Combining Oxidative Stress Markers and Expression of Surfactant Protein A in Lungs in the Diagnosis of Seawater Drowning
Abstract
:1. Introduction
2. Materials and Methods
2.1. Forensic Autopsy Cases
2.2. Sample Preparation
2.3. Analysis of Oxidative Stress Markers in the Lung
2.3.1. Determination of Malondialdehyde
2.3.2. Determination of Glutathione Levels
2.3.3. Determination of Pulmonary Surfactant Protein A
2.4. Statistical Analysis
3. Results
3.1. Sociodemographic Characteristics According to Types and Causes of Death
3.2. Oxidative Stress Markers in Lung Tissue
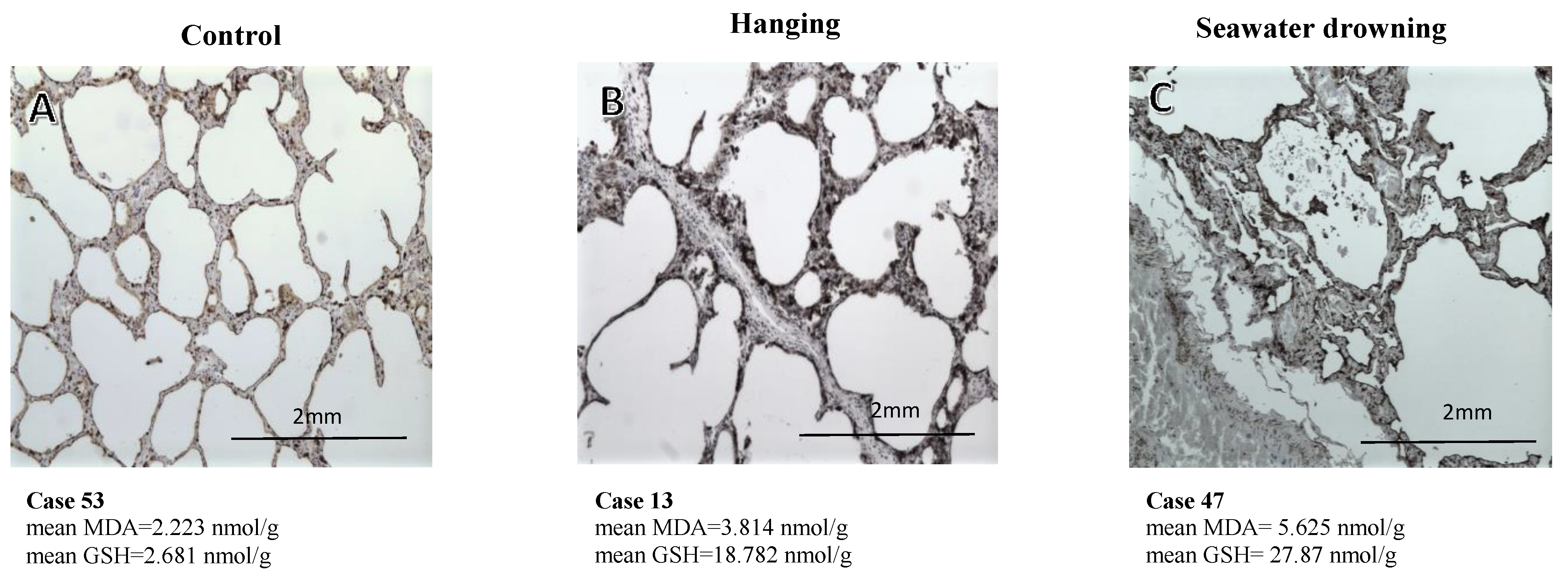
3.3. Immunohistochemical Expression of the SP-A Protein
3.4. Relationship between Oxidative Stress Levels and the Immunohistochemical Expression of SP-A
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Drowning. Available online: https://www.who.int/news-room/fact-sheets/detail/drowning (accessed on 1 January 2020).
- Girela-López, E.; Beltran-Aroca, C.M.; Dye, A.; Gill, J.R. Epidemiology and autopsy findings of 500 drowning deaths. Forensic Sci. Int. 2022, 330, 111137. [Google Scholar] [CrossRef]
- Torimitsu, S.; Yajima, D.; Inokuchi, G.; Makino, Y.; Motomura, A.; Chiba, F.; Yamaguchi, R.; Hoshioka, Y.; Tsuneya, S.; Iwase, H. Electrolyte analysis of pleural effusion for discrimination between seawater and freshwater drowning in decomposed bodies. J. Forensic Leg. Med. 2022, 90, 102389. [Google Scholar] [CrossRef]
- Van Beeck, E.F.; Branche, C.M.; Szpilman, D.; Modell, J.H.; Bierens, J.J.L.M. A new definition of drowning: Towards documentation and prevention of a global public health problem. Bull. World Health Organ. 2005, 83, 853–856. [Google Scholar]
- Szpilman, D.; Bierens, J.J.L.M.; Handley, A.J.; Orlowski, J.P. Drowning. N. Engl. J. Med. 2012, 366, 2102–2110. [Google Scholar] [CrossRef]
- Lee, D.H.; Park, J.H.; Choi, S.P.; Oh, J.H.; Wee, J.H. Clinical characteristics of elderly drowning patients. Am. J. Emerg. Med. 2019, 37, 1091–1095. [Google Scholar] [CrossRef]
- Bierens, J.; Tot, M.; Van Drenkelingen, R.; Lunetta, P.; Tipton, M.; Bierens, J.; Lunetta, P.; Tipton, M. Pathophysiology of drowning 85.1 variety in drowning scenarios 85.2 pathophysiological mechanisms. In Drowning; Springer: Berlin/Heidelberg, Germany, 2014; pp. 545–560. [Google Scholar] [CrossRef]
- Bierens, J.J.L.M.; Lunetta, P.; Tipton, M.; Warner, D.S. Physiology of drowning: A review. Physiology 2016, 31, 147–166. [Google Scholar] [CrossRef] [Green Version]
- Farrugia, A.; Ludes, B. Diagnostic of drowning in forensic Medicine. In Forensic Medicine—From Old Problems to New Challenges; InTech: Rang-Du-Fliers, France, 2011. [Google Scholar] [CrossRef] [Green Version]
- Papadodima, S.A.; Athanaselis, S.A.; Skliros, E.; Spiliopoulou, C.A. Forensic investigation of submersion deaths. Int. J. Clin. Pract. 2010, 64, 75–83. [Google Scholar] [CrossRef]
- Byard, R.W. Immersion deaths and drowning: Issues arising in the investigation of bodies recovered from water. Forensic Sci. Med. Pathol. 2015, 11, 323–325. [Google Scholar] [CrossRef] [Green Version]
- Piette, M.H.A.; De Letter, E.A. Drowning: Still a difficult autopsy diagnosis. Forensic Sci. Int. 2006, 163, 1–9. [Google Scholar] [CrossRef]
- Fortes, F.J.; Perez-Carceles, M.D.; Sibon, A.; Luna, A.; Laserna, J.J. Spatial distribution analysis of strontium in human teeth by laser-induced breakdown spectroscopy: Application to diagnosis of seawater drowning. Int. J. Legal Med. 2015, 129, 807–813. [Google Scholar] [CrossRef]
- Pollanen, M.S. Deciding the cause of death after autopsy—Revisited. J. Clin. Forensic. Med. 2005, 12, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Azparren, J.; Ortega, A.; Bueno, H.; Andreu, M. Blood strontium concentration related to the length of the agonal period in seawater drowning cases. Forensic Sci. Int. 2000, 108, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Betz, P.; Nerlich, A.; Penning, R.; Eisenmenger, W. Alveolar macrophages and the diagnosis of drowning. Forensic Sci. Int. 1993, 62, 217–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byard, R.W.; Houldsworth, G.; James, R.A.; Gilbert, J.D. Characteristic features of suicidal drownings: A 20-year study. Am. J. Forensic Med. Pathol. 2001, 22, 134–138. [Google Scholar] [CrossRef]
- Hürlimann, J.; Feer, P.; Elber, F.; Niederberger, K.; Dirnhofer, R.; Wyler, D. Diatom detection in the diagnosis of death by drowning. Int. J. Legal. Med. 2000, 114, 6–14. [Google Scholar] [CrossRef]
- Mueller, J.B. The drowning/near-drowning patient. In Preanesthetic Assessment 3; Birkhäuser: Boston, MA, USA, 1991; pp. 111–123. [Google Scholar]
- Pérez-Cárceles, M.D.; del Pozo, S.; Sibón, A.; Noguera, J.A.; Osuna, E.; Vizcaya, M.A.; Luna, A. Serum biochemical markers in drowning: Diagnostic efficacy of Strontium and other trace elements. Forensic Sci. Int. 2012, 214, 159–166. [Google Scholar] [CrossRef]
- Zhu, B.L.; Ishikawa, T.; Michiue, T.; Tanaka, S.; Zhao, D.; Li, D.R.; Quan, L.; Oritani, S.; Maeda, H. Differences in postmortem urea nitrogen, creatinine and uric acid levels between blood and pericardial fluid in acute death. Leg. Med. 2007, 9, 115–122. [Google Scholar] [CrossRef]
- Ramanathan, L.; Gozal, D.; Siegel, J.M. Antioxidant responses to chronic hypoxia in the rat cerebellum and pons. J. Neurochem. 2005, 93, 47–52. [Google Scholar] [CrossRef]
- Maiti, P.; Singh, S.B.; Sharma, A.K.; Muthuraju, S.; Banerjee, P.K.; Ilavazhagan, G. Hypobaric hypoxia induces oxidative stress in rat brain. Neurochem. Int. 2006, 49, 709–716. [Google Scholar] [CrossRef]
- Lewén, A.; Matz, P.; Chan, P.H. Free Radical Pathways in CNS Injury. J. Neurotruama 2009, 17, 871–890. [Google Scholar] [CrossRef]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef]
- Adam-Vizi, V. Production of Reactive Oxygen Species in Brain Mitochondria: Contribution by Electron Transport Chain and Non–Electron Transport Chain Sources. Antioxid. Redox Signal. 2005, 7, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Doménech, P.; Carbonell, L.; Pérez Cárceles, M.D.; Falcón, M.; Luna, A.; Osuna, E. Application of postmortem lipid peroxidation in heart tissue to the diagnosis of myocardial damage. Int. J. Leg. Med. 2004, 118, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Osuna, E.; Pérez-Cárceles, M.D.; García-Lorente, A.; Sánchez-Hanke, M.; Vieira, D.N.; Carvalho, L.; Püschel, K.; Luna, A. Lipid peroxidation in lung tissue after chest trauma and correlation with the duration of the post-trauma survival period. Int. J. Leg. Med. 1998, 111, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Holmskov, U.L. Collectins and collectin receptors in innate immunity. APMIS Suppl 2000, 100, 1–59. [Google Scholar] [CrossRef]
- Campobasso, C.P.; Colonna, M.F.; Zotti, F.; Sblano, S.; Dell’erba, A.S. An immunohistochemical study of pulmonary surfactant apoprotein A (SP-A) in forensic autopsy materials. Rom. J. Leg. Med. 2012, 20, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.L.; Ishida, K.; Quan, L.; Li, D.R.; Taniguchi, M.; Fujita, M.Q.; Maeda, H.; Tsuji, T. Pulmonary immunohistochemistry and serum levels of a surfactant-associated protein A in fatal drowning. Leg. Med. 2002, 4, 1–6. [Google Scholar] [CrossRef]
- Pérez-Cárceles, M.D.; Sibón, A.; Vizcaya, M.A.; Osuna, E.; Gómez-Zapata, M.; Luna, A.; Martínez-Díaz, F. Histological findings and immunohistochemical surfactant protein A (SP-A) expression in asphyxia: Its application in the diagnosis of drowning. Histol. Histopathol. 2008, 23, 1061–1068. [Google Scholar] [CrossRef]
- Radin, N.S. Preparation of lipid extracts. Methods Enzymol 1969, 14, 245–254. [Google Scholar] [CrossRef]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990, 186, 407–421. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Akerboom, T.P.M.; Sies, H. Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol. 1981, 77, 373–382. [Google Scholar] [CrossRef]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Yoshio, K.; Yoshitaka, F.; Hiroki, T.; Toyoaki, A. Monoclonal antibodies against human pulmonary surfactant apoproteins: Specificity and application in immunoassay. Biochim. Biophys. Acta-Lipids Lipid Metab. 1985, 836, 201–209. [Google Scholar] [CrossRef]
- Zhu, B.L.; Ishida, K.; Quan, L.; Fujita, M.Q.; Maeda, H. Immunohistochemistry of pulmonary surfactant apoprotein A in forensic autopsy: Reassessment in relation to the causes of death. Forensic Sci. Int. 2000, 113, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Szpilman, D.; Morgan, P.J. Management for the drowning patient. Chest 2021, 159, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S. Lung oedema in acute lung injury. In Anesthesia, Pain, Intensive Care and Emergency Medicine—A.P.I.C.E., Proceeding of the 20th Postgraduate Course in Critical Care Medicine Trieste, Italy, 18–21 November 2005; Springer: Berlin, Germany, 2005; pp. 345–355. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative stress mitigation by antioxidants - An overview on their chemistry and influences on health status. Eur. J. Med. Chem. 2021, 209, 112891. [Google Scholar] [CrossRef]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative stress in human pathology and aging: Molecular mechanisms and perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef]
- Thimmulappa, R.K.; Chattopadhyay, I.; Rajasekaran, S. Oxidative stress mechanisms in the pathogenesis of environmental lung diseases. Oxid. Stress Lung Dis. 2019, 2, 103–137. [Google Scholar] [CrossRef]
- Gu, M.; Mei, X.L.; Zhao, Y.N. Sepsis and Cerebral Dysfunction: BBB damage, neuroinflammation, oxidative stress, apoptosis and autophagy as key mediators and the potential therapeutic approaches. Neurotox Res. 2020, 39, 489–503. [Google Scholar] [CrossRef]
- Turcu, V.; Wild, P.; Hemmendinger, M.; Sauvain, J.J.; Bergamaschi, E.; Hopf, N.B.; Canu, I.G. Towards reference values for malondialdehyde on exhaled breath condensate: A systematic literature review and meta-analysis. Toxics 2022, 10, 258. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Sun, L.; Zhang, Y.; Wang, Y.; Zheng, J. Imbalanced GSH/ROS and sequential cell death. J. Biochem. Mol. Toxicol. 2022, 36, e22942. [Google Scholar] [CrossRef] [PubMed]
- Tahamtan, R.; Monfared, A.S.; Tahamtani, Y.; Tavassoli, A.; Akmali, M.; Mosleh-Shirazi, M.A.; Naghizadeh, M.M.; Ghasemi, D.; Keshavarz, M.; Haddadi, G.H. Radioprotective effect of melatonin on radiation-inducedlung injury and lipid peroxidation in rats. Cell J. 2015, 17, 111. [Google Scholar] [CrossRef] [PubMed]
- Olmeda, B.; Martínez-Calle, M.; Pérez-Gil, J. Pulmonary surfactant metabolism in the alveolar airspace: Biogenesis, extracellular conversions, recycling. Ann. Anat.-Anat. Anzeiger. 2017, 209, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, R.; Sestili, C.; Prosperini, G.; Cecchetto, G.; Vicini, E.; Viel, G.; Muciaccia, B. Markers of mechanical asphyxia: Immunohistochemical study on autoptic lung tissues. Int. J. Leg. Med. 2014, 128, 117–125. [Google Scholar] [CrossRef]
- Stemberga, V.; Stifter, S.; Cuculić, D.; Čoklo, M.; Bosnar, A. Immunohistochemical surfactant protein-A expression: Fatal drowning vs. postmortem immersion. Med. Hypotheses 2009, 72, 413–415. [Google Scholar] [CrossRef]
- Clarkson, P.M.; Thompson, H.S. Antioxidants: What role do they play in physical activity and health? Am. J. Clin. Nutr. 2000, 72, 637S–646S. [Google Scholar] [CrossRef]
- Aguilar, A.; Alvarez-Vijande, R.; Capdevila, S.; Alcoberro, J.; Alcaraz, A. Antioxidant patterns (superoxide dismutase, glutathione reductase, and glutathione peroxidase) in kidneys from non–heart-beating-donors: Experimental study. Transplant. Proc. 2007, 39, 249–252. [Google Scholar] [CrossRef]
- Iraz, M.; Ozerol, E.; Gulec, M.; Tasdemir, S.; Idiz, N.; Fadillioglu, E.; Naziroglu, M.; Akyol, O. Protective effect of caffeic acid phenethyl ester (CAPE) administration on cisplatin-induced oxidative damage to liver in rat. Cell Biochem. Funct. 2006, 24, 357–361. [Google Scholar] [CrossRef]
- Cheeseman, K.H.; Slater, T.F. An introduction to free radical biochemistry. Br. Med. Bull 1993, 49, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Sener, M.T.; Suleyman, H.; Hacimuftuoglu, A.; Polat, B.; Cetin, N.; Suleyman, B.; Akcay, F. Estimating the postmortem interval by the difference between oxidant/antioxidant parameters in liver tissue. Adv. Clin. Exp. Med. 2012, 21, 727–733. Available online: https://europepmc.org/article/med/23457129 (accessed on 25 October 2022). [PubMed]
| Cause and Type of Death | Number of Cases N = 93 | Male | Female | PMI (h) | Mean Weight of Both Lungs (g) | Right Lung Weight (g) | Left Lung Weight (g) | |
|---|---|---|---|---|---|---|---|---|
| n (%) 50.05 ± 19.28 (Mean ± SD) * | N = 78 (83.9) n (%) (Mean ± SD) | N = 15 (16.1) n (%) (Mean ± SD) | N = 93, n (%) 21.17 ± 8.56 (Mean ± SD) | N = 93, (Mean ± SD) | N = 93, (Mean ± SD) | N = 93, (Mean ± SD) | p ** | |
| Seawater drowning (SWD) | 35 (37.6) 50.83 ± 18.62 | 32 (91.4) 50.7 ± 18.7 | 3 (8.6) 52.0 ± 21.6 | 22.0 ± 11.6 | 724.9 ± 285.3 | 750.3 ± 304.4 | 699.5 ±276.1 | 0.074 |
| Other types of asphyxia | 33 (35.5) 48.9 ± 20.2 | 31 (93.9) 48.2 ± 20.6 | 2 (6.1) 59.0 ± 12.7 | 20.3 ± 7.6 | 474.1 ±183.7 | 510.0 ± 193.7 | 439.4 ± 176.6 | 0.009 |
| Hanging | 23 (24.7) 49.5 ±21.8 | 22 (95.7) 49.5 ± 22.3 | 1 (4.3) 50.0 | 19.5 ± 6.0 | 464.1 ± 191.6 | 502.7 ± 204.0 | 425.5 ± 181.7 | 0.011 |
| Suffocation | 6 (6.5) 52.0 ± 19.9 | 5 (83.3) 48.8 ± 20.4 | 1 (16.7) 68.0 | 25.1 ± 12.4 | 582.2 ± 58.8 | 627.5 ± 74.2 | 537.0 ± 67.8 | 0.180 |
| Strangulation | 4 (4.3) 51.2 ± 19.5 | 4 (100) 51.2 ± 19.5 | - | 22.0 ± 2.1 | 515.8 ± 101.5 | 528.3 ± 107.3 | 503.4 ± 98.5 | 0.231 |
| Other types of death | 25 (26.9) 50.4 ± 19.6 | 15 (60.0) 48.2 ± 20.6 | 10 (40.0) 52.5 ±15.2 | 19.4 ± 6.1 | 617.2 ± 253.9 | 672.7 ± 198.5 | 561.6 ± 177.1 | 0.008 |
| Multiple trauma | 17 (18.3) 53.1 ± 17.6 | 8 (47.1) 51.2 ± 20.0 | 9 (52.9) 54.8 ± 14.0 | 20.2 ± 4.6 | 637.9 ± 217.4 | 691.6 ± 232.7 | 584.1 ± 206.4 | 0.028 |
| Cardiovascular disease | 4 (4.3) 37.5 ± 29.5 | 3 (75.0) 39.6 ± 35.8 | 1 (25.0) 31 | 17.0 ±11.2 | 440.4 ± 99.8 | 480.0 ± 89.2 | 400.8 ± 107.0 | 0.053 |
| Gunshot | 4 (4.3) 41.2 ± 15.3 | 4 (100) 41.2 ± 15.3 | - | 14.2 ± 6.6 | 692.9 ± 79.5 | 745.9 ± 68.9 | 640.0 ± 89.1 | 0.012 |
| Asphyxia | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Oxidative Stress Markers Concentration Median (IQR) Mean ± SD Min–Max * | Total n = 93 | SWD n = 35 | Other Asphyxias, n = 33 | Other Causes of Death n = 25 | P1 | P2 | P3 | P4 | P5 |
| MDA concentration | |||||||||
| Right lung | 3.438 (2.560) 3.799 ± 2.267 0.624–10.379 | 3.473 (2.542) 3.967 ± 2.243 0.624–10.379 | 3.811 (2.743) 3.931 ± 2.133 0.781–9.520 | 2.819 (2.310) 3.366 ± 2.200 0.724–9.882 | 0.919 | 0.906 | 0.705 | 0.749 | 0.773 |
| Left lung | 3.800 (1.935) 4.012 ± 2.232 0.766–12.607 | 3.960 (2.183) 4.512 ± 2.254 0.766–1.199 | 3.774 (3.248) 4.065 ± 2.836 1.519–12.607 | 3.475 (1.528) 3.303 ± 1.265 0.859–5.151 | 0.529 | 0.623 | 0.438 | 0.287 | 0.910 |
| Mean both lungs | 3.493 (2.081) 3.905 ± 0.798 0.77–9.98 | 3.966 (2.251) 4.253 ± 2.012 1.772–9.981 | 3.436 (3.162) 3.404 ± 1.345 1.302–5.941 | 2.965 (1.722) 3.043 ± 1.358 0.387–5.423 | 0.042 | 0.707 | 0.023 | 0.233 | 0.047 |
| GSH concentration | |||||||||
| Right lung | 2.420 (56.556) 31.270 ± 43.679 0.000–159.3 | 56.350 (85.575) 52.205 ± 49.707 0.000–159.321 | 7.070 (34.120) 25.784 ± 39.161 0.0–146.854 | 3.278 (1.238) 4.551 ± 12.033 0.000–40.851 | 0.005 | 0.257 | 0.002 | 0.015 | 0.017 |
| Left lung | 9.31 (68.78) 37.023 ± 45.977 0.000–162.800 | 36.120 (78.741) 45.477 ± 48.117 0.000–162.811 | 3.510 (56.60) 33.22 ± 48.68 0.000–157.301 | 3.965 (61.64) 28.255 ± 38.428 0.000–100.681 | 0.534 | 0.494 | 0.284 | 0.233 | 0.314 |
| Mean both lungs | 23.420 (50.15) 34.147 ± 40.777 0.000–153.30 | 41.080 (81.0) 48.841 ± 45.877 0.000–153.30 | 21.325 (32.97) 29.507 ± 40.18 0.000–150.3 | 3.942 (30.82) 16.40 ± 21.17 0.000–66.96 | 0.046 | 0.210 | 0.030 | 0.233 | 0.041 |
| Asphyxia | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Degree of SP-A Immunostaining (Mean ± SD) * Immunostaining Degree (0–3) | Total N = 93 | SWD N = 35 | Other Asphyxia N = 33 | Other Causes of Death N = 25 | P1 | P2 | P3 | P4 | P5 |
| Right lung | |||||||||
| SP-A membrane | 1.345 ± 0.630 0–3 | 1.862 ± 0.6930–3 | 1.062 ± 0.4350–2 | 1.087 ± 0.2881–2 | 0.000 | 0.000 | 0.000 | 0.845 | 0.000 |
| SP-A space | 1.595 ± 0.9580–3 | 2.344 ± 0.7201–3 | 1.656 ± 0.6530–3 | 0.565 ± 0.5890–2 | 0.000 | 0.004 | 0.000 | 0.000 | 0.000 |
| Left lung | |||||||||
| SP-A membrane | 1.381 ± 0.5990–3 | 1.896 ± 0.5570–3 | 1.125 ± 0.4910–2 | 1.087 ± 0.2881–2 | 0.000 | 0.000 | 0.000 | 0.684 | 0.000 |
| SP-A space | 1.523 ± 0.9750–3 | 2.137 ± 0.9900–3 | 1.687 ± 0.5351–3 | 0.521 ± 0.5930–2 | 0.000 | 0.007 | 0.000 | 0.000 | 0.000 |
| Right Lung | Left Lung | ||||
|---|---|---|---|---|---|
| Causes of Death | Degree of SP-A Immunostaining * | Alveolar Membrane | Alveolar Space | Alveolar Membrane | Alveolar Space |
| SWD (%) | 0 | 5.9 | 0 | 3,4 | 10.3 |
| 1 | 8.8 | 20.6 | 10.3 | 10.3 | |
| 2 | 67.6 | 38.2 | 79.3 | 34.5 | |
| 3 | 17.6 | 41.2 | 6.9 | 44.8 | |
| Other types of asphyxia (%) | 0 | 6.3 | 3.1 | 6.3 | 0 |
| 1 | 81.3 | 34.4 | 75 | 34.4 | |
| 2 | 12.5 | 56.3 | 18.4 | 62.5 | |
| 3 | 0 | 6.3 | 18.8 | 3.1 | |
| Other types of death (%) | 0 | 0 | 47.8 | 0 | 52.2 |
| 1 | 91.3 | 47.8 | 91.3 | 43.5 | |
| 2 | 8.7 | 4.3.4 | 87 | 4.3 | |
| 3 | 0 | 0 | 0 | 0 | |
| p | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Upper Lobe Lung | MDA r (p) | GSH r (p) |
|---|---|---|
| Right lung | ||
| SP-A alveolar membrane | 0.281 (p = 0.011) | 0.315 (p = 0.007) |
| SP-A alveolar space | 0.287 (p = 0.009) | 0.183 (p = 0.124) |
| Left lung | ||
| SP-A alveolar membrane | 0.252 (p = 0.027) | 0.151 (p = 0.222) |
| SP-A alveolar space | 0.350 (p = 0.002) | 0.253 (p = 0.039) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Legaz, I.; Barrera-Pérez, E.; Sibón, A.; Martínez-Díaz, F.; Pérez-Cárceles, M.D. Combining Oxidative Stress Markers and Expression of Surfactant Protein A in Lungs in the Diagnosis of Seawater Drowning. Life 2023, 13, 159. https://doi.org/10.3390/life13010159
Legaz I, Barrera-Pérez E, Sibón A, Martínez-Díaz F, Pérez-Cárceles MD. Combining Oxidative Stress Markers and Expression of Surfactant Protein A in Lungs in the Diagnosis of Seawater Drowning. Life. 2023; 13(1):159. https://doi.org/10.3390/life13010159
Chicago/Turabian StyleLegaz, Isabel, Estefanía Barrera-Pérez, Agustín Sibón, Francisco Martínez-Díaz, and María D. Pérez-Cárceles. 2023. "Combining Oxidative Stress Markers and Expression of Surfactant Protein A in Lungs in the Diagnosis of Seawater Drowning" Life 13, no. 1: 159. https://doi.org/10.3390/life13010159
APA StyleLegaz, I., Barrera-Pérez, E., Sibón, A., Martínez-Díaz, F., & Pérez-Cárceles, M. D. (2023). Combining Oxidative Stress Markers and Expression of Surfactant Protein A in Lungs in the Diagnosis of Seawater Drowning. Life, 13(1), 159. https://doi.org/10.3390/life13010159








