Diagnostic Accuracy of an Indirect Enzyme Linked Immunosorbent Assay (iELISA) for Screening of Babesia bovis in Cattle from West Africa
Abstract
:1. Introduction
2. Materials and Methods
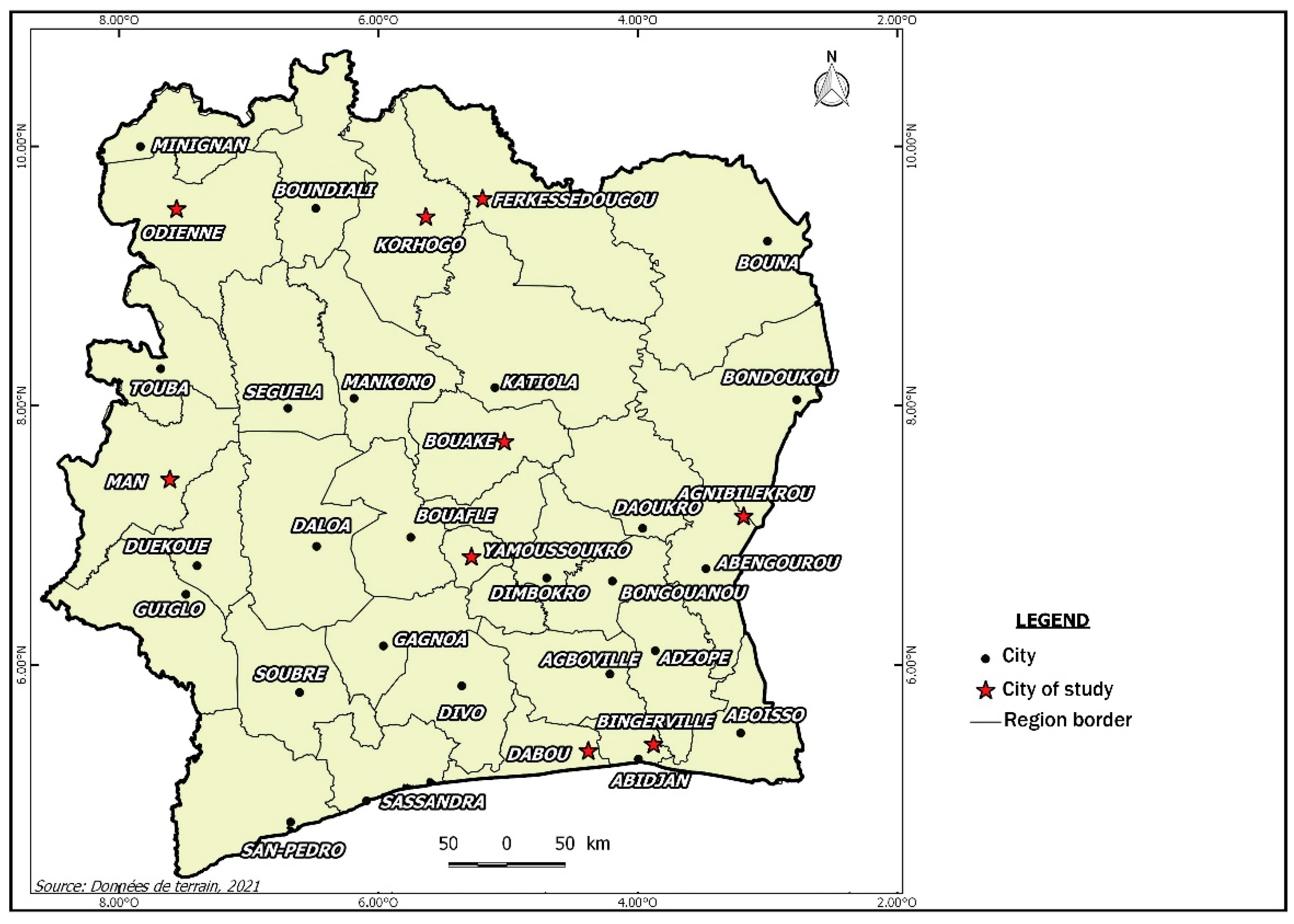
2.1. Study Area and Data Collection
2.2. Laboratory Assays
2.3. Statistical Analysis
3. Results
4. Discussion
4.1. Probability of Misclassification Given an Infectious Status
4.2. Probability of Misclassification Given a Test Result
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Node | Mean | Sd | MC Error | 2.5% | Median | 97.5% | Start | Sample | |
|---|---|---|---|---|---|---|---|---|---|
| bayesp | 0.5297 | 0.4991 | 0.001378 | 0.0 | 1.0 | 1.0 | 5001 | 150,000 | |
| p[1] | 0.03204 | 0.006148 | 1.566 × 10−5 | 0.0211 | 0.03166 | 0.04509 | 5001 | 150,000 | |
| p[2] | 0.003103 | 0.001889 | 6.4 × 10−6 | 5.681 × 10−4 | 0.002731 | 0.007777 | 5001 | 150,000 | |
| p[3] | 0.1131 | 0.01114 | 2.883 × 10−5 | 0.09222 | 0.1127 | 0.1358 | 5001 | 150,000 | |
| p[4] | 0.01634 | 0.004399 | 1.067 × 10−5 | 0.008867 | 0.01595 | 0.026 | 5001 | 150,000 | |
| p[5] | 0.07919 | 0.009465 | 2.57 × 10−5 | 0.06164 | 0.07886 | 0.09857 | 5001 | 150,000 | |
| p[6] | 0.02073 | 0.004975 | 1.29 × 10−5 | 0.01214 | 0.02033 | 0.03156 | 5001 | 150,000 | |
| p[7] | 0.5261 | 0.01756 | 4.726 × 10−5 | 0.4916 | 0.5261 | 0.5604 | 5001 | 150,000 | |
| p[8] | 0.2095 | 0.01435 | 3.649 × 10−5 | 0.182 | 0.2092 | 0.2383 | 5001 | 150,000 | |
| PCR | se[1] | 0.917 | 0.05656 | 0.001267 | 0.8282 | 0.9192 | 0.998 | 5001 | 150,000 |
| FS | se[2] | 0.8743 | 0.01694 | 1.349 × 10−4 | 0.8402 | 0.8746 | 0.9058 | 5001 | 150,000 |
| Elisa5 | se[3] | 0.2616 | 0.0203 | 2.625 × 10−4 | 0.2238 | 0.261 | 0.3025 | 5001 | 150,000 |
| PCR | sp[1] | 0.9484 | 0.02894 | 9.576 × 10−5 | 0.9023 | 0.9477 | 0.9972 | 5001 | 150,000 |
| FS | sp[2] | 0.2976 | 0.2267 | 0.003191 | 0.02554 | 0.2299 | 0.883 | 5001 | 150,000 |
| Elisa5 | sp[3] | 0.7946 | 0.172 | 0.002593 | 0.275 | 0.8569 | 0.9616 | 5001 | 150,000 |
| Dbar | Dhat | pD | DIC | |
|---|---|---|---|---|
| Res | 42.332 | 57.595 | −15.264 | 27.068 |
| th | 4.605 | 4.605 | 0.000 | 4.605 |
| total | 46.937 | 62.200 | −15.264 | 31.673 |
References
- World Organization for Animal Health (OIE). Principles and methods of validation of diagnostic assays for infectious diseases. In Manual of Diagnostic Tests for Aquatic Animals; OIE: Paris, France, 2021; pp. 10–30. [Google Scholar]
- Galon, E.M.; Zafar, I.; Ji, S.; Li, H.; Ma, Z.; Xuan, X. Molecular Reports of Ruminant Babesia in Southeast Asia. Pathogens 2022, 11, 915. [Google Scholar] [CrossRef]
- Egan, S.L.; Casey, L.; Taylor, J.M.; Austen, P.B.; Banks, A.S.; Northover, L.A.; Ahlstrom, U.M.; Ryan, P.J.; Irwin, I.C.L. Haemoprotozoan surveillance in peri-urban native and introduced wildlife from Australia. Curr. Res. Parasitol. Vector Borne Dis. 2021, 1, 100052. [Google Scholar] [CrossRef] [PubMed]
- Madder, M.; Adehan, S.; De Deken, R.; Adehan, R.; Lokossou, R. New foci of Rhipicephalus microplus in West Africa. Exp. Appl. Acarol. 2012, 56, 385–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toure, A.; Diaha, C.A.; Sylla, I.; Kouakou, K. Récente recomposition des populations de tiques prévalent en Côte d’Ivoire. Int. J. Biol. Chem. Sci. 2014, 8, 566–578. [Google Scholar] [CrossRef] [Green Version]
- Jacob_Pinaki, S.S.; Sengupta, P.P.; Krishnamoorthy, K.P.; Suresh, S.S.; Patil, A.G.S.; Chandu, J.K.; Chamuah, H.L.; Shome, B.R. Bovine babesiosis in India: Estimation of prevalence by systematic review and meta-analysis. Exp. Parasitol. 2022, 239, 108318. [Google Scholar] [CrossRef]
- Flahault, A.; Cadilhac, M.; Thomas, G. Sample size calculation should be performed for design accuracy in diagnostic test studies. J. Clin. Epidemiol. 2005, 58, 859–862. [Google Scholar] [CrossRef]
- Gallego-Lopez, G.M.; Lau, A.O.T.; Brown, W.C.; Johnson, W.C.; Ueti, M.W.; Suarez, C.E. Spherical Body Protein 2 truncated copy 11 as a specific Babesia bovis attenuation marker. Parasites Vectors 2018, 11, 169. [Google Scholar] [CrossRef]
- Terkawi, M.A.; Huyen, N.X.; Shinuo, C.; Inpankaew, T.; Maklon, K.; Aboulaila, M.; Ueno, A.; Goo, Y.-K.; Yokoyama, N.; Jittapalapong, S. Molecular and serological prevalence of Babesia bovis and Babesia bigemina in water buffaloes in the northeast region of Thailand. Vet. Parasitol. 2011, 178, 201–207. [Google Scholar] [CrossRef]
- Kostoulas, P.; Nielsen, S.S.; Branscum, A.J.; Johnson, W.O.; Dendukuri, N.; Dhand, N.K.; Ian, N.T.; Gardner, A. STARD-BLCM: Standards for the Reporting of Diagnostic accuracy studies that use Bayesian Latent Class Models. Prev. Vet. Med. 2017, 138, 37–47. [Google Scholar] [CrossRef]
- Elsohaby, I.; Kostoulas, P.; Elsayed, A.M.; Ahmed, H.A.; El-Diasty, M.M.; Wareth, G.; Ghanem, F.M.; Arango-Sabogal, J.C. Bayesian Evaluation of Three Serological Tests for Diagnosis of Brucella infections in Dromedary Camels Using Latent Class Models. Prev. Vet. Med. 2022, 208, 105771. [Google Scholar] [CrossRef]
- Pfukenyi, D.M.; Meletis, E.; Modise, B.; Ndengu, M.; Kadzviti, F.W.; Dipuo, K.; Moesi, K.; Polychronis Kostoulas, P.; Matope, G. Evaluation of the sensitivity and specificity of the lateral flow assay, Rose Bengal test and the complement fixation test for the diagnosis of brucellosis in cattle using Bayesian latent class analysis. Prev. Vet. Med. 2020, 181, 105075. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.; Colling, A.; Greiner, M. Design, statistical analysis and reporting standards for test accuracy studies for infectious diseases in animals: Progress, challenges and recommendations. Prev. Vet. Med. 2019, 162, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-C.A.; Al-Temimi, M.; Ying, J.; Muscat, J.; Olshan, A.F.; Zevallos, J.P.; Winn, D.M.; Li, G.; Sturgis, E.M.; Morgenstern, H.; et al. Risk Prediction Models for Head and Neck Cancer in the US Population from the INHANCE Consortium. Am. J. Epidemiol. 2020, 189, 330–342. [Google Scholar] [CrossRef]
- Wentzel, A.; Malan, L.; Känel, R.V.; Malan, N.T. Ethnicity-Specific Changes in Cardiac Troponin T in Response to Acute Mental Stress and Ethnicity-Specific Cut points for the R Wave of the a VL Lead: The SABPA Study. Am. J. Epidemiol. 2019, 188, 1444–1455. [Google Scholar] [CrossRef] [PubMed]
- Caraguel, C.G.; Stryhn, H.; Gagné, N.; Dohoo, I.R.; Hammell, K.I. Selection of a cutoff value for real-time polymerase chain reaction results to fit a diagnostic purpose: Analytical and epidemiologic approaches. J. Vet. Diagn. Investig. 2011, 23, 2–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkhatib, R.; Diab, M.; Moslem, B.; Corbier, C.; El Badaoui, M. Gait-Ground Reaction Force Sensors Selection Based on ROC Curve Evaluation. J. Comput. Commun. 2015, 3, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Rücker, G.; Steinhauser, S.; Schumacher, M. RE: “Selective Cut off reporting in studies of diagnostic test accuracy: A comparison of conventional and individual-patient-data meta-analyses of the patient health questionnaire–9 depression screening tool”. Am. J. Epidemiol. 2017, 186, 894–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oundo, J.W.; Masiga, D.; Bosch, Q.; Villinger, J.; Koenraadt, C.J.M.; Kalayou, S. Epidemiology of tick-borne pathogens of cattle and tick control practices in coastal Kenya. Prev. Vet. Med. 2022, 209, 105777. [Google Scholar] [CrossRef]
- Durocher, J.; Moore, R.K.; Castonguay, M.H.; Albaaj, A.; Le Blanc, S.J.; Dufour, S. Bayesian estimation of sensitivity and specificity of a milk pregnancy-associated glycoprotein ELISA test for pregnancy diagnosis between 23 and 27 days after insemination in Holstein dairy cows. J. Dairy Sci. 2022, 105, 6985–6996. [Google Scholar] [CrossRef]
- Dominguez, M.; Echaide, I.; De Echaide, S.T.; Wilkowsky, S.; Zabal, O.; Mosqueda, J.J.; Schnittger, L.; Florin-Christensen, M. Validation and field evaluation of a competitive enzyme-linked immunosorbent assay for diagnosis of Babesia bovis infections in Argentina. Clin. Vaccine Immunol. 2012, 19, 924–928. [Google Scholar] [CrossRef]
- Terkawi, M.A.; Thekisoe, O.M.; Katsande, C.; Latif, A.A.; Mans, B.J.; Matthee, O.; Mkize, N.; Mabogoane, N.; Marais, F.; Yokoyama, N. Serological survey of Babesia bovis and Babesia bigemina in cattle in South Africa. Vet. Parasitol. 2011, 182, 337–342. [Google Scholar] [CrossRef] [PubMed]
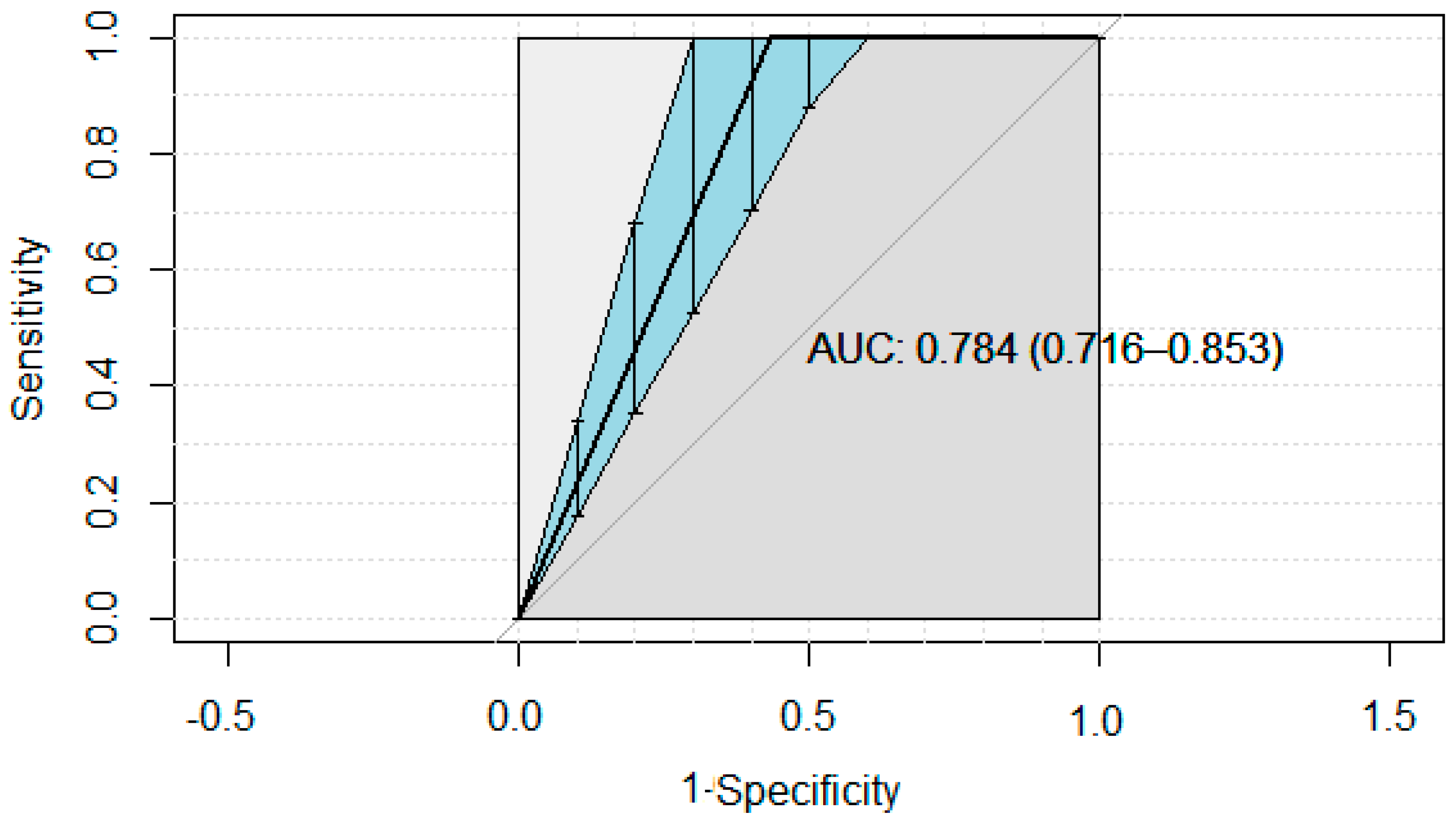
| Test Threshold Positivity | Se | 95% Cr. I. | Sp | 95% Cr. I. | NPV (%) | PPV (%) | J | DOR | LR+ | LR− |
|---|---|---|---|---|---|---|---|---|---|---|
| ELISA5 | 0.940 | 0.854–0.998 | 0.895 | 0.871–0.919 | 82.16 | 96.66 | 0.835 | 127.85 | 8.95 | 0.07 |
| ELISA10 | 0.852 | 0.767–0.916 | 0.842 | 0.263–0.993 | 63.73 | 94.58 | 0.694 | 31.7 | 5.39 | 0.17 |
| ELISA15 | 0.768 | 0.689–0.838 | 0.828 | 0.201–0.994 | 52.43 | 93.52 | 0.596 | 15.93 | 4.46 | 0.28 |
| ELISA20 | 0.731 | 0.650–0.796 | 0.859 | 0.252–0.996 | 49.65 | 94.37 | 0.59 | 16.71 | 5.18 | 0.31 |
| ELISA25 | 0.675 | 0.599–0.741 | 0.864 | 0.253–0.996 | 41.57 | 93.7 | 0.539 | 13.4 | 4.96 | 0.37 |
| ELISA30 | 0.623 | 0.545–0.688 | 0.910 | 0.383–0.998 | 42.71 | 95.72 | 0.533 | 16.87 | 6.92 | 0.41 |
| ELISA35 | 0.566 | 0.492–0.626 | 0.918 | 0.438–0.998 | 39.51 | 95.71 | 0.484 | 14.68 | 6.90 | 0.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toure, A.; Sanogo, M.; Sghiri, A.; Sahibi, H. Diagnostic Accuracy of an Indirect Enzyme Linked Immunosorbent Assay (iELISA) for Screening of Babesia bovis in Cattle from West Africa. Life 2023, 13, 203. https://doi.org/10.3390/life13010203
Toure A, Sanogo M, Sghiri A, Sahibi H. Diagnostic Accuracy of an Indirect Enzyme Linked Immunosorbent Assay (iELISA) for Screening of Babesia bovis in Cattle from West Africa. Life. 2023; 13(1):203. https://doi.org/10.3390/life13010203
Chicago/Turabian StyleToure, Alassane, Moussa Sanogo, Abdelmalek Sghiri, and Hamid Sahibi. 2023. "Diagnostic Accuracy of an Indirect Enzyme Linked Immunosorbent Assay (iELISA) for Screening of Babesia bovis in Cattle from West Africa" Life 13, no. 1: 203. https://doi.org/10.3390/life13010203







