.; Rangel Paniz, G.; Placone, N.T.; Thomasson, R.; Roy, H.; Chak, E.; et al. Fresh frozen plasma transfusion in acute variceal haemorrhage: Results from a multicentre cohort study. Liver Int. 2021, 41, 1901–1908. [Google Scholar] [CrossRef]
- Kwon, J.O.; MacLaren, R. Comparison of Fresh-Frozen Plasma, Four-Factor Prothrombin Complex Concentrates, and Recombinant Factor VIIa to Facilitate Procedures in Critically Ill Patients with Coagulopathy from Liver Disease: A Retrospective Cohort Study. Pharmacotherapy 2016, 36, 1047–1054. [Google Scholar] [CrossRef]
- Kumar, M.; Ahmad, J.; Maiwall, R.; Choudhury, A.; Bajpai, M.; Mitra, L.G.; Saluja, V.; Mohan Agarwal, P.; Bihari, C.; Shasthry, S.M.; et al. Thromboelastography-Guided Blood Component Use in Patients With Cirrhosis With Nonvariceal Bleeding: A Randomized Controlled Trial. Hepatology 2020, 71, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Chandok, N.; Watt, K.D. Pain management in the cirrhotic patient: The clinical challenge. Mayo Clin. Proc. 2010, 85, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Anekar, A.A.; Cascella, M. WHO Analgesic Ladder; Updated 15 May 2022; StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Rakoski, M.; Goyal, P.; Spencer-Safier, M.; Weissman, J.; Mohr, G.; Volk, M. Pain management in patients with cirrhosis. Clin. Liver Dis. 2018, 11, 135–140. [Google Scholar] [CrossRef] [PubMed]
- McGill, M.R.; James, L.P.; McCullough, S.S.; Moran, J.H.; Mathews, S.E.; Peterson, E.C.; Fleming, D.P.; Tripod, M.E.; Vazquez, J.H.; Kennon-McGill, S.; et al. Short-Term Safety of Repeated Acetaminophen Use in Patients With Compensated Cirrhosis. Hepatol. Commun. 2022, 6, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.P.; Jayasekera, C.; Nicoll, A. Analgesia for the cirrhotic patient: A literature review and recommendations. J. Gastroenterol. Hepatol. 2014, 29, 1356–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirouze, D.; Zipser, R.D.; Reynolds, T.B. Effect of inhibitors of prostaglandin synthesis on induced diuresis in cirrhosis. Hepatology 1983, 3, 50–55. [Google Scholar] [CrossRef]
- Schulte, B.; Tergast, T.L.; Griemsmann, M.; Menti, D.; Deveci, N.; Kahlhöfer, J.; Dörge, P.; Hüffner, L.; Kraft, A.R.M.; Behrendt, P.; et al. Metamizole-Associated Risks in Decompensated Hepatic Cirrhosis. Dtsch. Arztebl. Int. 2022. [Google Scholar] [CrossRef]
- Elia, C.; Graupera, I.; Barreto, R.; Solà, E.; Moreira, R.; Huelin, P.; Ariza, X.; Solé, C.; Pose, E.; Baiges, A.; et al. Severe acute kidney injury associated with non-steroidal anti-inflammatory drugs in cirrhosis: A case-control study. J. Hepatol. 2015, 63, 593–600. [Google Scholar] [CrossRef]
- Weersink, R.A.; Bouma, M.; Burger, D.M.; Drenth, J.P.H.; Harkes-Idzinga, S.F.; Hunfeld, N.G.M.; Metselaar, H.J.; Monster-Simons, M.H.; Taxis, K.; Borgsteede, S.D. Evidence-Based Recommendations to Improve the Safe Use of Drugs in Patients with Liver Cirrhosis. Drug Saf. Int. J. Med. Toxicol. Drug Exp. 2018, 41, 603–613. [Google Scholar] [CrossRef] [Green Version]
- Krcevski Skvarc, N.; Morlion, B.; Vowles, K.E.; Bannister, K.; Buchsner, E.; Casale, R.; Chenot, J.F.; Chumbley, G.; Drewes, A.M.; Dom, G.; et al. European clinical practice recommendations on opioids for chronic noncancer pain—Part 2: Special situations. Eur. J. Pain 2021, 25, 969–985. [Google Scholar] [CrossRef]
- Tegeder, I.; Lotsch, J.; Geisslinger, G. Pharmacokinetics of opioids in liver disease. Clin. Pharmacokinet. 1999, 37, 17–40. [Google Scholar] [CrossRef] [PubMed]
- Haberer, J.P.; Schoeffler, P.; Couderc, E.; Duvaldestin, P. Fentanyl pharmacokinetics in anaesthetized patients with cirrhosis. Br. J. Anaesth. 1982, 54, 1267–1270. [Google Scholar] [CrossRef] [PubMed]
- Tandon, P.; Montano-Loza, A.J.; Lai, J.C.; Dasarathy, S.; Merli, M. Sarcopenia and frailty in decompensated cirrhosis. J. Hepatol. 2021, 75 (Suppl. 1), S147–S162. [Google Scholar] [CrossRef] [PubMed]
- Alukal, J.J.; Thuluvath, P.J. Gastrointestinal Failure in Critically Ill Patients With Cirrhosis. Am. J. Gastroenterol. 2019, 114, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Lo, E.K.K.; Xu, J.H.; Zhan, Q.; Zeng, Z.; El-Nezami, H. The Emerging Role of Branched-Chain Amino Acids in Liver Diseases. Biomedicines 2022, 10, 1444. [Google Scholar] [CrossRef]
- Angeli, P.; Ginès, P.; Wong, F.; Bernardi, M.; Boyer, T.D.; Gerbes, A.; Moreau, R.; Jalan, R.; Sarin, S.K.; Piano, S.; et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: Revised consensus recommendations of the International Club of Ascites. J. Hepatol. 2015, 62, 968–974. [Google Scholar] [CrossRef] [Green Version]
- Angeli, P.; Garcia-Tsao, G.; Nadim, M.K.; Parikh, C.R. News in pathophysiology, definition and classification of hepatorenal syndrome: A step beyond the International Club of Ascites (ICA) consensus document. J. Hepatol. 2019, 71, 811–822. [Google Scholar] [CrossRef] [Green Version]
- Gupta, K.; Bhurwal, A.; Law, C.; Ventre, S.; Minacapelli, C.D.; Kabaria, S.; Li, Y.; Tait, C.; Catalano, C.; Rustgi, V.K. Acute kidney injury and hepatorenal syndrome in cirrhosis. World J. Gastroenterol. 2021, 27, 3984–4003. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines on the management of hepatic encephalopathy. J. Hepatol. 2022, 77, 807–824. [Google Scholar] [CrossRef]
- DeAngelis, G.A.; Khot, R.; Haskal, Z.J.; Maitland, H.S.; Northup, P.G.; Shah, N.L.; Caldwell, S.H. Bleeding Risk and Management in Interventional Procedures in Chronic Liver Disease. J. Vasc. Interv. Radiol. JVIR 2016, 27, 1665–1674. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-H.; Lee, C.-H.; Chang, C. Spontaneous Bacterial Peritonitis in Decompensated Liver Cirrhosis—A Literature Review. Livers 2022, 2, 214–232. [Google Scholar] [CrossRef]
- Arroyo, V.; Moreau, R.; Jalan, R.; Ginès, P. Acute-on-chronic liver failure: A new syndrome that will re-classify cirrhosis. J. Hepatol. 2015, 62, S131–S143. [Google Scholar] [CrossRef] [PubMed]
- Klein, L.M.; Chang, J.; Gu, W.; Manekeller, S.; Jansen, C.; Lingohr, P.; Praktiknjo, M.; Kalf, J.C.; Schulz, M.; Spengler, U.; et al. The Development and Outcome of Acute-on-Chronic Liver Failure After Surgical Interventions. Liver Transplant. 2020, 26, 227–237. [Google Scholar] [CrossRef] [PubMed]



| Section 1. Preoperative evaluation and management | |
| 1. | What pathophysiological characteristics do patients with ACLD 1 present? |
| 2. | How could we diagnose patients with ACLD 1 before surgery? |
| 3. | What tools are available to assess the risk of surgical mortality before surgery? |
| 4. | How could we intervene to reduce surgical risk before elective procedures? |
| 5. | How can we evaluate and manage coagulation disorders? |
| Section 2. Surgical procedures | |
| 6. | What recommendations should be considered for surgical procedures? |
| 7. | What considerations should be taken into account for elective surgery? |
| 8. | What concerns should be considered for emergent surgery? |
| Section 3. Intraoperative management | |
| 9. | Is the anesthetic technique different? |
| 10. | Is regional anesthesia recommended? |
| 11. | Is cardiovascular monitoring necessary during surgery? |
| 12. | What is the best fluid replacement strategy and vasopressor during surgery? |
| Section 4. General recommendations in postoperative | |
| 13. | Is a liberal or restrictive transfusion strategy recommended in acute bleeding? |
| 14. | Is venous thromboembolism prophylaxis recommendable after surgery? |
| 15. | What is the treatment of postoperative pain? |
| 16. | What nutritional recommendations should be made after surgery? |
| Section 5. Postoperative management of hepatic decompensation and ACLD complications | |
| 17. | How should we manage an acute kidney injury after surgery? |
| 18. | How should we treat hepatic encephalopathy after surgery? |
| 19. | How should we manage ascites after surgery? |
| 20. | Can ACLD 1 patients develop Acute-on-Chronic Liver Failure after surgery? |
| Anaesthetic Drug | Pros | Cons |
|---|---|---|
| Sevoflurane | Fewest complications | |
| Isoflurane | Halogenated drug with lower hepatic metabolism. Little disturbance of hepatic blood flow. | |
| Desflurane | Little disturbance of hepatic blood flow. | |
| Propofol | Wake-up time slightly longer in ACLD patients due to their added extrahepatic metabolism. | |
| Diazepam, Clonazepam, Midazolam | Prolonged effect in ACLD patients. | |
| Lorazepam, Oxacepam, Temazepam | Minimally affected by liver disease. | |
| Succinylcholine | Longer elimination half-life in ACLD patients. | |
| Vecuronium | Longer elimination half-life in ACLD patients. | |
| Rocuronium | Longer elimination half-life in ACLD patients. | |
| Atracurium, Cis-atracurium, Mivacurium | Metabolization without liver involvement. | |
| Fentanyl, Sufentanil, Remifentanil | Metabolism not affected in ACLD patients. | Can cause HE. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canillas, L.; Pelegrina, A.; Álvarez, J.; Colominas-González, E.; Salar, A.; Aguilera, L.; Burdio, F.; Montes, A.; Grau, S.; Grande, L.; et al. Clinical Guideline on Perioperative Management of Patients with Advanced Chronic Liver Disease. Life 2023, 13, 132. https://doi.org/10.3390/life13010132
Canillas L, Pelegrina A, Álvarez J, Colominas-González E, Salar A, Aguilera L, Burdio F, Montes A, Grau S, Grande L, et al. Clinical Guideline on Perioperative Management of Patients with Advanced Chronic Liver Disease. Life. 2023; 13(1):132. https://doi.org/10.3390/life13010132
Chicago/Turabian StyleCanillas, Lidia, Amalia Pelegrina, Juan Álvarez, Elena Colominas-González, Antonio Salar, Lluís Aguilera, Fernando Burdio, Antonio Montes, Santiago Grau, Luis Grande, and et al. 2023. "Clinical Guideline on Perioperative Management of Patients with Advanced Chronic Liver Disease" Life 13, no. 1: 132. https://doi.org/10.3390/life13010132
APA StyleCanillas, L., Pelegrina, A., Álvarez, J., Colominas-González, E., Salar, A., Aguilera, L., Burdio, F., Montes, A., Grau, S., Grande, L., & Carrión, J. A. (2023). Clinical Guideline on Perioperative Management of Patients with Advanced Chronic Liver Disease. Life, 13(1), 132. https://doi.org/10.3390/life13010132








