Abstract
Since the start of the SARS-CoV-2 pandemic, several scores have been proposed to identify infected individuals at a higher risk of progression and death. The most famous is the 4C score. However, it was developed in early 2020. Our study aimed to evaluate the accuracy of the 4C score during the wave in which the Omicron variant was prevalent. An observational study was conducted at an Italian University Hospital between 1 January and 31 July 2022. A receiver operating characteristic (ROC) curve analysis was performed to evaluate the ability of the 4C score to predict mortality. Overall, 1186 people were recruited, of which 160 (13.5%) died. According to the 4C score, 177 (11.6%) were classified as having a low risk of mortality, 302 (25.5%) were intermediate, 596 (50.3%) were high, and 151 (12.7%) were very high. The ROC curve of the 4C score showed an AUC (95% CI) value of 0.78 (0.74–0.82). At the criterion value of > 10, the sensitivity was 76.2% and the specificity was 62.67%. Similar to previous studies, the 4C mortality score performed well in our sample, and it is still a useful tool for clinicians to identify patients with a high risk of progression. However, clinicians must be aware that the mortality rate reported in the original studies was higher than that observed in our study.
Keywords:
SARS-CoV-2; COVID-19; 4C score; mortality; predictive score; Omicron variant; vaccine; antiviral treatment 1. Introduction
Since the beginning of the SARS-CoV-2 pandemic, infected people have reached half a billion with more than six million deaths. Asymptomatic or paucisymptomatic forms of infection occurred in most of the infected individuals [1]. The most prevalent symptoms of coronavirus disease 19 (COVID-19) are a fever, a cough, and dyspnea; a low proportion of patients complain of gastrointestinal symptoms, anosmia, dysgeusia, headaches, and skin lesions [2,3,4]. In addition, the infection can be associated with life-threatening systemic inflammation, respiratory failure, and multiorgan dysfunctions [5].
Many treatments have been proposed to reduce the disease progression in people with COVID-19. However, most of them have been rejected after different studies confirmed their futility (e.g., hydroxychloroquine and lopinavir/ritonavir). A massive vaccination campaign drained overcrowded hospitals. More than 12.99 billion doses have been administered since the start of the pandemic. However, vaccine uptake is now dramatically decreasing due to a reduced risk perception and the softening of enforcement policies. As a result, a risk of new waves of hospital congestion is around the corner. Unnecessary hospital admissions carry an increased risk of bed rest, immobilization, and nosocomial infections.
For this reason, a punctual and reliable classification of patients is mandatory to reduce hospitalization and health-system overloads. Several scores have been proposed to identify infected individuals at a higher risk of progression and death [6,7,8,9]. In September 2020, Knight et al. published their score (the 4C score); this was developed with a cohort of patients recruited from the ISARIC Coronavirus Clinical Characterisation Consortium (ICARIC-4C), which selected patients from 260 hospitals across England, Scotland, and Wales [10]. Jones et al. externally validated the score by using the records of the McMaster Multi-Regional Hospital Coronavirus Registry (COREG), a multicenter data registry from Ontario, Canada [11]. The 4C score is characterized by nine items (i.e., age, sex at birth, number of comorbidities, respiratory rate, peripheral oxygen saturation, Glasgow Coma Scale, urea, and C-reactive protein (CRP)). The score, ranging from 0–21, predicts in-hospital mortality from a low risk to a very high risk. It is easy and quick to apply, and few data are needed. However, the 4C score has many limitations. First, it is limited to determining in-patient hospital mortality and is not designed to be applicable to an outpatient setting. Second, it was developed using data collected between February and May 2020 from infections caused by a wild-type strain that is associated with a more severe disease compared with that caused by the Omicron variant. Third, no vaccines and early therapies (monlupiravir, nirmatrelvir/ritonavir, remdesivir, or monoclonal antibodies) were previously available. Finally, the 4C score considers the burden of comorbidities according to the presence of none (0 points), one (1 point), or more than one (2 points) without differentiating the type of comorbidities. For all these reasons, we believed that an external validation considering the latest changes in epidemiology and available treatments was necessary. Our study aimed to evaluate the accuracy of the 4C score in patients with SARS-CoV-2 infections during the wave in which the Omicron variant was prevalent.
2. Materials and Methods
2.1. Study Design and Sample
An observational study was conducted at an Italian University Hospital between 1 January and 31 July 2022. All people included in the study were evaluated by an infectious disease consultant when admitted to the emergency room, an infectious disease ward, or other wards for in-hospital infections.
The inclusion criteria were: (i) age ≥ 18 years; (ii) confirmed diagnosis of SARS-CoV-2 infection by a polymerase chain reaction (PCR) or third-generation antigenic test; (iii) collection of the variables needed for the computation of the 4C score; and (iv) having at least four weeks of follow-up.
Information on the medical history, symptoms, computer tomography (CT) findings, blood test results, cause/s of hospital admission, disease progression (need for oxygen supplementation, non-invasive or invasive ventilation, or death), and treatment were collected.
2.2. Outcome
The primary outcome was all-cause in-hospital mortality. Following the findings of the original manuscripts [10,11], we did not select a limit on the time until death. Therefore, people with more than four weeks of follow-up, but who were hospitalized at the time of the analysis were considered to be alive.
2.3. Statistical Analysis
The quantitative variables were summarized with medians and 25–75 percentiles (IQR); the qualitative ones were summarized by absolute and relative (percentage) frequencies. The Shapiro–Wilk test was used to assess the normality of the quantitative data. Differences in the quantitative variables were evaluated using the Mann–Whitney test; Pearson chi-squared or Fisher exact tests were used to assess the differences in the qualitative covariates. A receiver operating characteristic (ROC) curve analysis was performed to evaluate the ability of the 4C score to predict mortality. A two-tailed p-value less than 0.05 was considered to be statistically significant. Data analyses were carried out using STATA 17 (StataCorp, TX, USA).
3. Results
Overall, 1186 people were recruited. The median (IQR) age was 74 (62–83) years old and 54.3% were males. Of them, 160 (13.5%) died; these were older than those who survived (median (IQR) age of 81.5 (70–88) years old vs. 73 (60–83) years old; p < 0.0001) (Table 1).

Table 1.
Characteristics of 1186 people with SARS-CoV-2 infections stratified by survival.
The patients who died had a higher prevalence of chronic kidney disease, COPD, dementia, hematologic tumors, and cardiovascular diseases. Furthermore, people who died had a lower percentage of a complete vaccination course. The median levels of CRP and urea were significantly higher in the patients who did not survive. In addition, there was a higher percentage of people who complained about dyspnea at the moment of the first evaluation who died. Having ground-glass opacity (GGO) or consolidation at the CT scan were also more common in the group of those who died.
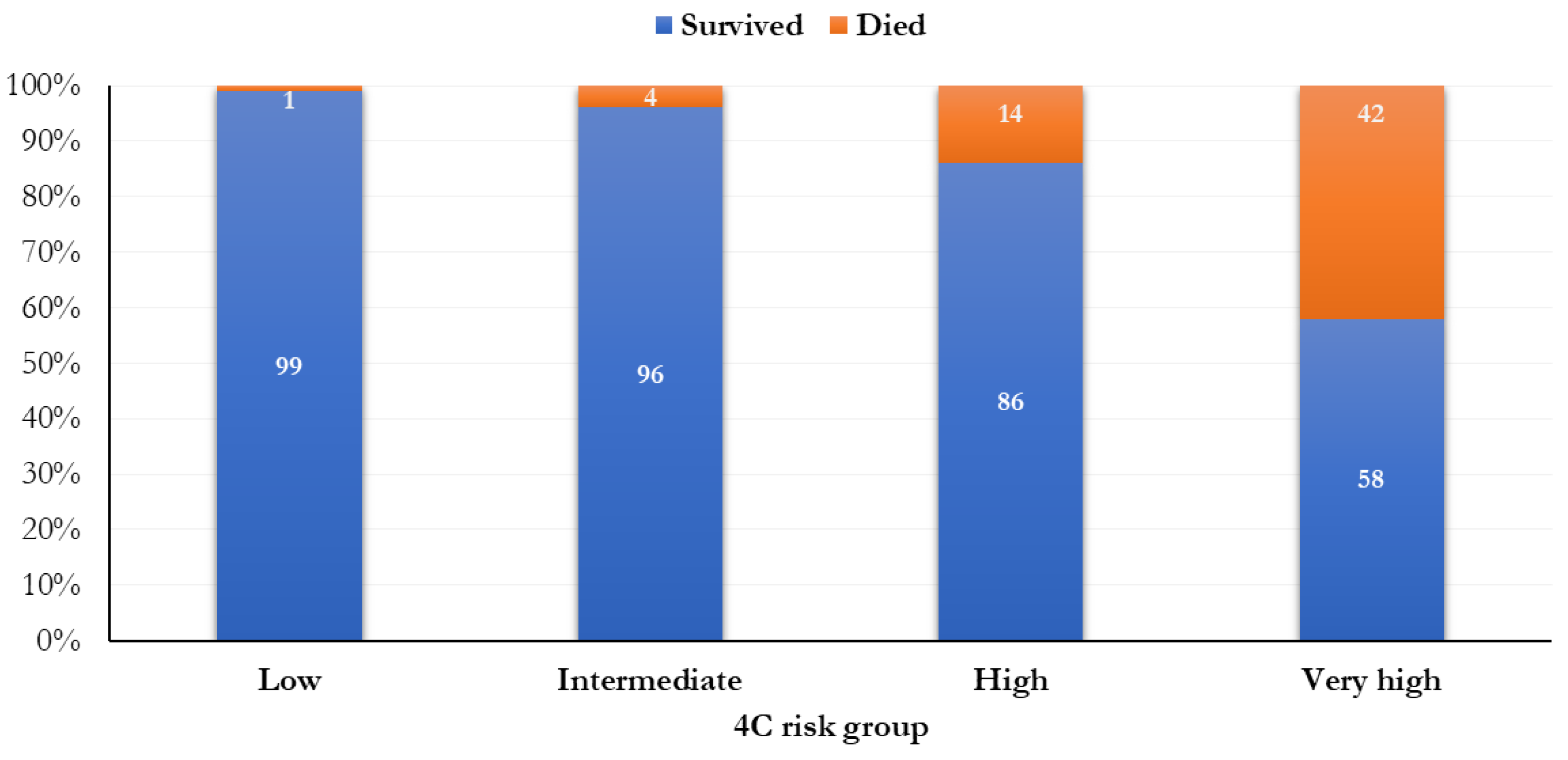
A total of 137 patients (11.6%) were classified as having a low risk of mortality (0–3 points), 302 (25.5%) were intermediate (4–8 points), 596 (50.3%) were high (9–14 points), and 151 (12.7%) were very high (≥ 15 points) (Figure 1). The mortality was 0.7% (1 person) in people with a low risk of mortality, 4.3% (13 subjects) in those with an intermediate risk of death, 13.9% (83 people) in people with a high risk of death, and 41.7% (63 subjects) in people with a very high risk of death, according to the 4C score.
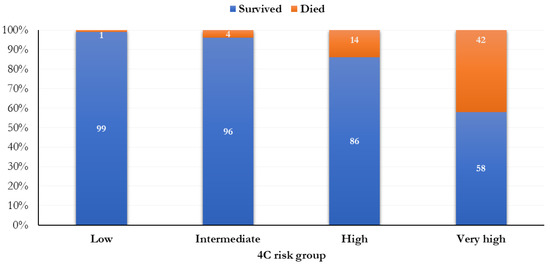
Figure 1.
Frequency distribution of the mortality by 4C risk groups.
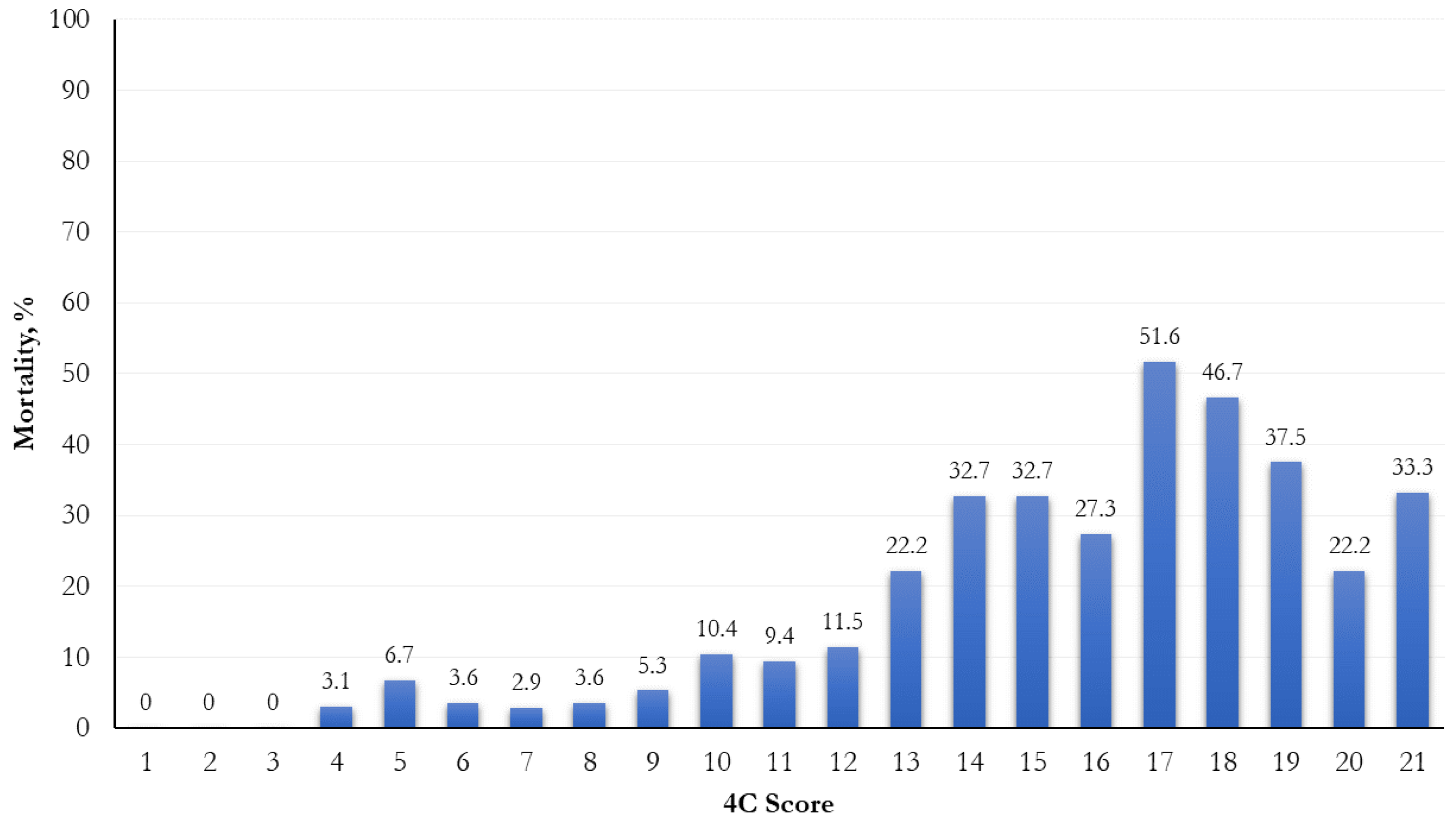
The mortality was significantly higher when a higher risk score was attributed (p < 0.0001; Figure 1). In relation to different 4C score cut-points (range: 0–21), the most increased mortality (51.6%) was observed in the 17th cut-point (Figure 2).
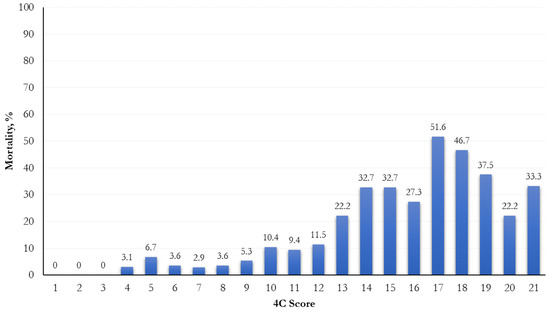
Figure 2.
Frequency distribution of mortality by 4C score cut-points.
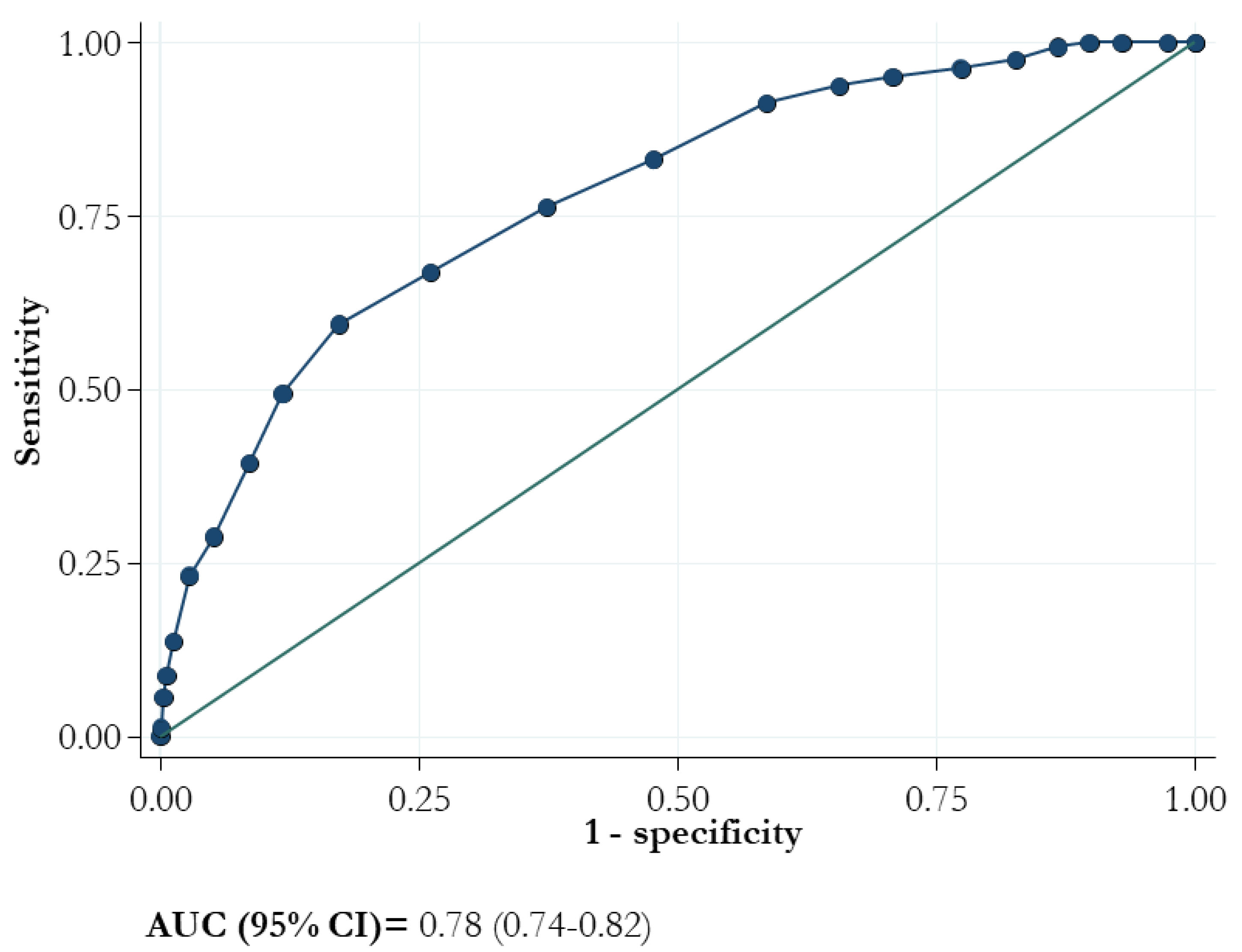
The ROC curve of the 4C score showed an AUC (95% CI) value of 0.78 (0.74–0.82; Figure 3).
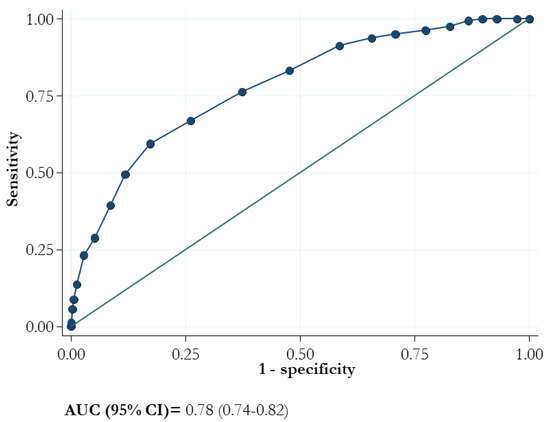
Figure 3.
Area under the receiver operating characteristic curve (AUC) for mortality and 4C score.
The sensitivity of the model for each value of 4C score ranged from 100.0 to 28.75% whereas the specificity ranged from 0 to 94.83%, respectively (Table 2). At the criterion value of > 10, the sensitivity was 76.2% and the specificity was 62.67%. The lower two cut-offs (3 and 8) demonstrated negative likelihood ratios of 0.047 and 0.21, respectively, and the positive likelihood ratios exceeded 4 for values higher than 13.

Table 2.
Criterion values and coordinates of the ROC curve.
Finally, we divided the subjects, depending on whether they had received an early antiviral treatment or not (Table 3). Those who received an early antiviral treatment had a lower mortality than those who did not.

Table 3.
Comparison of 4C score and mortality between people who received an early antiviral treatment and people who did not.
4. Discussion
The aim of our study was to evaluate the ability of the 4C score to predict mortality in COVID-19 patients. Our findings suggested that the 4C mortality score is a useful tool for COVID-19 patients admitted to hospital wards.
As described by Jones et al., we confirmed that mortality was higher with increasing 4C scores. Moreover, although the AUC was not high (0.78), its value was acceptable and similar to previous studies [10,11].
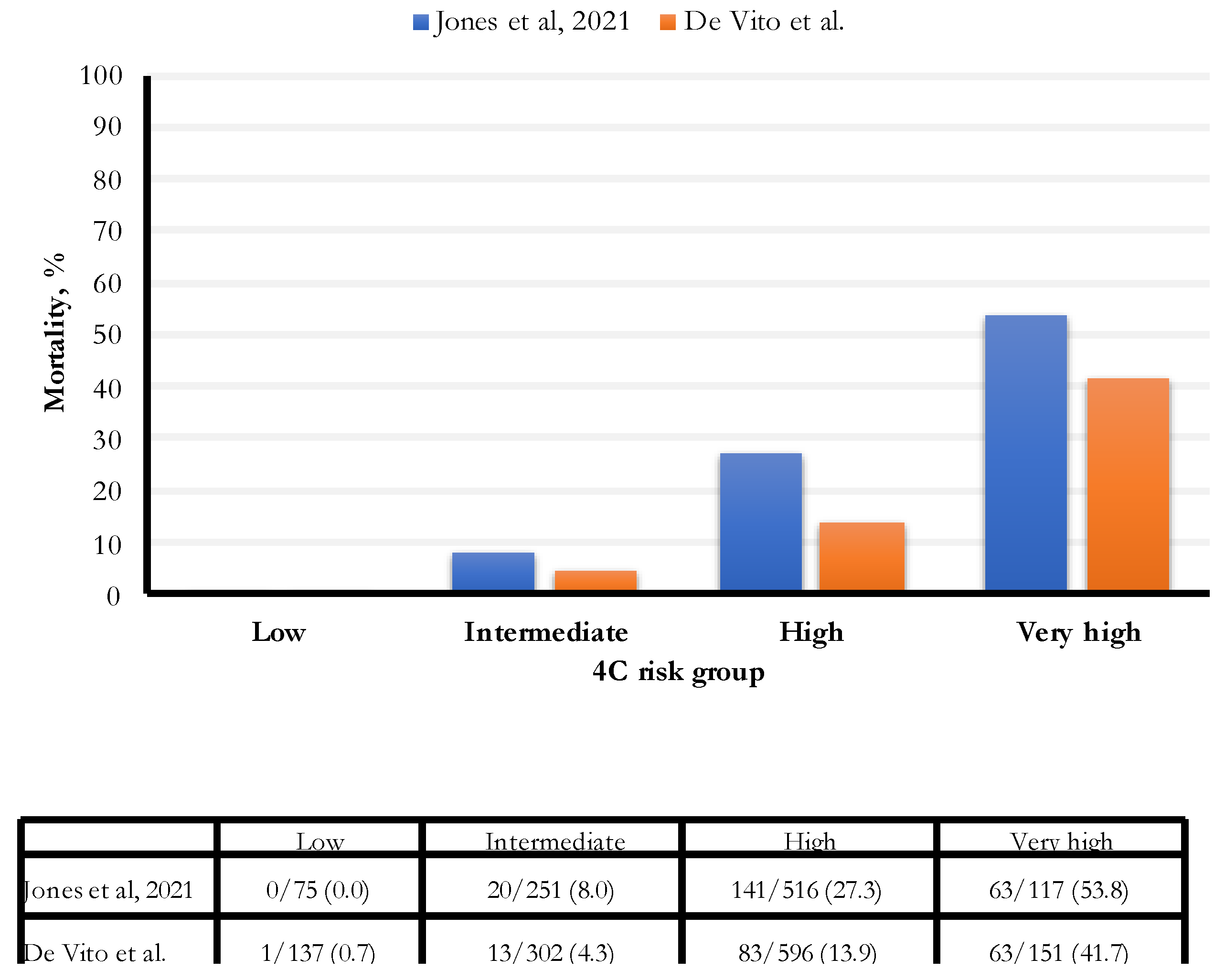
In our study, the hospital all-cause mortality was 13.5%, an estimate lower than those found in two recent studies (16.9% and 23.4%, respectively, Gordon and Jones) (Figure 4). In particular, the percentage of people with an intermediate risk was 46% lower (8% vs. 4.3%) in our study; for people with a high risk of death, the percentage was 49% lower (27.3% vs. 13.9%). Finally, for people with a very high risk of death, the rate was 22.5% lower (53.8% vs. 41.7%). The established cut-off for a very high risk of death was 15 points; we observed the most increased death risk for 17 points and above.
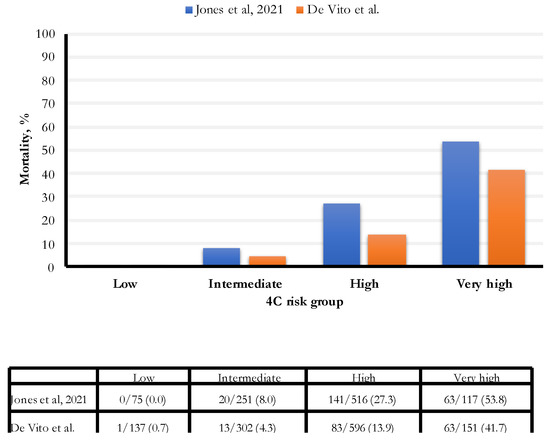
Figure 4.
Comparison of 4C score categories between Jones et al. [11] and De Vito et al. cohorts.
These differences could be explained by the data collection period. During these two years, substantial changes in the management of COVID-19 occurred. In addition, several vaccines were commercially distributed, reducing the risk of severe illness and death [12]. In this regard, Watson et al. created a mathematical model to analyze the impact of the anti-SARS-CoV-2 vaccination program considering data from 185 countries. As a result, they estimated that vaccinations prevented 14.4 million deaths globally, confirming that vaccination altered the course of the pandemic [13].
Moreover, the principal strain present in Italy was B.1.1.529 (Omicron) and its subvariants during the study period; Omicron has been proven to cause a lower incidence of severe disease and mortality compared with previous variants [14,15].
In addition, three different antiviral therapies have now been approved to avoid the progression of the disease: monlupiravir, nirmatrelvir/ritonavir, and remdesivir [16,17,18,19]. Monlupiravir, a small-molecule ribonucleoside prodrug of hydroxy-cytidine (NHC) with a high genetic barrier [20], was demonstrated to have high efficacy in reducing the disease progression in a clinical trial [16]. Although the chosen population was young in the clinical trial and had a lower comorbidity burden, real-life studies confirmed the efficacy and safety of monlupiravir [21,22,23]. Nirmatrelvir/ritonavir(r) is a protease inhibitor targeting the SARS-CoV-2 3-chymotrypsin-like cysteine protease enzyme (M pro). This target enzyme is essential for viral replication [24]. However, nirmatrelvir is metabolized by CYP3A4; thus, to increase the T1/2, it is associated with a booster (ritonavir) that enhances the nirmatrelvir/r. On the other hand, the addition of ritonavir causes various drug–drug interactions. The clinical trial showed excellent efficacy and safety, confirmed by real-life studies [17,22]. Remdesivir is a direct-acting nucleotide prodrug inhibitor of the SARS-CoV-2 RNA-dependent RNA polymerase. It was the first antiviral approved for treating hospitalized patients who needed oxygen supplementation and who had confirmed evidence of COVID-19-related pneumonia. In January 2022, the results of the PINETREE trial were published, demonstrating how a short dose of remdesivir (3 days instead of 5) could reduce the disease progression and hospitalization by 87% with good safety [18]. For these reasons, we believe that the use of these treatments has had a significant impact on reducing the number of people who experienced the disease progression and who died from COVID-19. Furthermore, during the period of our study, monoclonal antibodies were available. The available treatments were casirivimab/imdevimab (Ronapreve®), tixagevimab/cilgavimab (Evusheld®), and sotrovimab (Xevudy®) [25,26,27]. However, the efficacy of casirivimab/imdevimab and sotrovimab in preventing the disease progression of people infected by the Omicron variant and its subvariants (BA.2, BA.4, and BA.5) is unclear [28]; however, the data about the use of tixagevimab/cilgavimab are more reassuring. In our center, tixagevimab/cilgavimab was not available during the months of our study. Several drugs for patients with severe COVID-19 can also reduce mortality [29,30,31,32,33]. As shown in Table 2, people treated with an early antiviral treatment had a lower mortality rate than those who were not. For this reason, new scores that consider these treatments are needed.
An important limitation of our study was related to its monocentric nature. However, we believe it could be generalized to all countries where antiviral and monoclonal antibodies are available. Another significant limitation was that the scores were calculated retrospectively and not at the evaluation time. The situation is ever-changing, and we will continue to update our tools according to the evolving ecology and management of the infection.
5. Conclusions
Similar to previous studies, the 4C mortality score performed well in our sample, and it is still a useful tool for clinicians to identify patients with a high risk of progression. However, clinicians must be aware that the mortality rate reported in the original studies was higher than that observed in our study. Finally, new scores for predicting disease progression that consider early antiviral treatments and vaccinations are needed.
Author Contributions
Conceptualization: A.D.V., A.C. and G.M.; methodology: A.D.V., L.S., M.P. and G.S.; validation: G.S., I.M., S.B. and G.M.; formal analysis: L.S., M.P. and G.S.; investigation: A.C., V.F., B.Z., M.F., M.C.M., A.B. and C.D.C.; resources: I.M., B.Z., A.C. and V.F.; data curation: S.B., M.F., A.B., M.C.M. and C.D.C.; writing—original draft preparation: A.D.V., A.C., M.P., L.S. and G.S.; writing—review and editing: G.M., V.F., S.B., I.M., M.F., B.Z., A.B., M.C.M. and C.D.C.; visualization: A.D.V. and V.F.; supervision: G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee (Comitato Etico Indipendente - Azienda Ospedaliero Universitaria di Cagliari) with the protocol code PG/2022/20481.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- He, J.; Guo, Y.; Mao, R.; Zhang, J. Proportion of asymptomatic coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. J. Med. Virol. 2020, 93, 820–830. [Google Scholar] [CrossRef]
- De Vito, A.; Fiore, V.; Princic, E.; Geremia, N.; Napodano, C.M.P.; Muredda, A.A.; Maida, I.; Madeddu, G.; Babudieri, S. Predictors of infection, symptoms development, and mortality in people with SARS-CoV-2 living in retirement nursing homes. PLoS ONE 2021, 16, e0248009. [Google Scholar] [CrossRef] [PubMed]
- Geremia, N.; De Vito, A.; Gunnella, S.; Fiore, V.; Princic, E.; Napodano, C.P.; Madeddu, G.; Babudieri, S. A Case of Vasculitis-Like Skin Eruption Associated with COVID-19. Infect. Dis. Clin. Pract. 2020, 28, e30–e31. Available online: https://journals.lww.com/infectdis/Fulltext/9000/A_Case_of_Vasculitis_Like_Skin_Eruption_Associated.98743.aspx (accessed on 23 October 2020). [CrossRef]
- Vaira, L.A.; De Vito, A.; Lechien, J.R.; Chiesa-Estomba, C.M.; Mayo-Yàñez, M.; Calvo-Henrìquez, C.; Saussez, S.; Madeddu, G.; Babudieri, S.; Boscolo-Rizzo, P.; et al. New Onset of Smell and Taste Loss Are Common Findings Also in Patients with Symptomatic COVID-19 after Complete Vaccination. Laryngoscope 2022, 132, 419–421. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Connors, J.M.; Levy, J.H. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflamm. Res. 2020, 69, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- King, J.T., Jr.; Yoon, J.S.; Rentsch, C.T.; Tate, J.P.; Park, L.S.; Kidwai-Khan, F.; Skanderson, M.; Hauser, R.G.; Jacobson, D.A.; Erdos, J.; et al. Development and validation of a 30-day mortality index based on pre-existing medical administrative data from 13,323 COVID-19 patients: The Veterans Health Administration COVID-19 (VACO) Index. PLoS ONE 2020, 15, e0241825. [Google Scholar] [CrossRef]
- Garibaldi, B.T.; Fiksel, J.; Muschelli, J.; Robinson, M.L.; Rouhizadeh, M.; Perin, J.; Schumock, G.; Nagy, P.; Gray, J.H.; Malapati, H.; et al. Patient Trajectories among Persons Hospitalized for COVID-19: A Cohort Study. Ann. Intern. Med. 2021, 174, 33–41. [Google Scholar] [CrossRef]
- Goodacre, S.; Thomas, B.; Sutton, L.; Burnsall, M.; Lee, E.; Bradburn, M.; Loban, A.; Waterhouse, S.; Simmonds, R.; Biggs, K.; et al. Derivation and validation of a clinical severity score for acutely ill adults with suspected COVID-19: The PRIEST observational cohort study. PLoS ONE 2021, 16, e0245840. [Google Scholar] [CrossRef]
- Wynants, L.; Van Calster, B.; Collins, G.S.; Riley, R.D.; Heinze, G.; Schuit, E.; Bonten, M.M.J.; Dahly, D.L.; Damen, J.A.; Debray, T.P.A.; et al. Prediction models for diagnosis and prognosis of covid-19: Systematic review and critical appraisal. BMJ 2020, 369, 29. [Google Scholar] [CrossRef]
- Knight, S.R.; Ho, A.; Pius, R.; Buchan, I.; Carson, G.; Drake, T.M.; Dunning, J.; Fairfield, C.J.; Gamble, C.; Green, C.A.; et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4C Mortality Score. BMJ 2020, 370, 22. Available online: https://www.bmj.com/content/370/bmj.m3339 (accessed on 16 October 2022). [CrossRef]
- Jones, A.; Pitre, T.; Junek, M.; Kapralik, J.; Patel, R.; Feng, E.; Dawson, L.; Tsang, J.L.Y.; Duong, M.; Ho, T.; et al. External validation of the 4C mortality score among COVID-19 patients admitted to hospital in Ontario, Canada: A retrospective study. Sci. Rep. 2021, 11, 18638. Available online: https://www.nature.com/articles/s41598-021-97332-1 (accessed on 16 October 2022). [CrossRef]
- Chi, W.-Y.; Li, Y.-D.; Huang, H.-C.; Chan, T.E.H.; Chow, S.-Y.; Su, J.-H.; Ferrall, L.; Hung, C.-F.; Wu, T.-C. COVID-19 vaccine update: Vaccine effectiveness, SARS-CoV-2 variants, boosters, adverse effects, and immune correlates of protection. J. Biomed. Sci. 2022, 29, 82. Available online: https://pubmed.ncbi.nlm.nih.gov/36243868/ (accessed on 16 October 2022). [CrossRef] [PubMed]
- Watson, O.J.; Barnsley, G.; Toor, J.; Hogan, A.B.; Winskill, P.; Ghani, A.C. Global impact of the first year of COVID-19 vaccination: A mathematical modelling study. Lancet Infect. Dis. 2022, 22, 1293–1302. Available online: http://www.thelancet.com/article/S1473309922003206/fulltext (accessed on 26 November 2022). [CrossRef] [PubMed]
- Nyberg, T.; Ferguson, N.M.; Nash, S.G.; Webster, H.H.; Flaxman, S.; Andrews, N.; Hinsley, W.; Bernal, J.L.; Kall, M.; Bhatt, S.; et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: A cohort study. Lancet 2022, 399, 1303–1312. Available online: http://www.thelancet.com/article/S0140673622004627/fulltext (accessed on 17 October 2022). [CrossRef] [PubMed]
- Ughi, N.; Bernasconi, D.P.; Del Gaudio, F.; Dicuonzo, A.; Maloberti, A.; Giannattasio, C.; Tarsia, P.; Puoti, M.; Scaglione, F.; Beltrami, L.; et al. Trends in all-cause mortality of hospitalized patients due to SARS-CoV-2 infection from a monocentric cohort in Milan (Lombardy, Italy). J. Public Health 2022, 30, 1985–1993. Available online: https://pubmed.ncbi.nlm.nih.gov/35004128/ (accessed on 22 December 2022). [CrossRef]
- Jayk Bernal, A.; Gomes da Silva, M.M.; Musungaie, D.B.; Kovalchuk, E.; Gonzalez, A.; Delos Reyes, V.; Martín-Quirós, A.; Caraco, Y.; Williams-Diaz, A.; Brown, M.L.; et al. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N. Engl. J. Med. 2022, 386, 509–520. Available online: https://www.nejm.org/doi/full/10.1056/NEJMoa2116044 (accessed on 15 March 2022). [CrossRef]
- Hammond, J.; Leister-Tebbe, H.; Gardner, A.; Abreu, P.; Bao, W.; Wisemandle, W.; Baniecki, M.; Hendrick, V.M.; Damle, B.; Simón-Campos, A.; et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. N. Engl. J. Med. 2022, 386, 1397–1408. Available online: https://www.nejm.org/doi/full/10.1056/NEJMoa2118542 (accessed on 15 March 2022). [CrossRef]
- Gottlieb, R.L.; Vaca, C.E.; Paredes, R.; Mera, J.; Webb, B.J.; Perez, G.; Oguchi, G.; Ryan, P.; Nielsen, B.U.; Brown, M.; et al. Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients. N. Engl. J. Med. 2022, 386, 305–315. Available online: https://www.nejm.org/doi/full/10.1056/NEJMoa2116846 (accessed on 15 March 2022). [CrossRef]
- De Vito, A.; Colpani, A.; Saderi, L.; Puci, M.; Zauli, B.; Fiore, V.; Fois, M.; Meloni, M.C.; Bitti, A.; Di Castri, C.; et al. Impact of Early SARS-CoV-2 Antiviral Therapy on Disease Progression. Viruses 2023, 15, 71. Available online: https://www.mdpi.com/1999-4915/15/1/71/htm (accessed on 3 January 2023). [CrossRef]
- Malone, B.; Campbell, E.A. Molnupiravir: Coding for catastrophe. Nat. Struct. Mol. Biol. 2021, 28, 706–708. Available online: https://www.nature.com/articles/s41594-021-00657-8 (accessed on 15 March 2022). [CrossRef]
- De Vito, A.; Colpani, A.; Bitti, A.; Zauli, B.; Meloni, M.C.; Fois, M.; Denti, L.; Bacciu, S.; Marcia, C.; Maida, I.; et al. Safety and efficacy of molnupiravir in SARS-CoV-2 infected patients: A real-life experience. J. Med. Virol. 2022, 94, 5582–5588. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/jmv.28011 (accessed on 1 August 2022). [CrossRef]
- Gentile, I.; Scotto, R.; Shiano Moriello, N.; Pinchera, B.; Villari, R.; Trucillo, E.; Ametrano, L.; Fusco, L.; Castaldo, G.; Buonomo, A.R.; et al. Nirmatrelvir/Ritonavir and Molnupiravir in the Treatment of Mild/Moderate COVID-19: Results of a Real-Life Study. Vaccines 2022, 10, 1731. Available online: http://www.ncbi.nlm.nih.gov/pubmed/36298596 (accessed on 1 December 2022). [CrossRef]
- Wong, C.K.H.; Au, I.C.H.; Lau, K.T.K.; Lau, E.H.Y.; Cowling, B.J.; Leung, G.M. Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: An observational study. Lancet 2022, 400, 1213–1222. Available online: http://www.thelancet.com/article/S0140673622015860/fulltext (accessed on 31 October 2022). [PubMed]
- Saravolatz, L.D.; Depcinski, S.; Sharma, M. Molnupiravir and Nirmatrelvir-Ritonavir: Oral COVID Antiviral Drugs. Clin. Infect. Dis. 2022. Available online: https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciac180/6542722 (accessed on 15 March 2022).
- Abani, O.; Abbas, A.; Abbas, F.; Abbas, M.; Abbasi, S.; Abbass, H.; Abbott, A.; Abdallah, N.; Abdelaziz, A.; Abdelfattah, M.; et al. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2022, 399, 665–676. Available online: http://www.thelancet.com/article/S0140673622001635/fulltext (accessed on 16 October 2022).
- Gupta, A.; Gonzalez-Rojas, Y.; Juarez, E.; Casal, M.C.; Moya, J.; Falci, D.R.; Sarkis, E.; Solis, J.; Zheng, H.; Scott, N.; et al. Effect of Sotrovimab on Hospitalization or Death Among High-risk Patients with Mild to Moderate COVID-19: A Randomized Clinical Trial. JAMA 2022, 327, 1236–1246. Available online: https://jamanetwork.com/journals/jama/fullarticle/2790246 (accessed on 16 October 2022). [CrossRef] [PubMed]
- Ginde, A.A.; Paredes, R.; Murray, T.A.; Engen, N.; Grandits, G.; Vekstein, A.; Ivey, N.; Mourad, A.; Sandkovsky, U.; Gottlieb, R.L.; et al. Tixagevimab-cilgavimab for treatment of patients hospitalised with COVID-19: A randomised, double-blind, phase 3 trial. Lancet Respir. Med. 2022, 10, 972–984. Available online: http://www.thelancet.com/article/S2213260022002156/fulltext (accessed on 26 November 2022).
- Takashita, E.; Yamayoshi, S.; Simon, V.; van Bakel, H.; Sordillo, E.M.; Pekosz, A.; Fukushi, S.; Suzuki, T.; Maeda, K.; Halfmann, P.; et al. Efficacy of Antibodies and Antiviral Drugs against Omicron BA.2.12.1, BA.4, and BA.5 Subvariants. N. Engl. J. Med. 2022, 387, 468–470. Available online: https://www.nejm.org/doi/full/10.1056/NEJMc2207519 (accessed on 26 November 2022). [CrossRef]
- De Vito, A.; Poliseno, M.; Colpani, A.; Zauli, B.; Puci, M.V.; Santantonio, T.; Meloni, M.C.; Fois, M.; Fanelli, C.; Saderi, L.; et al. Reduced risk of death in people with SARS-CoV-2 infection treated with remdesivir: A nested case-control study. Curr. Med. Res. Opin. 2022, 38, 2029–2033. Available online: https://pubmed.ncbi.nlm.nih.gov/36170020/ (accessed on 16 October 2022). [CrossRef]
- De Vito, A.; Saderi, L.; Fiore, V.; Geremia, N.; Princic, E.; Fanelli, C.; Muredda, A.A.; Napodano, C.P.; Moi, G.; Maida, I.; et al. Early treatment with low-molecular-weight heparin reduces mortality rate in SARS-CoV-2 patients. Panminerva Med. 2022. Available online: https://pubmed.ncbi.nlm.nih.gov/35622392/ (accessed on 1 August 2022). [CrossRef]
- Rodriguez-Guerra, M.; Jadhav, P.; Vittorio, T.J. Current treatment in COVID-19 disease: A rapid review. Drugs Context 2021, 10, 2020-10-3. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7850293/ (accessed on 16 October 2022). [CrossRef]
- Mazzitelli, M.; Arrighi, E.; Serapide, F.; Pelle, M.C.; Tassone, B.; Lionello, R.; Marrazzo, G.; Laganà, D.; Costanzo, F.S.; Matera, G.; et al. Use of subcutaneous tocilizumab in patients with COVID-19 pneumonia. J. Med. Virol. 2021, 93, 32–34. Available online: https://pubmed.ncbi.nlm.nih.gov/32410234/ (accessed on 28 June 2022). [CrossRef] [PubMed]
- Balena, F.; Bavaro, D.F.; Fabrizio, C.; Bottalico, I.F.; Calamo, A.; Santoro, C.R.; Brindicci, G.; Bruno, G.; Mastroianni, A.; Greco, S.; et al. Tocilizumab and corticosteroids for COVID-19 treatment in elderly patients. J. Gerontol. Geriatr. 2020, 68, 197–203. Available online: http://www.jgerontology-geriatrics.com/article/view/283 (accessed on 9 August 2022). [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).