Development and Diversity of Epibiont Assemblages on Cultivated Sugar Kelp (Saccharina latissima) in Relation to Farming Schedules and Harvesting Techniques
Abstract
:1. Introduction
2. Materials and Methods
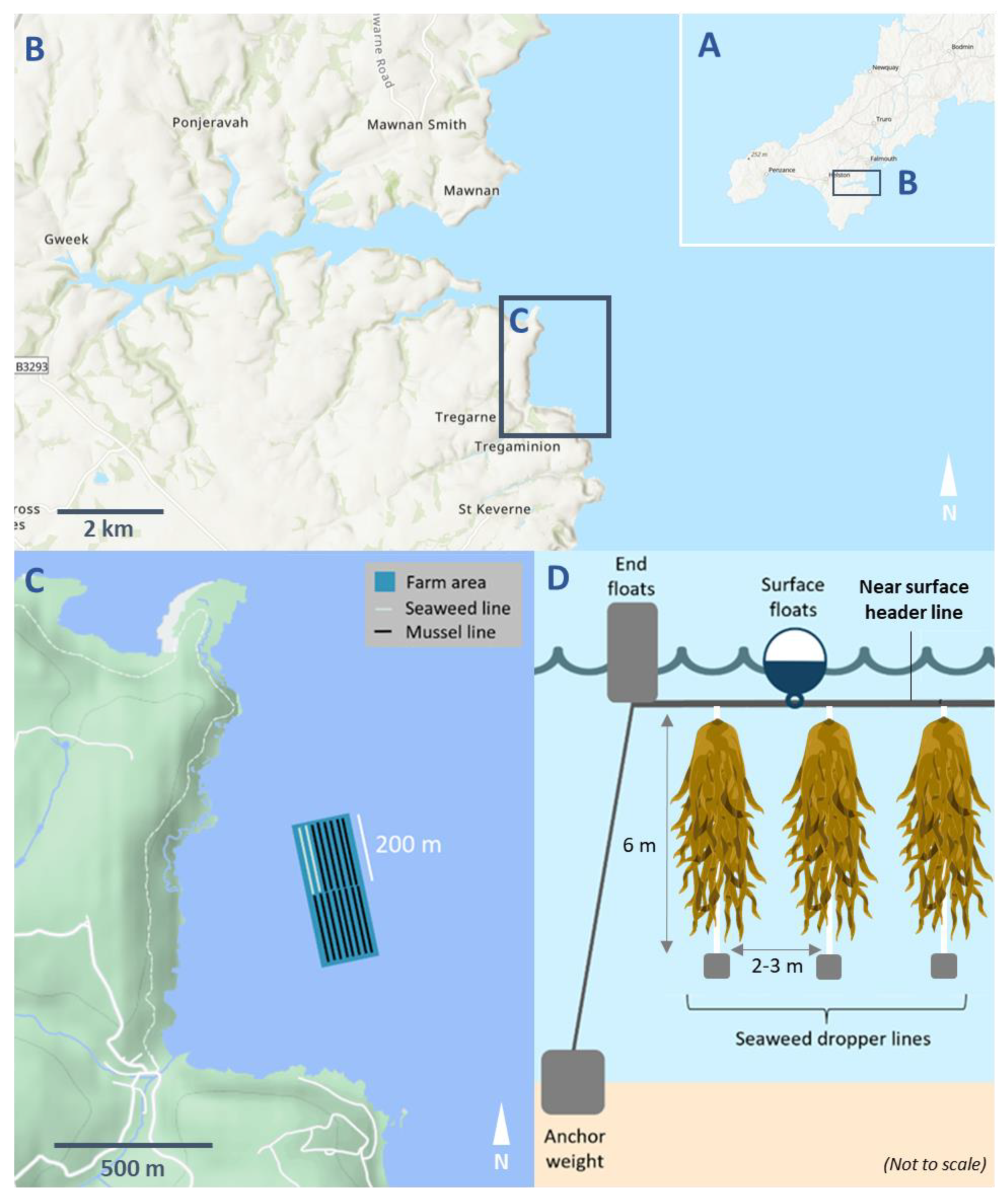
2.1. Study Site and Farm Setup
2.2. Sample Collection
2.2.1. Monthly Sampling
2.2.2. Regrowth Sampling
2.3. Sample Processing
2.4. Statistical Analysis
3. Results
3.1. Epibiont Assemblage Development
Differences between Blades and Holdfasts in Monthly Time Series of Epibiont Assemblage Development
3.2. Regrowth Treatments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2020: Sustainability in Action; The State of World Fisheries and Aquaculture (SOFIA); FAO: Rome, Italy, 2020; ISBN 978-92-5-132692-3. [Google Scholar]
- Schlarb-Ridley, B.; Parker, B. A Road Map for Algal Technologies in the UK. 2013. Available online: https://www.ifm.eng.cam.ac.uk/uploads/Roadmapping/UK-Roadmap-for-Algal-Technologies.pdf (accessed on 8 January 2023).
- Capuzzo, E.; McKie, T. Seaweed in the UK and Abroad—Status, Products, Limitations, Gaps and CEFAS Role. 2016. Available online: https://www.gov.uk/government/publications/the-seaweed-industry-in-the-uk-and-abroad (accessed on 8 January 2023).
- Buschmann, A.H.; Camus, C.; Infante, J.; Neori, A.; Israel, Á.; Hernández-González, M.C.; Pereda, S.V.; Gomez-Pinchetti, J.L.; Golberg, A.; Tadmor-Shalev, N.; et al. Seaweed production: Overview of the global state of exploitation, farming and emerging research activity. Eur. J. Phycol. 2017, 52, 391–406. [Google Scholar] [CrossRef]
- Wood, D.; Capuzzo, E.; Kirby, D.; Mooney-McAuley, K.; Kerrison, P. UK macroalgae aquaculture: What are the key environmental and licensing considerations? Mar. Policy 2017, 83, 29–39. [Google Scholar] [CrossRef]
- Hasselström, L.; Visch, W.; Gröndahl, F.; Nylund, G.M.; Pavia, H. The impact of seaweed cultivation on ecosystem services—A case study from the west coast of Sweden. Mar. Pollut. Bull. 2018, 133, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Alleway, H.; Gillies, C.L.; Bishop, M.; Gentry, R.R.; Theuerkauf, S.J.; Jones, R. The Ecosystem Services of Marine Aquaculture: Valuing Benefits to People and Nature. Bioscience 2018, 69, 59–68. [Google Scholar] [CrossRef]
- Theuerkauf, S.J.; Barrett, L.T.; Alleway, H.K.; Costa-Pierce, B.A.; Gelais, A.S.; Jones, R.C. Habitat value of bivalve shellfish and seaweed aquaculture for fish and invertebrates: Pathways, synthesis and next steps. Rev. Aquac. 2021, 14, 54–72. [Google Scholar] [CrossRef]
- Campbell, I.; Macleod, A.; Sahlmann, C.; Neves, L.; Funderud, J.; Øverland, M.; Hughes, A.D.; Stanley, M. The Environmental Risks Associated with the Development of Seaweed Farming in Europe—Prioritizing Key Knowledge Gaps. Front. Mar. Sci. 2019, 6, 107. [Google Scholar] [CrossRef]
- Langton, R.; Augyte, S.; Price, N.; Forster, J.; Noji, T.; Grebe, G.; St. Gelais, A.; Byron, C.J.; Gelais, A.S.; Byron, C.J. An Ecosystem Approach to the Culture of Seaweed. NOAA Technical Memorandum NMFS-F/SPO-195. 2019. Available online: https://repository.library.noaa.gov/view/noaa/35734 (accessed on 8 January 2023).
- Wilding, C.; Tillin, H.; Corrigan, S.E.; Stuart, E.; Ashton, I.A.; Felstead, P.; Lubelski, A.; Burrows, M.; Smale, D. Seaweed Aquaculture and Mechanical Harvesting: An Evidence Review to Support Sustainable Management. 2021. Available online: http://publications.naturalengland.org.uk/publication/5191133599891456 (accessed on 8 January 2023).
- Corrigan, S.; Brown, A.R.; Ashton, I.G.C.; Smale, D.A.; Tyler, C.R. Quantifying habitat provisioning at macroalgal cultivation sites. Rev. Aquac. 2022, 14, 1671–1694. [Google Scholar] [CrossRef]
- Forbes, H.; Shelamoff, V.; Visch, W.; Layton, C. Farms and forests: Evaluating the biodiversity benefits of kelp aquaculture. J. Appl. Phycol. 2022, 34, 3059–3067. [Google Scholar] [CrossRef]
- Daniels, C.; Ashton, I.; Brown, R.; Martin, J.T.; Ryan, C.; Mynott, K.; Kaye, S.R. (Eds.) Supporting Mariculture Development: Evidence for Informed Regulation; Policy Brief. 2020. Available online: https://www.exeter.ac.uk/media/universityofexeter/research/microsites/sustainableaquaculturefutures/docs/POLICY_STATEMENT_Supporting_Mariculture_Development_.pdf (accessed on 8 January 2023).
- Dürr, S.; Watson, D.I. Biofouling and Antifouling in Aquaculture. Biofouling 2009, 267–287. [Google Scholar] [CrossRef]
- Bannister, J.; Sievers, M.; Bush, F.; Bloecher, N. Biofouling in marine aquaculture: A review of recent research and developments. Biofouling 2019, 35, 631–648. [Google Scholar] [CrossRef]
- Visch, W.; Nylund, G.M.; Pavia, H. Growth and biofouling in kelp aquaculture (Saccharina latissima): The effect of location and wave exposure. J. Appl. Phycol. 2020, 32, 3199–3209. [Google Scholar] [CrossRef]
- Matsson, S.; Christie, H.; Fieler, R. Variation in biomass and biofouling of kelp, Saccharina latissima, cultivated in the Arctic, Norway. Aquaculture 2019, 506, 445–452. [Google Scholar] [CrossRef]
- Walls, A.; Edwards; Firth, L.; Johnson, M. Successional changes of epibiont fouling communities of the cultivated kelp Alaria esculenta: Predictability and influences. Aquac. Environ. Interact. 2017, 9, 57–71. [Google Scholar] [CrossRef] [Green Version]
- Park, C.S.; Hwang, E.K. Seasonality of epiphytic development of the hydroid Obelia geniculata on cultivated Saccharina japonica (Laminariaceae, Phaeophyta) in Korea. J. Appl. Phycol. 2011, 24, 433–439. [Google Scholar] [CrossRef]
- Fitridge, I.; Dempster, T.; Guenther, J.; de Nys, R. The impact and control of biofouling in marine aquaculture: A review. Biofouling 2012, 28, 649–669. [Google Scholar] [CrossRef]
- Radulovich, R.; Umanzor, S.; Cabrera, R.; Mata, R. Tropical seaweeds for human food, their cultivation and its effect on biodiversity enrichment. Aquaculture 2015, 436, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Buschmann, A.H.; Correa, J.A.; Westermeier, R.; Hernández-González, M.d.C.; Norambuena, R. Red algal farming in Chile: A review. Aquaculture 2001, 194, 203–220. [Google Scholar] [CrossRef]
- da Gama, B.A.; Santos, R.P.D.A.; Pereira, R.C. The effect of epibionts on the susceptibility of the red seaweed Cryptonemia seminervis to herbivory and fouling. J. Bioadhesion Biofilm Res. 2008, 24, 209–218. [Google Scholar] [CrossRef]
- Krumhansl, K.A.; Lee, J.M.; Scheibling, R.E. Grazing damage and encrustation by an invasive bryozoan reduce the ability of kelps to withstand breakage by waves. J. Exp. Mar. Biol. Ecol. 2011, 407, 12–18. [Google Scholar] [CrossRef]
- Peteiro, C.; Freire, Ó. Biomass yield and morphological features of the seaweed Saccharina latissima cultivated at two different sites in a coastal bay in the Atlantic coast of Spain. J. Appl. Phycol. 2012, 25, 205–213. [Google Scholar] [CrossRef]
- Førde, H.; Forbord, S.; Handå, A.; Fossberg, J.; Arff, J.; Johnsen, G.; Reitan, K.I. Development of bryozoan fouling on cultivated kelp (Saccharina latissima) in Norway. J. Appl. Phycol. 2015, 28, 1225–1234. [Google Scholar] [CrossRef] [Green Version]
- Rolin, C.; Inkster, R.; Laing, J.; McEvoy, L. Regrowth and biofouling in two species of cultivated kelp in the Shetland Islands, UK. J. Appl. Phycol. 2017, 29, 2351–2361. [Google Scholar] [CrossRef] [Green Version]
- Bak, U.G.; Mols-Mortensen, A.; Gregersen, O. Production method and cost of commercial-scale offshore cultivation of kelp in the Faroe Islands using multiple partial harvesting. Algal Res. 2018, 33, 36–47. [Google Scholar] [CrossRef]
- Bernard, M. Seaweed Diseases and Pests. Wageningen Marine Research, Proseaweed Dossier (project AF-16202). 2018. Available online: https://edepot.wur.nl/475726 (accessed on 8 January 2023).
- Gutow, L.; Poore, A.; Poblete, M.A.D.; Villalobos, V.; Thiel, M. Small burrowing amphipods cause major damage in a large kelp. Proc. R. Soc. B Boil. Sci. 2020, 287, 20200330. [Google Scholar] [CrossRef] [PubMed]
- Forbord, S.; Matsson, S.; Brodahl, G.E.; Bluhm, B.A.; Broch, O.J.; Handå, A.; Metaxas, A.; Skjermo, J.; Steinhovden, K.B.; Olsen, Y. Latitudinal, seasonal and depth-dependent variation in growth, chemical composition and biofouling of cultivated Saccharina latissima (Phaeophyceae) along the Norwegian coast. J. Appl. Phycol. 2020, 32, 2215–2232. [Google Scholar] [CrossRef] [Green Version]
- Hurd, C.L.; Durante, K.M.; Chia, F.-S.; Harrison, P.J. Effect of bryozoan colonization on inorganic nitrogen acquisition by the kelps Agarum fimbriatum and Macrocystis integrifolia. Mar. Biol. 1994, 121, 167–173. [Google Scholar] [CrossRef]
- Pica, D.; Bloecher, N.; Dell’Anno, A.; Bellucci, A.; Pinto, T.; Pola, L.; Puce, S. Dynamics of a biofouling community in finfish aquaculture: A case study from the South Adriatic Sea. Biofouling 2019, 35, 696–709. [Google Scholar] [CrossRef]
- Montalto, V.; Rinaldi, A.; Ape, F.; Mangano, M.; Gristina, M.; Sarà, G.; Mirto, S. Functional role of biofouling linked to aquaculture facilities in Mediterranean enclosed locations. Aquac. Environ. Interact. 2020, 12, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Paclibare, J.; Evelyn, T.; Albright, L.; Prosperi-Porta, L. Clearing of the kidney disease bacterium Renibacterium salmoninarum from seawater by the blue mussel Mytilus edulis, and the status of the mussel as a reservoir of the bacterium. Dis. Aquat. Org. 1994, 18, 129–133. [Google Scholar] [CrossRef]
- Fitridge, I. The Ecology of Hydroids (Hydrozoa: Cnidaria) in Port Phillip Bay, Australia, and Their Impacts as Fouling Species in Longline Mussel Culture. Ph.D. Thesis, The University of Melbourne, Melbourne, Australia, 2011. [Google Scholar]
- Hickman, N.; Sause, B. Culture of the Blue Mussel (Mytilus edulis Planulatus) in Port Phillip Bay, Victoria Australia. III: Larval Settlement; Report No. 75; Marine Science Laboratories: Queenscliff, Australia, 1984. [Google Scholar]
- Dalby, J.E.; Young, C.M. Variable effects of ascidian competitors on oysters in a Florida epifaunal community. J. Exp. Mar. Biol. Ecol. 1993, 167, 47–57. [Google Scholar] [CrossRef]
- Ross, K.A.; Thorpe, J.P.; Norton, T.A.; Brand, A.R. Fouling in Scallop Cultivation: Help or Hindrance? J. Shellfish Res. 2002, 21, 539–548. [Google Scholar]
- Leblanc, A.R.; Landry, T.; Miron, G. Fouling Organisms of the Blue Mussel Mytilus edulis: Their Effect on Nutrient Uptake and Release. J. Shellfish Res. 2003, 22, 633–638. [Google Scholar]
- Walls, A.; Kennedy, R.; Fitzgerald, R.; Blight, A.; Johnson, M.; Edwards, M.D. Potential novel habitat created by holdfasts from cultivated Laminaria digitata: Assessing the macroinvertebrate assemblages. Aquac. Environ. Interact. 2016, 8, 157–169. [Google Scholar] [CrossRef] [Green Version]
- Visch, W.; Kononets, M.; Hall, P.O.; Nylund, G.M.; Pavia, H. Environmental impact of kelp (Saccharina latissima) aquaculture. Mar. Pollut. Bull. 2020, 155, 110962. [Google Scholar] [CrossRef]
- Walls, A.; Edwards; Firth, L.; Johnson, M. Ecological priming of artificial aquaculture structures: Kelp farms as an example. J. Mar. Biol. Assoc. U.K. 2018, 99, 729–740. [Google Scholar] [CrossRef]
- Lane, C.E.; Mayes, C.; Druehl, L.D.; Saunders, G.W. A multi-gene molecular investigation of the kelp (laminariales, phaeophyceae) supports substantial taxonomic re-organization1. J. Phycol. 2006, 42, 493–512. [Google Scholar] [CrossRef]
- Anderson, M.J.; Diebel, C.E.; Blom, W.M.; Landers, T.J. Consistency and variation in kelp holdfast assemblages: Spatial patterns of biodiversity for the major phyla at different taxonomic resolutions. J. Exp. Mar. Biol. Ecol. 2005, 320, 35–56. [Google Scholar] [CrossRef]
- Teagle, H.; Moore, P.J.; Jenkins, H.; Smale, D.A. Spatial variability in the diversity and structure of faunal assemblages associated with kelp holdfasts (Laminaria hyperborea) in the northeast Atlantic. PLoS ONE 2018, 13, e0200411. [Google Scholar] [CrossRef] [Green Version]
- Clarke, K.R.; Gorley, R.N. Getting Started with PRIMER V7; PRIMER-E: Plymouth, UK, 2015. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; University of Auckland/PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Anderson, M.J. Permutational Multivariate Analysis of Variance (PERMANOVA); Balakrishnan, N., Colton, T., Everitt, B., Piegorsch, W., Ruggeri, F., Teugels, J.L., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 1–15. [Google Scholar] [CrossRef]
- Bué, M.; Smale, D.A.; Natanni, G.; Marshall, H.; Moore, P.J. Multiple-scale interactions structure macroinvertebrate assemblages associated with kelp understory algae. Divers. Distrib. 2020, 26, 1551–1565. [Google Scholar] [CrossRef]
- Arnold, M.; Teagle, H.; Brown, M.P.; Smale, D.A. The structure of biogenic habitat and epibiotic assemblages associated with the global invasive kelp Undaria pinnatifida in comparison to native macroalgae. Biol. Invasions 2015, 18, 661–676. [Google Scholar] [CrossRef]
- King, N.; Moore, P.; Wilding, C.; Jenkins, H.; Smale, D. Multiscale spatial variability in epibiont assemblage structure associated with stipes of kelp Laminaria hyperborea in the northeast Atlantic. Mar. Ecol. Prog. Ser. 2021, 672, 33–44. [Google Scholar] [CrossRef]
- Hayward, P.; Ryland, J. British Anascan Bryozoans; Kermack, D.M., Barnes, R.S.K., Eds.; The Linnaen Society of London/Academic Press: London, UK, 1979; Volume 14. [Google Scholar]
- Hargrave, M.S.; Nylund, G.M.; Enge, S.; Pavia, H. Co-cultivation with blue mussels increases yield and biomass quality of kelp. Aquaculture 2021, 550, 737832. [Google Scholar] [CrossRef]
- Thiel, M.; Vásquez, J.A. Are kelp holdfasts islands on the ocean floor?—Indication for temporarily closed aggregations of peracarid crustaceans. In Island, Ocean and Deep-Sea Biology; Springer: Dordrecht, The Netherlands, 2000; pp. 45–54. [Google Scholar] [CrossRef]
- Eklöf, J.S.; De La Torre-Castro, M.; Nilsson, C.; Rönnbäck, P. How do seaweed farms influence local fishery catches in a seagrass-dominated setting in Chwaka Bay, Zanzibar? Aquat. Living Resour. 2006, 19, 137–147. [Google Scholar] [CrossRef]
- Hill, J. Jassa Falcata An Amphipod. In Marine Life Information Network: Biology and Sensitivity Key Information Reviews; Tyler-Walters, H., Hiscock, K., Eds.; Marine Biological Association of the United Kingdom: Plymouth, UK, 2000. [Google Scholar]
- Moore, P.G. Levels of Heterogeneity and the Amphipod Fauna of Kelp Holdfasts. In The Ecology of Rocky Coasts: Essays presented to J.R. Lewis, D.Sc; Moore, P.G., Seed, R., Eds.; Hodder & Stoughton Ltd.: London, UK, 1985. [Google Scholar]
- Nair, K.K.C.; Anger, K. Experimental studies on the life cycle of Jassa falcata (Crustacea, Amphipoda). Helgol. Mar. Res. 1979, 32, 444–452. [Google Scholar] [CrossRef] [Green Version]
- Nair, K.C.; Anger, K. Seasonal variation in population structure and biochemical composition of Jassa falcata (Crustacea, Amphipoda) off the island of Helgoland (North Sea). Estuar. Coast. Mar. Sci. 1980, 11, 505–513. [Google Scholar] [CrossRef]
- Hargrave, M.S.; Ekelund, A.; Nylund, G.M.; Pavia, H. Filtration and fertilisation effects of the bivalves Mytilus edulis and Magallana gigas on the kelp Saccharina latissima in tank culture. J. Appl. Phycol. 2021, 33, 3927–3938. [Google Scholar] [CrossRef]
- Knowler, D.; Chopin, T.; Martínez-Espiñeira, R.; Neori, A.; Nobre, A.; Noce, A.; Reid, G. The economics of Integrated Multi-Trophic Aquaculture: Where are we now and where do we need to go? Rev. Aquac. 2020, 12, 1579–1594. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Zahedi, S.; Mohammadi, A. Integrated multitrophic aquaculture (IMTA) as an environmentally friendly system for sustainable aquaculture: Functionality, species, and application of biofloc technology (BFT). Environ. Sci. Pollut. Res. 2022, 29, 67513–67531. [Google Scholar] [CrossRef]
- Christie, H.; Jørgensen, N.M.; Norderhaug, K.M.; Waage-Nielsen, E. Species distribution and habitat exploitation of fauna associated with kelp (Laminaria hyperborea) along the Norwegian Coast. J. Mar. Biol. Assoc. UK 2003, 83, 687–699. [Google Scholar] [CrossRef]
- Moore, P. Particulate matter in the sublittoral zone of an exposed coast and its ecological significance with special reference to the fauna inhabiting kelp holdfasts. J. Exp. Mar. Biol. Ecol. 1972, 10, 59–80. [Google Scholar] [CrossRef]
- Arroyo, N.L.; Maldonado, M.; Benito, J. Distribution patterns of meiofauna associated with a sublittoral Laminaria bed in the Cantabrian Sea (north-eastern Atlantic). Mar. Biol. 2003, 144, 231–242. [Google Scholar] [CrossRef]
- Bunker, F.S.D.; Brodie, J.A.; Maggs, C.A.; Bunker, A.R. Saccharina latissima. In Seaweeds of Britain and Ireland, 2nd ed.; Princeton University Press: Princeton, NJ, USA, 2017; ISBN 9780995567337. [Google Scholar]
- Defra Sustainable Farming Incentive Opens for—GOV.UK. Available online: https://www.gov.uk/government/news/sustainable-farming-incentive-opens-for-applications (accessed on 6 November 2022).
- Defra Funding for Farmers and Land Managers—GOV.UK. Available online: https://www.gov.uk/guidance/funding-for-farmers (accessed on 6 November 2022).






| Treatment/ Control Name | Seeding Date | Number of Replicates (n) | Description |
|---|---|---|---|
| May2020 (control) | November 2019 | 6 | Lines from November 2019 seeding, harvested as usual in May 2020 |
| Left2019 (treatment) | November 2019 | 3 | Lines from November 2019 seeding left in the farm an extra year until May 2021 with no harvesting treatment |
| BareOct20 (treatment) | November 2019 | 3 | Lines originally seeded in November 2019, left in the farm until October 2020, when they were stripped and left in again until May 2021 |
| 10cmOct20 (treatment) | November 2019 | 3 | Lines originally seeded in November 2019, left in the farm until October 2020, when they were cut at 10 cm above their meristems and left in again until May 2021 |
| May2021 (control) | October 2020 | 3 | New lines seeded in October 2020, harvested as usual in May 2021 |
| PERMANOVA | PERMDISP | ||||||
|---|---|---|---|---|---|---|---|
| Response Metric | Transformation | df | p | F | p | F | Post Hoc Significant Differences between Months |
| (Uv) Taxa richness | N/A | 4, 25 | 0.001 | 27.245 | 0.359 | 1.3115 | All different except May–June, June–July, July–August |
| (Uv) Mobile epibiont abundance | N/A | 4, 25 | 0.001 | 100.56 | 0.049 | 5.1546 | All different except April–May |
| (Uv) Mobile and algal epibiont biomass | N/A | 4, 25 | 0.001 | 39.471 | 0.003 | 8.5812 | All different except April–May |
| (Uv) Sessile epibiont coverage of blade (%) | N/A | 4, 25 | 0.001 | 24.787 | 0.011 | 5.8651 | All different except June–July, June–August, July–August |
| (Uv) Kelp biomass | N/A | 4, 25 | 0.001 | 87.757 | 0.014 | 6.2136 | All different |
| (Uv) Holdfast habitable volume | N/A | 4, 25 | 0.001 | 7.947 | 0.42 | 1.5967 | All different except April–May, May–June, June–July, July–August |
| (Uv) Blade surface area | N/A | 4, 25 | 0.001 | 10.028 | 0.417 | 1.7751 | All different except May–June, May–July, May–August, June–July, June–August, July–August |
| (Mv) Total assemblage (presence–absence) | Presence–absence | 4, 25 | 0.001 | 14.243 | 0.265 | 1.3878 | All different except May–June |
| (Mv) Mobile epibiont abundance | Fourth root | 4, 25 | 0.001 | 37.333 | 0.067 | 2.7666 | All different |
| (Mv) Mobile and algal epibiont biomass | Fourth root | 4, 25 | 0.001 | 10.093 | 0.813 | 0.44907 | All different |
| (Mv) Sessile epibiont coverage of blade (%) | Square root and dummy variable added = 0.4 | 4, 25 | 0.001 | 28.871 | 0.712 | 0.6851 | All different |
| PERMANOVA | PERMDISP | |||||||
|---|---|---|---|---|---|---|---|---|
| Response Metric | Transformation | Factors | df | p | F | p | F | Post Hoc Significance Differences between Kelp Structures within Months |
| (Uv) Taxa richness | N/A | Month | 4 | 0.001 | 34.987 | 0.001 | 8.8306 | N/A |
| Kelp structure | 1 | 0.001 | 24.013 | 0.004 | 7.8454 | N/A | ||
| Month × kelp structure | 4 | 0.001 | 12.909 | N/A | N/A | No difference except in April and July | ||
| (Uv) Mobile epibiont abundance | N/A | Month | 4 | 0.001 | 99.755 | 0.001 | 108.52 | N/A |
| Kelp structure | 1 | 0.001 | 28414 | 0.001 | 145.88 | N/A | ||
| Month × kelp structure | 4 | 0.001 | 99.389 | N/A | N/A | All different except in April and May | ||
| (Uv) Mobile and algal epibiont biomass | N/A | Month | 4 | 0.001 | 42.098 | 0.001 | 40.775 | N/A |
| Kelp structure | 1 | 0.001 | 118.78 | 0.001 | 75.951 | N/A | ||
| Month × kelp structure | 4 | 0.001 | 36.051 | N/A | N/A | All different except in April and May | ||
| (Uv) Sessile epibiont coverage (%) | N/A | Month | 4 | 0.001 | 58.195 | 0.001 | 16.936 | N/A |
| Kelp structure | 1 | 0.001 | 78.339 | 0.005 | 13.538 | N/A | ||
| Month × kelp structure | 4 | 0.001 | 13.978 | N/A | N/A | All different except in May | ||
| (Uv) Kelp structure biomass | N/A | Month | 4 | 0.001 | 393.84 | 0.001 | 39.665 | N/A |
| Kelp structure | 1 | 0.001 | 40.696 | 0.001 | 65.251 | N/A | ||
| Month × kelp structure | 4 | 0.001 | 36.059 | N/A | N/A | All different | ||
| (Mv) Total assemblage (presence-absence) | Presence–absence | Month | 4 | 0.001 | 22.981 | 0.001 | 12.607 | N/A |
| Kelp structure | 1 | 0.001 | 37.167 | 0.708 | 0.708 | N/A | ||
| Month × kelp structure | 4 | 0.001 | 9.2638 | N/A | N/A | All different except in April | ||
| (Mv) Mobile epibiont abundance | Fourth root | Month | 4 | 0.001 | 23.512 | 0.001 | 14.907 | N/A |
| Kelp structure | 1 | 0.001 | 27.054 | 0.001 | 31.268 | N/A | ||
| Month × kelp structure | 4 | 0.001 | 6.745 | N/A | N/A | All different except in April | ||
| (Mv) Mobile and algal epibiont biomass | Fourth root | Month | 4 | 0.001 | 22.904 | 0.256 | 1.7008 | N/A |
| Kelp structure | 1 | 0.001 | 48.941 | 0.105 | 3.0193 | N/A | ||
| Month × kelp structure | 4 | 0.001 | 8.0059 | N/A | N/A | All different | ||
| (Mv) Sessile epibiont coverage (%) | Square root dummy variable added = 0.4 | Month | 4 | 0.001 | 19.025 | 0.001 | 22.106 | N/A |
| Kelp structure | 1 | 0.001 | 22.903 | 0.255 | 0.84876 | N/A | ||
| Month × kelp structure | 4 | 0.001 | 10.915 | N/A | N/A | All different except in August | ||
| PERMANOVA | PERMDISP | ||||||
|---|---|---|---|---|---|---|---|
| Response Metric | Transformation | df | p | F | p | F | Post Hoc Significant Differences between Treatments |
| (Uv) Taxa richness | N/A | 3, 11 | 0.001 | 14.401 | 0.146 | 2.9333 | May2020 and May2021 different from Left2019 and 10cmOct2020 |
| (Uv) Mobile epibiont abundance | N/A | 3, 11 | 0.024 | 4.0763 | 0.002 | 13.744 | May2020 different from other treatments |
| (Uv) Mobile and algal epibiont biomass | N/A | 3, 11 | 0.002 | 23.752 | 0.013 | 6.9953 | May2020 different from other treatments |
| (Uv) Sessile epibiont coverage of the blade (%) | N/A | 3, 11 | 0.001 | 25.198 | 0.003 | 11.83 | May2020 and May2021 different from Left2019 and 10cmOct2020 |
| (Uv) Kelp biomass | N/A | 3, 11 | 0.01 | 16.45 | 0.089 | 3.6096 | May2020 different from other treatments |
| (Uv) Holdfast habitable volume | N/A | 3, 11 | 0.02 | 4.5757 | 0.046 | 4.2501 | May2020 different from other treatments |
| (Uv) Blade surface area | N/A | 3, 11 | 0.006 | 10.558 | 0.014 | 6.9989 | May2020 different from other treatments |
| (Mv) Total assemblage (presence-absence) | Presence–absence | 3, 11 | 0.001 | 11.632 | 0.7 | 0.62771 | May2020 different from other treatments |
| (Mv) Mobile epibiont abundance | Fourth root | 3, 11 | 0.001 | 19.683 | 0.762 | 0.7985 | May2020 different from other treatments |
| (Mv) Mobile and algal epibiont biomass | Fourth root | 3, 11 | 0.001 | 10.882 | 0.35 | 2.0862 | May2020 different from other treatments |
| (Mv) Sessile epibiont coverage of blade (%) | Square root | 3, 11 | 0.001 | 8.8321 | 0.445 | 1.5613 | May2020 different from Left2019 and 10cmOct2020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrigan, S.; Brown, A.R.; Tyler, C.R.; Wilding, C.; Daniels, C.; Ashton, I.G.C.; Smale, D.A. Development and Diversity of Epibiont Assemblages on Cultivated Sugar Kelp (Saccharina latissima) in Relation to Farming Schedules and Harvesting Techniques. Life 2023, 13, 209. https://doi.org/10.3390/life13010209
Corrigan S, Brown AR, Tyler CR, Wilding C, Daniels C, Ashton IGC, Smale DA. Development and Diversity of Epibiont Assemblages on Cultivated Sugar Kelp (Saccharina latissima) in Relation to Farming Schedules and Harvesting Techniques. Life. 2023; 13(1):209. https://doi.org/10.3390/life13010209
Chicago/Turabian StyleCorrigan, Sophie, A. Ross Brown, Charles R. Tyler, Catherine Wilding, Carly Daniels, Ian G. C. Ashton, and Dan A. Smale. 2023. "Development and Diversity of Epibiont Assemblages on Cultivated Sugar Kelp (Saccharina latissima) in Relation to Farming Schedules and Harvesting Techniques" Life 13, no. 1: 209. https://doi.org/10.3390/life13010209
APA StyleCorrigan, S., Brown, A. R., Tyler, C. R., Wilding, C., Daniels, C., Ashton, I. G. C., & Smale, D. A. (2023). Development and Diversity of Epibiont Assemblages on Cultivated Sugar Kelp (Saccharina latissima) in Relation to Farming Schedules and Harvesting Techniques. Life, 13(1), 209. https://doi.org/10.3390/life13010209








