Oxidative Stress Parameters and Morphological Changes in Japanese Medaka (Oryzias latipes) after Acute Exposure to OA-Group Toxins
Abstract
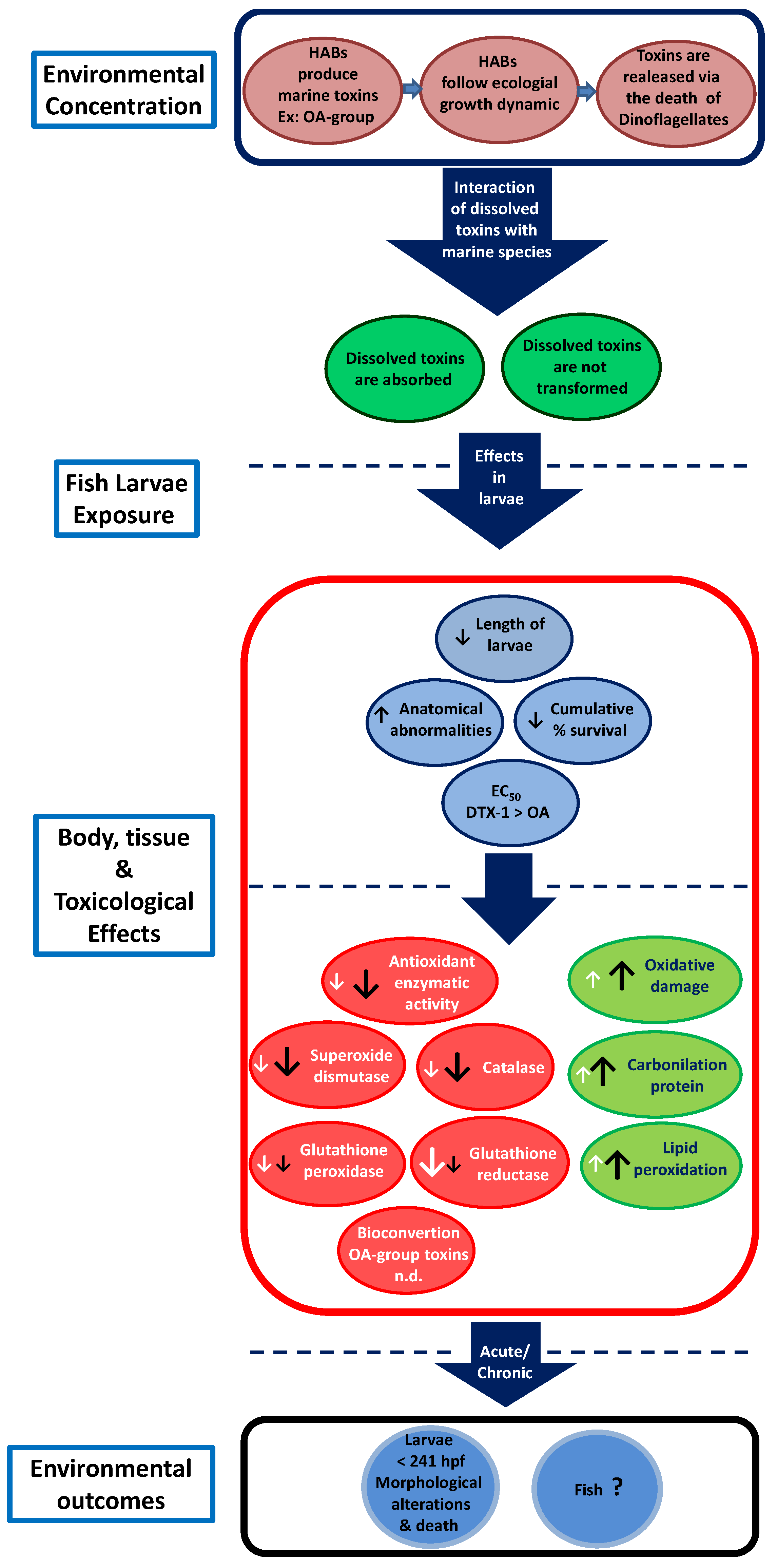
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fish Husbandry and Embryo Collection
2.3. Median Lethal Concentration (LC50)
2.4. Morphological Abnormalities
2.5. Calculation of the Median Lethal and Median Effective Concentrations
2.6. Evaluating Time Course of Adverse Effects in Older Larvae
2.7. Determination of Biochemical Biomarkers
2.8. Oxidative Damage
2.9. Protein Concentration
2.10. Mass Spectrometer Coupled to High-Resolution Liquid Chromatography
2.11. Statistical Analysis
3. Results
3.1. Analysis of OA-Group Toxins
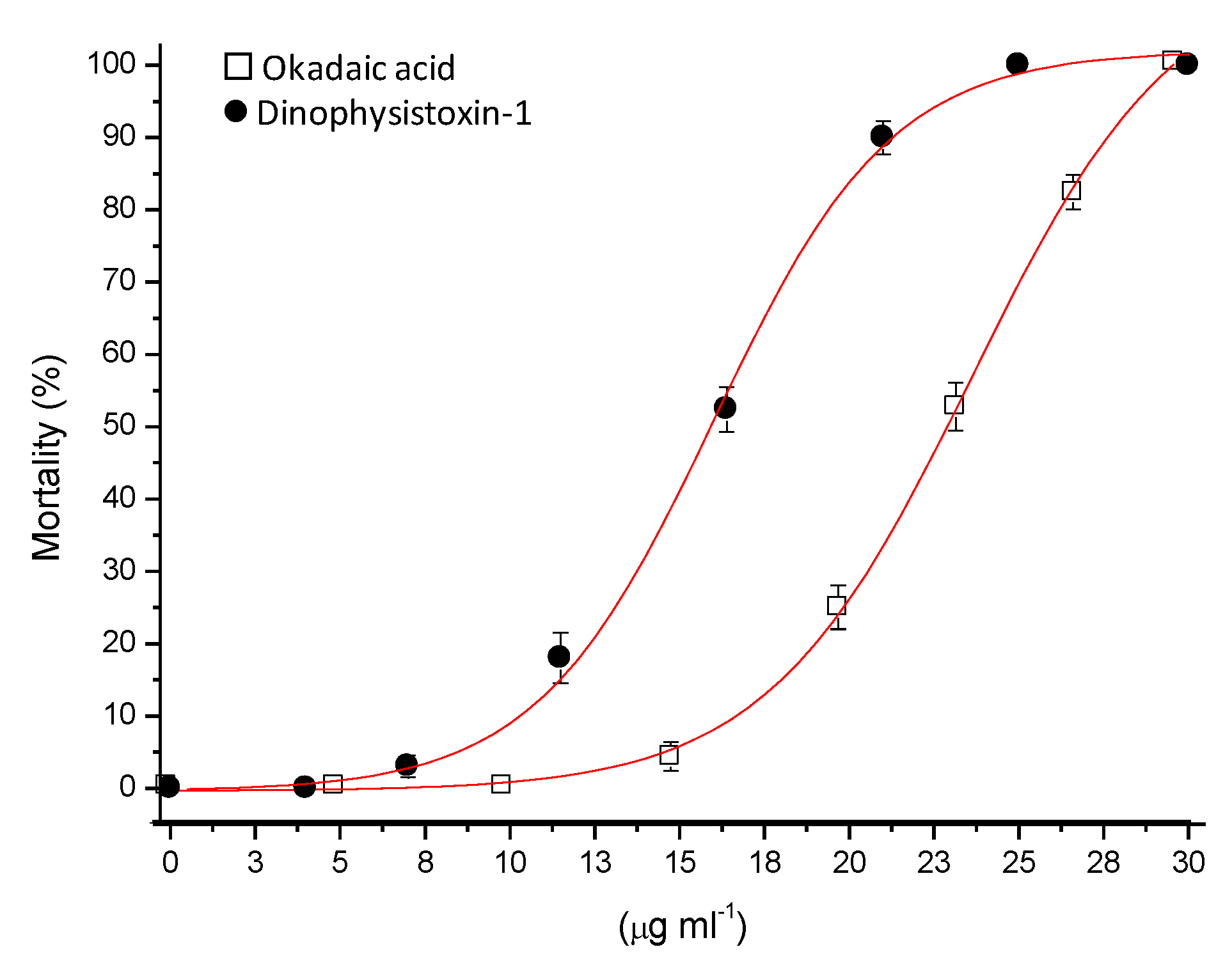
3.2. Effects of Okadaic Acid and Dinophysistoxin-1 on Medaka Larvae
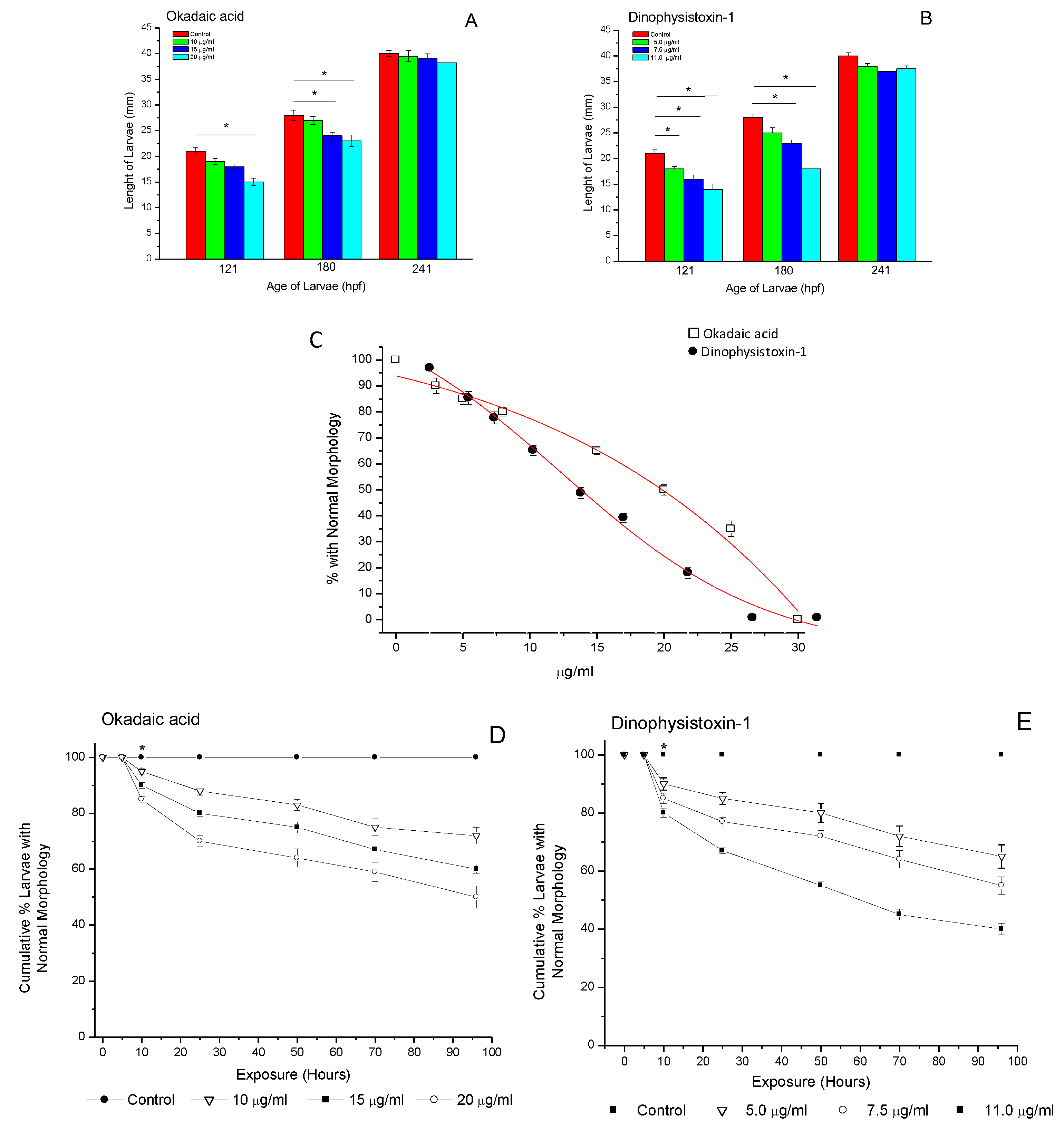
3.3. Effects of OA-Group Toxins on the Growth and Development of Older Larvae
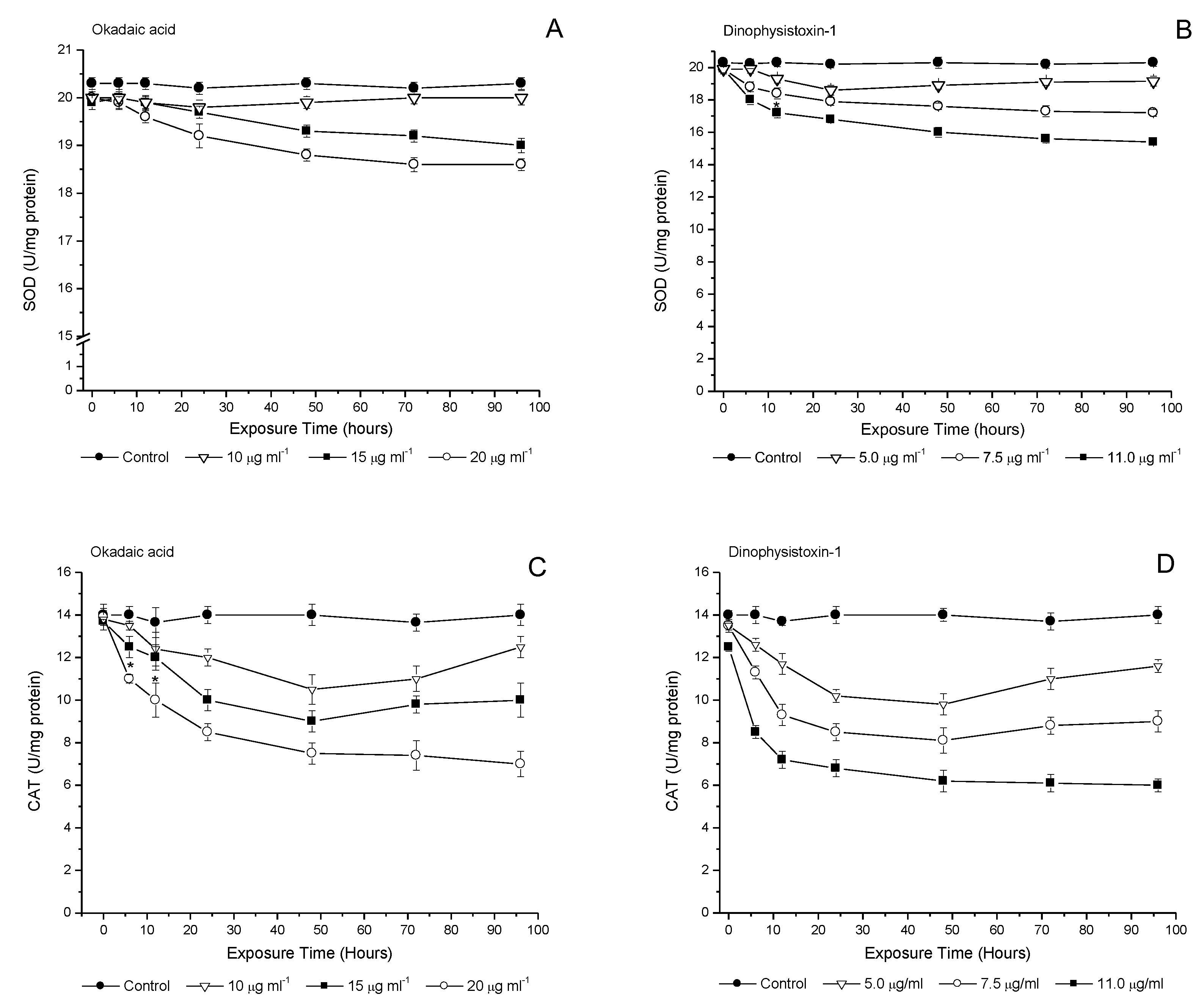
3.4. Temporal Evaluation of Incubation with OA-Group Toxins on Antioxidant Enzymes
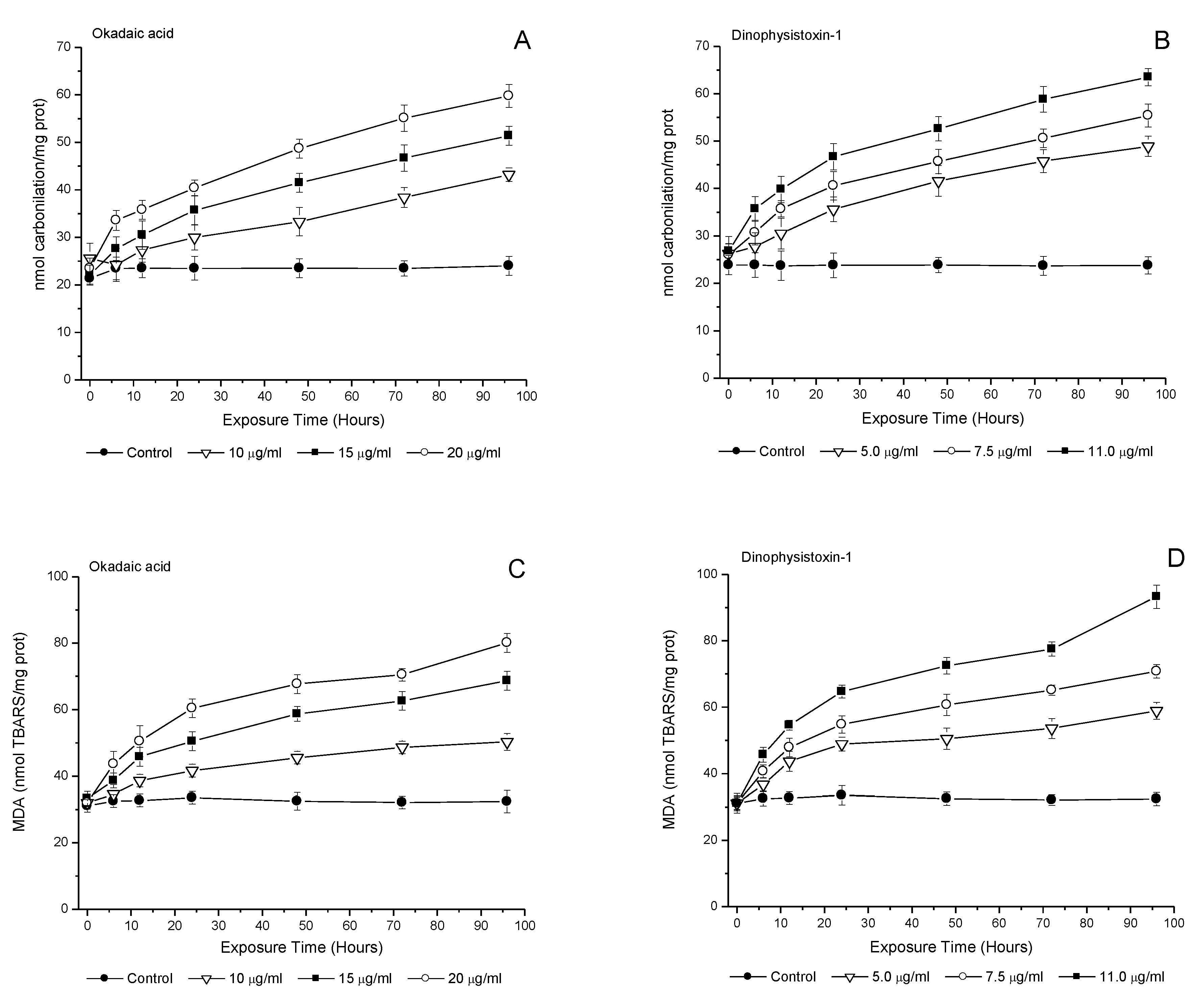
3.5. Effect of OA-Group Toxins on Protein Carbonylation and Lipid Peroxidation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hayes, N.M.; Simpson, G.L.; Haig, H.A.; Leavitt, P. Effects of lake warming on the seasonal risk of toxic cyanobacteria exposure. Limnol. Oceanogr. Lett. 2020, 5, 393–402. [Google Scholar] [CrossRef]
- Marampouti, C.; Buma, A.C.J.; de Boer, M.K. Mediterranean alien harmful algal blooms: Origins and impacts. Environ. Sci. Pollut. Res. Int. 2020, 28, 3837–3851. [Google Scholar] [CrossRef] [PubMed]
- Nwankwegu, A.S.; Li, Y.; Huang, Y.; Wei, J.; Norgbey, E.; Sarpong, L.; Lai, Q.; Wang, W. Harmful algal blooms under changing climate and constantly increasing anthropogenic actions: The review of management implications. 3 Biotech 2019, 9, 449. [Google Scholar] [CrossRef] [PubMed]
- Griffith, A.W.; Gobler, C.J. Harmful algal blooms: A climate change co-stressor in marine and freshwater ecosystems. Harmful Algae 2020, 91, 101590. [Google Scholar] [CrossRef]
- Wells, M.L.; Karlson, B.; Wulff, A.; Kudela, R.; Trick, C.; Asnaghi, V.; Berdalet, E.; Cochlan, W.; Davidson, K.; De Rijcke, M.; et al. Future HAB science: Directions and challenges in a changing climate. Harmful Algae 2020, 91, 101632. [Google Scholar] [CrossRef] [PubMed]
- Reguera, B.; Riobó, P.; Rodríguez, F.; Díaz, P.A.; Pizarro, G.; Paz, B.; Franco, J.M.; Blanco, J. Dinophysis Toxins: Causative Organisms, Distribution and Fate in Shellfish. Mar. Drugs. 2014, 12, 394–461. [Google Scholar] [CrossRef]
- Campos, A.; Freitas, M.; de Almeida, A.M.; Martins, J.C.; Domínguez-Pérez, D.; Osório, H.; Vasconcelos, V.; Reis Costa, P. OMICs Approaches in Diarrhetic Shellfish Toxins Research. Toxins 2020, 12, 493. [Google Scholar] [CrossRef]
- Garcia, C.; Oyaneder-Terrazas, J.; Figueroa, D.; Díaz, C.; Mora, R.; Contreras, H.R. Oxidative effects in aquatic organisms exposed to lipophilic marine biotoxins. In Advances in Marine Biology, 1st ed.; Kovács, A., Nagy, P., Eds.; Nova Science Publishers: New York, NY, USA, 2017; Volume 1, pp. 91–135. [Google Scholar]
- Contreras, H.R.; García, C. Inter-species variability of okadaic acid group toxicity in relation to the content of fatty acids detected in different marine vectors. Food Addit. Contam. Part A 2019, 36, 464–482. [Google Scholar] [CrossRef]
- Pereira-Alves, T.P.; Mafra, L.L. Diel variations in cell abundance and trophic transfer of diarrheic toxins during massive Dinophysis bloom in southern Brazil. Toxins 2018, 10, 232. [Google Scholar] [CrossRef] [Green Version]
- Oyaneder-Terrazas, J.; Polanco, C.; Figueroa, D.; Barriga, A.; García, C. In vitro biotransformation of OA-group and PTX-group toxins in visceral and non-visceral tissues of Mytilus chilensis and Ameghinomya antiqua. Food Addit. Contam. Part A 2020, 37, 1216–1228. [Google Scholar] [CrossRef]
- EFSA. Opinion of the Scientific Panel on Contaminants in the Food chain on a request from the European Commission on marine biotoxins in shellfish–azaspiracids. EFSA J. 2008, 6, 723. [Google Scholar] [CrossRef]
- O’Mahony, M. EU Regulatory Risk Management of Marine Biotoxins in the Marine Bivalve Mollusc Food-Chain. Toxins 2018, 10, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uhrig, R.G.; Moorhead, G.B. Okadaic acid and microcystin insensitive PPP-family phosphatases may represent novel biotechnology targets. Plant Signal Behav. 2011, 6, 2057–2059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Twiner, M.J.; Doucette, G.J.; Pang, Y.; Fang, C.; Forsyth, C.J.; Miles, C.O. Structure-Activity Relationship Studies Using Natural and Synthetic Okadaic Acid/Dinophysistoxin Toxins. Mar. Drugs 2016, 14, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abal, P.; Louzao, M.C.; Suzuki, T.; Watanabe, R.; Vilariño, N.; Carrera, C.; Botana, A.M.; Vieytes, M.R.; Botana, L.M. Toxic Action Reevaluation of Okadaic Acid, Dinophysistoxin-1 and Dinophysistoxin-2: Toxicity Equivalency Factors Based on the Oral Toxicity Study. Cell Physiol. Biochem. 2018, 49, 743–757. [Google Scholar] [CrossRef] [Green Version]
- Espiña, B.; Louzao, M.; Cagide, E.; Alfonso, A.; Vieytes, M.R.; Yasumoto, T.; Botana, L.M. The methyl ester of okadaic acid is more potent than okadaic acid in disrupting the actin cytoskeleton and metabolism of primary cultured hepatocytes. Br. J. Pharmacol. 2010, 159, 337–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opsahl, J.A.; Ljostveit, S.; Solstad, T.; Risa, K.; Roepstorff, P.; Fladmark, K.E. Identification of dynamic changes in proteins associated with the cellular cytoskeleton after exposure to okadaic acid. Mar. Drugs 2013, 11, 1763–1782. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Cárcamo, D.; García, C.; Contreras, H.R. Toxins of Okadaic Acid-Group Increase Malignant Properties in Cells of Colon Cancer. Toxins 2020, 12, 179. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, M.; O’Halloran, J.; O’Brien, N.M.; Van Pelt, F.F.N.A.M. Does the marine biotoxin okadaic acid cause DNA fragmentation in the blue mussel and the pacific oyster? Mar. Environ. Res. 2014, 101, 153–160. [Google Scholar] [CrossRef]
- Fujiki, H.; Sueoka, E.; Watanabe, T.; Suganuma, M. The concept of the okadaic acid class of tumor promoters is revived in endogenous protein inhibitors of protein phosphatase 2A, SET and CIP2A, in human cancers. J. Cancer Res. Clin. Oncol. 2018, 144, 2339–2349. [Google Scholar] [CrossRef]
- Tor Nielsen, L.; Krock, B.; Hansen, P.J. Production and excretion of okadaic acid, pectenotoxin-2 and a novel dinophysistoxin from the DSP-causing marine dinoflagellate Dinophysis acuta–Effects of light, food availability and growth phase. Harmful Algae 2013, 23, 34–45. [Google Scholar] [CrossRef]
- Souid, G.; Souayed, N.; Haouas, Z.; Maaroufi, K. Does the phycotoxin okadaic acid cause oxidative stress damages and histological alterations to seabream (Sparus aurata)? Toxicon 2018, 144, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Prieto, A.I.; Pichardo, S.; Jos, A.; Moreno, I.; Camean, A.M. Time-dependent oxidative stress responses after acute exposure to toxic cyanobacterial cells containing microcystins in tilapia fish (Oreochromis niloticus) under laboratory conditions. Aquat. Toxicol. 2007, 84, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Pavagadhi, S.; Gong, Z.; Hande, M.P.; Dionysiou, D.D.; de la Cruz, A.A.; Balasubramanian, R. Biochemical response of diverse organs in adult Danio rerio (zebrafish) exposed to sub-lethal concentrations of microcystin-LR and microcystin-RR: A balneation study. Aquat. Toxicol. 2012, 109, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, D.; Signore, A.; Araneda, O.; Contreras, H.R.; Concha, M.; García, C. Toxicity and differential oxidative stress effects on zebrafish larvae following exposure to toxins from the okadaic acid group. J. Toxicol. Environ. Health A 2020, 83, 573–588. [Google Scholar] [CrossRef] [PubMed]
- Prego-Faraldo, M.V.; Valdiglesias, V.; Laffon, B.; Mendez, J.; Eirin-Lopez, J.M. Early Genotoxic and Cytotoxic Effects of the Toxic Dinoflagellate Prorocentrum lima in the Mussel Mytilus galloprovincialis. Toxins 2016, 8, 159. [Google Scholar] [CrossRef] [Green Version]
- Valdiglesias, V.; Prego-Faraldo, M.V.; Pásaro, E.; Méndez, J.; Laffon, B. Okadaic acid: More than a diarrheic toxin. Mar. Drugs 2013, 11, 4328–4349. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wang, P.; Hou, H.; Wen, H.; Zhou, H.; Gao, F.; Wu, J.; Qiu, Z.; Li, L. Histone Modification Is Involved in Okadaic Acid (OA) Induced DNA Damage Response and G2-M Transition Arrest in Maize. PLoS ONE 2016, 11, e0155852. [Google Scholar] [CrossRef] [Green Version]
- Hellou, J.; Neil, N.N.; Moon, T.W. Glutathione, glutathione S-transferase, and glutathione conjugates, complementary markers of oxidative stress in aquatic biota. Environ. Sci. Pollut. Res. 2012, 19, 2007–2023. [Google Scholar] [CrossRef]
- Kirchen, R.V.; West, W.R. The Japanese Medaka: Care and Development; Carolina Biological Supply: Burlington, NC, USA, 1976. [Google Scholar]
- Shima, A.; Mitani, H. Medaka as a research organism: Past, present and future. Mech. Dev. 2004, 121, 599–604. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chiang, C.Y.; Tsai, H.J. Zebrafish and Medaka: New model organisms for modern biomedical research. J. Biomed. Sci. 2016, 23, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasconcelos, V.; Azevedo, J.; Silva, M.; Ramos, V. Effects of marine toxins on the reproduction and early stages development of aquatic organisms. Mar. Drugs 2010, 8, 59–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, S.D.; Kurobe, T.; Hammock, B.G.; Lam, C.H.; Wu, G.; Vasylieva, N.; Gee, S.J.; Hammock, B.D.; Teh, S.J. Developmental effects of fipronil on Japanese Medaka (Oryzias latipes) embryos. Chemosphere 2017, 166, 511–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwamatsu, T. Stages of normal development in the medaka Oryzias latipes. Mech. Dev. 2004, 121, 605–618. [Google Scholar] [CrossRef]
- Porazinski, S.R.; Wang, H.; Furutani-Seiki, M. Dechorionation of medaka embryos and cell transplantation for the generation of chimeras. J. Vis. Exp. 2010, 46, 2055. [Google Scholar] [CrossRef] [Green Version]
- Jung, G.; Hug, M.; Halter, C.; Friesenhengst, A.; Walzer, J.; Czerny, T. Diffusion of small molecules into medaka embryos improved by electroporation. BMC Biotechnol. 2013, 13, 53. [Google Scholar] [CrossRef] [Green Version]
- Liedtke, D.; Erhard, I.; Schartl, M. Snail gene expression in the medaka, Oryzias latipes. Gene Expr. Patterns 2011, 11, 181–189. [Google Scholar] [CrossRef]
- OECD. Test N° 210: Fish Early–Life Stage Toxicity Test, OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2013. [Google Scholar]
- Zhang, D.L.; Hu, C.X.; Li, D.H.; Liu, Y.D. Zebrafish locomotor capacity and brain acetylcholinesterase activity is altered by Aphanizomenon flos-aquae DC-1 aphantoxins. Aquat. Toxicol. 2013, 138, 139–149. [Google Scholar] [CrossRef]
- Iwamatsu, T.; Nakamura, H.; Ozato, K.; Wakamatsu, Y. Normal Growth of the “See-Through” Medaka. Zool. Sci. 2003, 20, 607–615. [Google Scholar] [CrossRef]
- Roubach, R.; Gomes, L.C.; Val, A.L. Safest level of tricaine methane- sulfonate (MS-222) to induce anesthesia in juveniles of matrinxa Brycon cephalus. Acta Amaz. 2001, 31, 159–163. [Google Scholar] [CrossRef]
- Escoffier, N.; Gaudin, J.; Mezhoud, K.; Huet, H.; Château-Joubert, S.; Turquet, J.; Crespeau, F.; Edery, M. Toxicity to medaka fish embryo development of okadaic acid and crude extracts of Prorocentrum dinoflagellates. Toxicon 2007, 49, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- Tschirren, L.; Siebenmann, S.; Pietsch, C. Toxicity of Ochratoxin to Early Life Stages of Zebrafish (Danio rerio). Toxins 2018, 10, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MotulsKy, H.J.; Christopoulus, A. Fitting model to biological data using linear and nonlinear regression. In A Practical Guide to Curve Fitting; GraphPad Software Inc.: San Diego, CA, USA, 2003; pp. 1–296. [Google Scholar]
- Zhao, L.; Guo, D.; Lin, J.; Liu, R. Responses of catalase and superoxide dismutase to low-dose quantum dots on molecular and cellular levels. Ecotoxicol. Environ. Saf. 2019, 181, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Simos, Y.V.; Verginadis, I.I.; Toliopoulos, I.K.; Velalopoulou, A.P.; Karagounis, I.V.; Karkabounas, S.C.; Evangelou, A.M. Effects of catechin and epicatechin on superoxide dismutase and glutathione peroxidase activity, in vivo. Redox Rep. 2012, 17, 181–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowaltowski, A.J.; Vercesi, A.E.; Rhee, S.G.; Netto, L.E. Catalases and thioredoxin peroxidase protect Saccharomyces cerevisiae against Ca(2+)-induced mitochondrial membrane permeabilization and cell death. FEBS Lett. 2000, 473, 177–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.C.; Han, G.Y. A procedure for quantitative determination of tris(2-carboxyethyl)phosphine, an odorless reducing agent more stable and effective than dithiothreitol. Anal Biochem. 1994, 220, 5–10. [Google Scholar] [CrossRef]
- Gupta, B.L.; Baquer, N.Z. Hexokinase, glucose-6-phosphate dehydrogenase and antioxidant enzymes in diabetic reticulocytes: Effects of insulin and vanadate. Biochem. Mol. Biol. Int. 1998, 46, 1145–1152. [Google Scholar] [CrossRef]
- Parraguez, V.H.; Atlagich, M.; Araneda, O.F.; García, C.; Muñoz, A.; De Los Reyes, M.; Urquieta, B. Effects of antioxidant vitamins on newborn and placental traits in gestations at high altitude: Comparative study in high and low altitude native sheep. Reprod. Fertil. 2011, 23, 285–296. [Google Scholar] [CrossRef] [Green Version]
- Zeb, A.; Ullah, F. A Simple Spectrophotometric Method for the Determination of Thiobarbituric Acid Reactive Substances in Fried Fast Foods. J. Anal. Methods Chem. 2016, 2016, 9412767. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- EURLMB European Union Reference Laboratory for Marine Biotoxins. EU-Harmonised Standard Operating Procedure for Determination of Lipophilic Marine Biotoxins in Molluscs by LC-MS/MS. Version. 5. 2015. Available online: http://www.aecosan.msssi.gob.es/AECOSAN/docs/documntos/laboratorios/LNRBM/ARCHIVO2EUHarmonised-SOP-LIPOLCMSMS_Version5.Pdf (accessed on 4 February 2016).
- Wells, M.L.; Trainer, V.L.; Smayda, T.J.; Karlson, B.S.; Trick, C.G.; Kudela, R.M.; Ishikawa, A.; Bernard, S.; Wulff, A.; Anderson, D.M.; et al. Harmful algal blooms and climate change: Learning from the past and present to forecast the future. Harmful Algae 2015, 49, 68–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hennon, G.M.M.; Dyhrman, S.T. Progress and promise of omics for predicting the impacts of climate change on harmful algal blooms. Harmful Algae 2020, 91, 101587. [Google Scholar] [CrossRef] [PubMed]
- Ralston, D.K.; Moore, S.K. Modeling harmful algal blooms in a changing climate. Harmful Algae 2020, 91, 101729. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, G.; Díaz, P.A.; Godoy, M.; Araya, M.; Ganuza, I.; Pino, R.; Álvarez, F.; Rengel, J.; Hernández, C.; Uribe, E.; et al. Paralytic Shellfish Toxins in Surf Clams Mesodesma donacium during a Large Bloom of Alexandrium catenella Dinoflagellates Associated to an Intense Shellfish Mass Mortality. Toxins 2019, 11, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, P.; Krock, B.; Hansen, P.J.; Vismann, B. Effects of the DSP-toxic dinoflagellate Dinophysis acuta on clearance and respiration rate of the blue mussel, Mytilus edulis. PLoS ONE 2020, 15, e0230176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO/WHO Food and Agriculture Organization/World Health Organization. Technical Paper on Toxicity Equivalency Factors for Marine Biotoxins Associated with Bivalve Molluscs; FAO/WHO: Rome, Italy, 2016; p. 108. [Google Scholar]
- Botana, L.M.; Hess, P.; Munday, R.; Nathalie, A.; De Grasse, S.L.; Feeley, M.; Suzuki, T.; van der Berg, M.; Fattori, V.; Garrido-Gamarra, E.; et al. Derivation of toxicity equivalency factors for marine biotoxins associated with bivalve molluscs. Trends Food Sci Technol. 2017, 50, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Fernández, D.A.; Louzao, M.C.; Fraga, M.; Vilariño, N.; Vieytes, M.R.; Botana, L.M. Experimental basis for the high oral toxicity of dinophysistoxin 1: A comparative study of DSP. Toxins 2014, 6, 211–228. [Google Scholar] [CrossRef] [Green Version]
- Gong, Y.; Zhang, K.; Geng, N.; Wu, M.; Li, X.; Liu, R.; Challis, J.; Codling, G.; Genbo Xu, E.; Giesy, J.P. Molecular mechanisms of zooplanktonic toxicity in the okadaic acid-producing dinoflagellate Prorocentrum lima. Environ. Pollut. 2021, 279, 116942. [Google Scholar] [CrossRef]
- Kaushik, S.; Kaur, J. Chronic cold exposure affects the antioxidant defense system in various rat tissues. Clin. Chim. Acta 2003, 333, 69–77. [Google Scholar] [CrossRef]
- Gallagher, E.P.; Sheehy, K.M. Altered glutathione-S-transferase catalytic activities in female brown bullheads from a contaminated site in central Florida lakes. Mar. Environ. Res. 2000, 50, 399–403. [Google Scholar] [CrossRef]
- Hou, J.; Li, L.; Xue, T.; Long, M.; Su, Y.; Wu, N. Hepatic positive and negative antioxidant responses in zebrafish after intraperitoneal administration of toxic microcystin-LR. Chemosphere 2015, 120, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Rossignoli, A.E.; Blanco, J. Subcellular distribution of okadaic acid in the digestive gland of Mytilus galloprovincialis: First evidences of lipoprotein binding to okadaic acid. Toxicon 2010, 55, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yang, W.; Chen, Y.; Yang, Z. Effect of purified microcystin on oxidative stress of silver carp Hypophthalmichthys molitrix larvae under different ammonia concentrations. Biochem. Syst. Ecol. 2011, 39, 536–543. [Google Scholar] [CrossRef]
- de Morais Calado, S.L.G.; Souza Santos, T.P.; Borges Leite, J.; Wojciechowski, M.N.; Junior, D.C.; Bozza, V.; de Magalhaes, F.; Cestari, M.M.; Prodocimo, V.; Silva de Assis, H.C. Depuration time and sublethal effects of microcystins in a freshwater fish from water supply reservoir. Chemosphere 2018, 210, 805–815. [Google Scholar] [CrossRef]
- Dos Santos Carvalho, C.; Bernusso, V.A.; Selistre de Araujo, H.S.; Gaeta Espindola, E.L.; Fernandes, M.N. Biomarker responses as indication of contaminant effects in Oreochromis niloticus. Chemosphere 2012, 89, 60–69. [Google Scholar] [CrossRef]
- Corriere, M.; Soliño, L.; Costa, P.R. Effects of the Marine Biotoxins Okadaic Acid and Dinophysistoxins on Fish. J. Mar. Sci. Eng. 2021, 9, 293. [Google Scholar] [CrossRef]
- Corriere, M.; Baptista, M.; Paula, J.R.; Repolho, T.; Rosa, R.; Costa, P.R.; Soliño, L. Impaired fish swimming performance following dietary exposure to the marine phycotoxin okadaic acid. Toxicon 2020, 179, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Larsen, K.; Petersen, D.; Wilkins, A.L.; Samdal, I.A.; Sandvik, M.; Rundberget, T.; Goldstone, D.; Arcus, V.; Hovgaard, P.; Rise, F.; et al. Clarification of the C-35 stereochemistries of dinophysistoxin-1 and dinophysistoxin-2 and its consequences for binding to protein phosphatase. Chem. Res. Toxicol. 2007, 20, 868–875. [Google Scholar] [CrossRef]
- Sripathi, S.R.; He, W.; Um, H.Y.; Moser, T.; Dehnbostel, S.; Kindt, K.; Goldman, J.; Frost, M.C.; Jahng, W.J. Nitric oxide leads to cytoskeletal reorganization in the retinal pigment epithelium under oxidative stress. Adv. Biosci. Biotechnol. 2012, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- Low, I.C.; Loh, T.; Huang, Y.; Virshup, D.M.; Pervaiz, S. Ser70 phosphorylation of Bcl-2 by selective tyrosine nitration of PP2A-B56delta stabilizes its antiapoptotic activity. Blood 2014, 124, 2223–2234. [Google Scholar] [CrossRef]
- Kankaanpaa, H.; Leinio, S.; Olin, M.; Sjovall, O.; Meriluoto, J.; Lehtonen, J. Accumulation and depuration of cyanobacterial toxin nodularin and biomarker responses in the mussel Mytilus edulis. Chemosphere 2007, 68, 210–1220. [Google Scholar] [CrossRef] [PubMed]
- El Ghazali, I.; Saqrane, S.; Carvalho, A.P.; Ouahid, Y.; Oudra, B.; Fernandez Del Campo, F.; Vasconcelos, V. Compensatory Growth Induced in Zebrafish Larvae after Pre-Exposure to a Microcystis aeruginosa Natural Bloom Extract Containing Microcystins. Int. J. Mol. Sci. 2009, 10, 133–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibegelins, B.W.; Chorus, I. Accumulation of cyanobacterial toxins in freshwater “seafood” and its consequences for public health: A review. Environ. Pollut. 2007, 150, 177–192. [Google Scholar]
- Cazenave, J.M.; Bistoni, D.L.; Zwirnmann, E.; Wunderlin, D.A.; Wiegand, C. Attenuating effects of natural organic matter on microcystin toxicity in zebra fish (Danio rerio) embryos-benefits and costs of microcystin detoxication. Environ. Toxicol. 2006, 21, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Persson, K.J.; Legrand, C.; Olsson, T. Detection of nodularin in European flounder (Platichthys flesus) in the west coast of Sweden: Evidence of nodularin mediated oxidative stress. Harmful Algae 2009, 8, 832–838. [Google Scholar] [CrossRef]
- Huguet, A.; Drapeau, O.; Rousselet, F.; Quenault, H.; Fessard, V. Differences in Toxic Response Induced by Three Variants of the Diarrheic Shellfish Poisoning Phycotoxins in Human Intestinal Epithelial Caco-2 Cells. Toxins 2020, 12, 783. [Google Scholar] [CrossRef]
- Díaz, P.A.; Perez-Santo, I.; Alvarez, G.; Garreaud, R.; Pinilla, E.; Diaz, M.; Sandoval, A.; Araya, M.; Alvarez, F.; Rengel, J.; et al. Multiscale physical background to an exceptional harmful algal Bloom of Dinophysis acuta in a fjord system. Sci Total Environ. 2021, 773, 145621. [Google Scholar] [CrossRef]


| Abnormalities | Control | Exposed OA * | Exposed DTX-1 * |
|---|---|---|---|
| Edema | 0 | 10 | 15 |
| Cyclopia | 0 | 5–10 | 10 |
| Lethargy | 0 | 40 | 40 |
| Paresis | 0 | 70 | 70 |
| Touch responses | 0 | 60 | 80 |
| Craniofacial deformities | 0 | 5 | 5–10 |
| Pericardial edema | 0 | 5 | 10–15 |
| Spinal deformities | 0 | 5–8 | 10 |
| Reduced eye pigmentation | 0 | 5 | 10 |
| Developmental rate | |||
| Shortening of the anterior-posterior axis | 0 | 10–15 | 20–25 |
| Developmental delay | 0 | 30 | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueroa, D.; Ríos, J.; Araneda, O.F.; Contreras, H.R.; Concha, M.L.; García, C. Oxidative Stress Parameters and Morphological Changes in Japanese Medaka (Oryzias latipes) after Acute Exposure to OA-Group Toxins. Life 2023, 13, 15. https://doi.org/10.3390/life13010015
Figueroa D, Ríos J, Araneda OF, Contreras HR, Concha ML, García C. Oxidative Stress Parameters and Morphological Changes in Japanese Medaka (Oryzias latipes) after Acute Exposure to OA-Group Toxins. Life. 2023; 13(1):15. https://doi.org/10.3390/life13010015
Chicago/Turabian StyleFigueroa, Diego, Javiera Ríos, Oscar F. Araneda, Héctor R. Contreras, Miguel L. Concha, and Carlos García. 2023. "Oxidative Stress Parameters and Morphological Changes in Japanese Medaka (Oryzias latipes) after Acute Exposure to OA-Group Toxins" Life 13, no. 1: 15. https://doi.org/10.3390/life13010015
APA StyleFigueroa, D., Ríos, J., Araneda, O. F., Contreras, H. R., Concha, M. L., & García, C. (2023). Oxidative Stress Parameters and Morphological Changes in Japanese Medaka (Oryzias latipes) after Acute Exposure to OA-Group Toxins. Life, 13(1), 15. https://doi.org/10.3390/life13010015








