Abstract
Heart failure (HF) is categorized arbitrarily based on the left ventricular ejection fraction (LVEF) in HF with reduced (HFrEF; LVEF < 40%), mildly reduced (HFmrEF; LVEF 40–49%), or preserved ejection fraction (HFpEF; LVEF ≥ 50%). In this opinion paper, based on (patho)physiological considerations, we contend that the neurohormonal overactivity syndrome (NOHS), which is present in all symptomatic HF patients irrespective of their LVEF, not only contributes to the development of signs and symptoms but it is also a major determinant of patients’ outcomes. In this regard, NHOS is the only currently available treatment target in HF and should be combatted in most patients with the combined use of diuretics and neurohormonal inhibitors (β-blockers, angiotensin receptor-neprilysin inhibitor/angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, mineralocorticoid antagonists, and sodium-glucose co-transporter 2 inhibitors). Unfortunately, despite the advances in therapeutics, HF mortality remains high. Probably machine learning approaches could better assess the multiple and higher-dimension interactions leading to the HF syndrome and define clusters of HF treatment efficacy.
1. Introduction
Heart failure (HF) is a clinical syndrome related to high morbidity and mortality [1]. According to the traditional view, HF is categorized based on the left ventricular ejection fraction (LVEF) in HF with reduced (HFrEF; LVEF < 40%), mid-range (HFmrEF; LVEF 40–49%), or preserved ejection fraction (HFpEF; LVEF ≥ 50%). Nevertheless, LVEF categorization has several limitations (i.e., imprecise physiological implications, substantial intra- and inter-observer variability between LVEF measurements, arbitrary LVEF cut-offs, LVEF transitions) and has been challenged (i.e., epidemiological, clinical, pathophysiological, and therapeutic features are common across the HF spectrum) [2,3,4,5,6]. In this regard, the neurohormonal overactivity syndrome (NHOS), which is present in all symptomatic HF patients, irrespective of the LVEF, contributes to the development of signs and symptoms (please see Pathophysiology section), is a major determinant of outcomes [7,8] (Figure 1) and is the only currently available treatment target to reduce rehospitalizations and prolong survival [5].
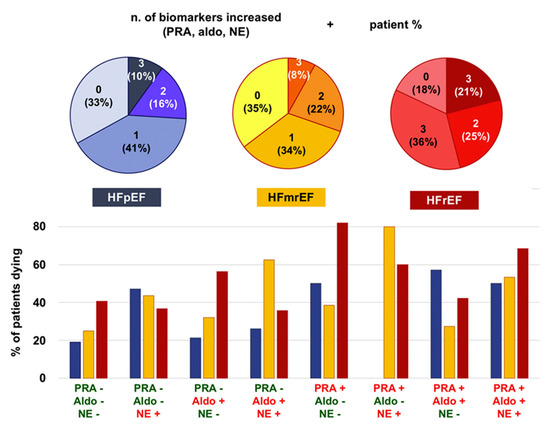
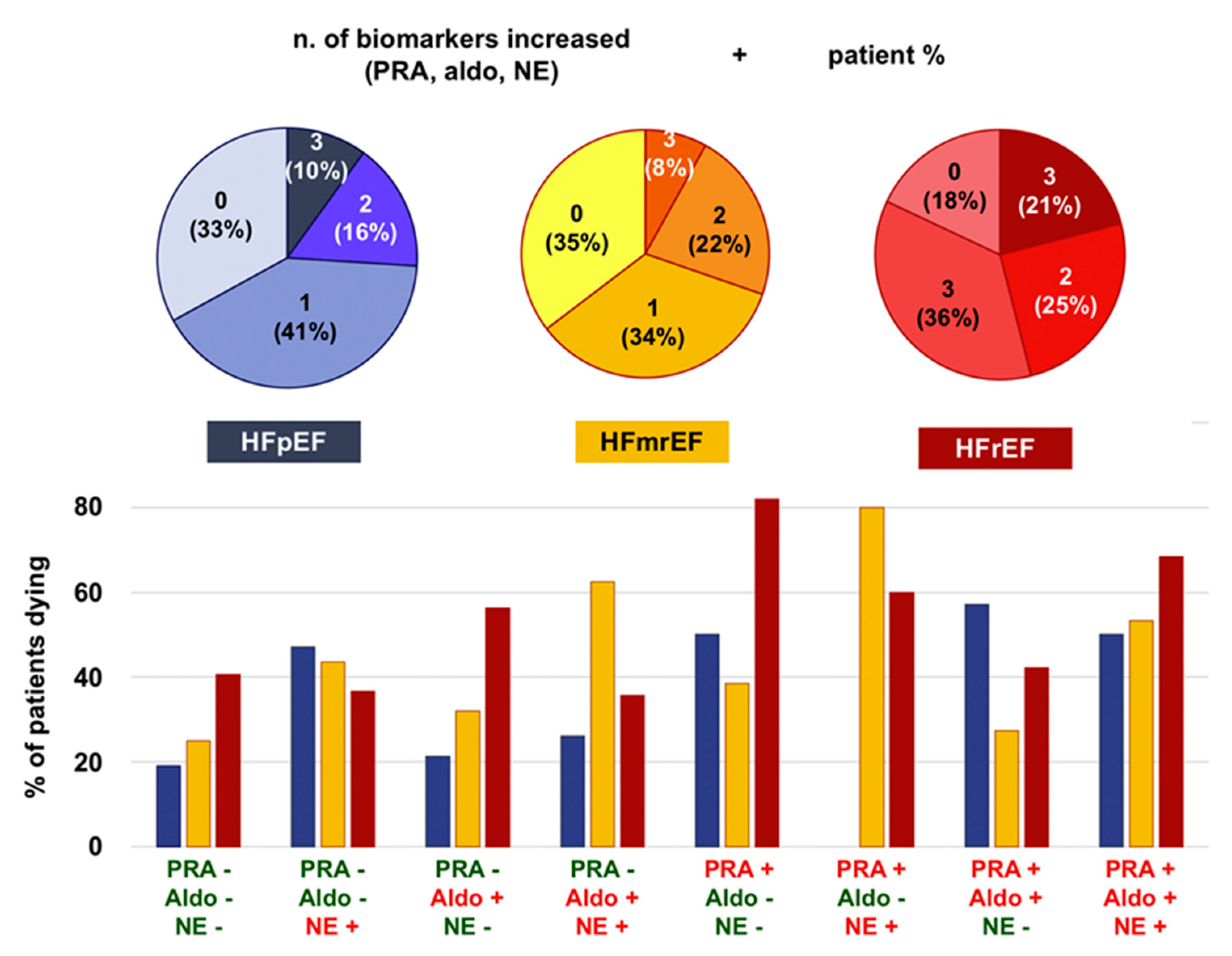
Figure 1.
Sympathetic and renin-angiotensin-aldosterone system activation across ejection fraction categories and their prognostic impact. Aldo, aldosterone; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; NE, norepinephrine NT-proBNP, N-terminal fraction of pro-B-type natriuretic peptide PRA, plasma renin activity. Adapted with permission from Ref. [8]. © 2019 Elsevier B.V.
2. Pathophysiology
Risk factors (hypertension, coronary artery disease, obesity, and others) always precede the development of HF and are associated with a high HF incidence, whereas comorbidities (atrial fibrillation, diabetes mellitus, anemia, chronic kidney disease, depression, pulmonary diseases, sleep-disordered breathing, and others) may precede or develop after HF and usually co-exist with HF in groups of two or more (multi-morbidity) [9]. The complex interaction between risk factors, comorbidities, and disease modifiers (sex, genes, and others) may lead to cardiac damage (myocardial dysfunction) and HF [10].
Traditionally, the principal hemodynamic mechanisms following cardiac damage have included low cardiac output at rest or even at exercise (“forward failure”) as well as elevated (left and/or right) cardiac filling pressures (“backward failure”) [11]. Rarely cardiac output is increased in HF due to vasodilation and decreased peripheral resistance (high high-output HF) [12]. The studies on patients with low- and high-output HF, support the idea that preservation or maintenance of the arterial blood pressure is the main trigger for the development of neurohormonal overactivity (activation of the sympathetic nervous system (SNS) and the renin-angiotensin aldosterone system (RAAS)) [13,14]) signaling to the kidney to retain sodium and water which seen in all forms of HF [15]. However prolonged neurohormonal overactivity results in endothelial dysfunction, myocardial fibrosis, skeletal myopathy, and inflammation [16]. The concentration of circulating proinflammatory cytokines is increased, presumably due to endotoxin-induced immune activation resulting from bowel oedema, myocardial production due to haemodynamic overload, and peripheral extramyocardial production due to tissue hypoperfusion and hypoxia [17] and may stimulate the production of reactive oxygen species (ROS) via induction of the NADPH oxidase [18]. The above result in neurohormonal overactivity syndrome (NHOS), which is characterized by exercise intolerance, limited by dyspnea and fatigue (Figure 2). Congestion, the cardinal derangement underlying NHOS, is due to renal retention of sodium and water and leads to intravascular and interstitial fluid volume expansion and redistribution [19]. Although initial sympathetic-driven vasoconstriction supports organ perfusion pressure in the short term, a more progressive aggregation of interstitial compartment fluid also arises which supports a compensatory expansion of intravascular plasma volume. A fast component, which is noticed shortly prior to decompensation, is triggered by the sympathetic nervous system overactivity and leads to an intercompartmental fluid shift into the central circulation with an upcoming increase in central filling pressures [20].
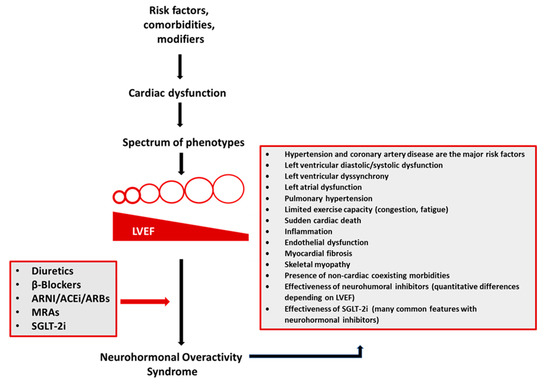
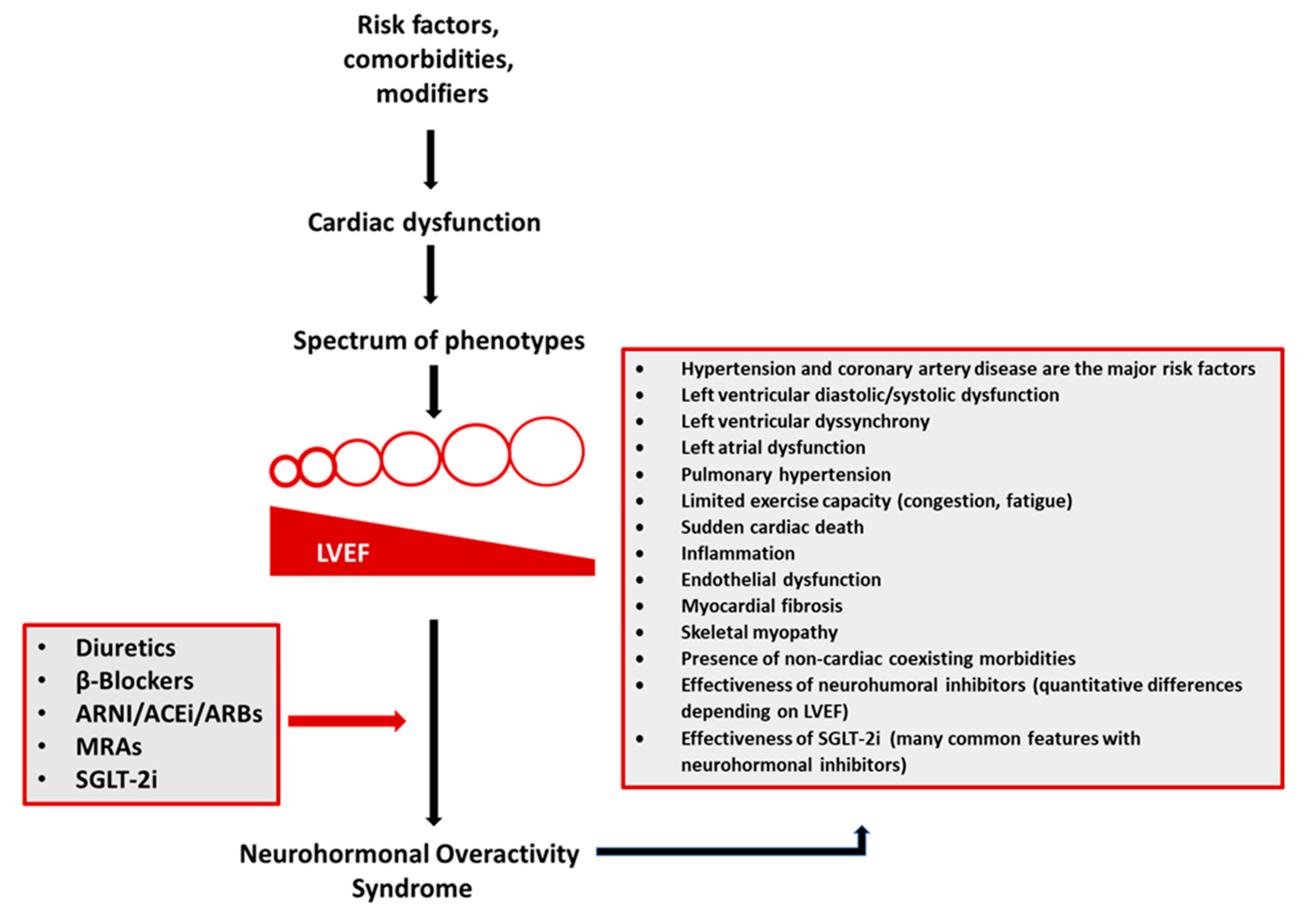
Figure 2.
A complex interplay between risk factors (coronary artery disease, hypertension, obesity, and others), comorbidities (atrial fibrillation, diabetes mellitus, chronic kidney disease, depression, pulmonary diseases, sleep-disordered breathing, anemia, and others), and disease modifiers (sex, genes, and others) leads to cardiac damage manifested by a spectrum of phenotypes. Diverse heart failure (HF) phenotypes converge to the neurohormonal overactivity syndrome (NOHS). Treatment with diuretics and neurohormonal inhibitors (β-blockers, angiotensin receptor-neprilysin inhibitor (ARNI)/angiotensin-converting enzyme inhibitors (ACEi)/angiotensin receptor blockers (ARBs), mineralocorticoid antagonists (MRAs), and sodium-glucose co-transporter 2 inhibitors (SGLT-2i)) is the only currently available treatment to combat NHOS and, therefore, decrease morbidity and mortality in most HF patients.
The detrimental consequences of the prolonged activation of the RAAS and SNS are partly counteracted by the activation of neurohumoral factors such as natriuretic peptides (atrial (ANP), brain (BNP), and C-type natriuretic peptides (CNP)), which exhibit cardioprotective effects (i.e., diuretic, natriuretic, and vasodilatory actions) [21,22]. Unfortunately, although patients with chronic HF have an increase in ANP and BNP production, the proportion of inactive molecules is increased along with an increase in natriuretic peptide-degrading enzymes and the receptor-mediated clearance of natriuretic peptides [23].
The advanced stage of NHOS is characterized by the development of multiorgan dysfunction due to the development of an abnormal bidirectional relationship between the heart and several organs such as the brain (cardiocerebral syndrome) [24], the kidney (cardiorenal syndrome) [25] or the liver (cardiohepatic syndrome) [26] and leading to a vicious cycle.
Finally, sudden cardiac death (SCD) is a cardinal manifestation of the NHOS syndrome [27]. Heart disease disposes patients towards malignant ventricular arrhythmias by causing neural remodeling at the level of the myocardium, the intrinsic cardiac ganglia, spinal cord, extracardiac intrathoracic sympathetic ganglia, extrathoracic ganglia, and the brainstem, as well as the higher centers and the cortex [28]. Abnormal metabolism and oxidative stress in the cardiomyocytes and noncardiac myocytes tissues also may lead to ventricular arrhythmias and SCD [29]. Treatment with neurohumoral inhibitors dramatically reduces the risk of SCD emphasizing the tight link between neurohormonal overactivity and SCD [30]. In particular, an analysis including 40,195 HF patients from 12 clinical trials conducted over a period of 19 years, demonstrated that the use of angiotensin-converting–enzyme inhibitors, angiotensin-receptor blockers, angiotensin receptor-neprilysin inhibitors, beta-blockers, and mineralocorticoid-receptor antagonists was associated with 44% lower risk of SCD [30]. Several recent meta-analyses of randomized controlled trials on sodium-glucose co-transporter 2 inhibitors (SGLT-2i) revealed a trend towards reduced risk of ventricular tachycardia or fibrillation and sudden cardiac death in patients with type 2 diabetes mellitus as well as patients with HF [31,32,33,34]. Nevertheless, in the majority of these meta-analyses, statistical significance was not reached, presumably due to the low number of events [35].
3. Clinical Implications
NHOS is the only currently available treatment target in HF and should be combatted in most patients with the combined use of diuretics and neurohormonal inhibitors (β-blockers/angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, mineralocorticoid antagonists, and SGLT-2i) [5,36,37,38,39]. The novel SGLT-2i block glucose reabsorption in the renal proximal tubule, a mechanism independent of insulin that plays an important role in glycemic regulation in diabetes [40]. Aside from the target effects of SGLT-2i, a wide variety of favorable effects is described for the kidney and the heart, despite the fact that cardiac tissue does not express SGLT-2 channels. SGLT-2i decrease the hazard of death from cardiac or vascular causes and hospitalization for HF in patients with HF. Initially, these medicines were thought to exert actions similar to diuretics or hemodynamically active drugs, but they do not rapidly decrease natriuretic peptides or cardiac filling pressures, and they demonstrate minimal early benefit on symptoms, exercise tolerance, or quality of life. Clinically, the profile of SGLT-2i looks similar to that of neurohormonal inhibitors, whose favorable effects are observed gradually during sustained therapy. Indeed, in experimental in vivo animal models, SGLT-2i produce a distinctive pattern of cellular effects (attenuation of proinflammatory pathways, amelioration of oxidative stress, mitigation of mitochondrial dysfunction, and a reduction in myocardial fibrosis), which is similar to that of neurohormonal inhibitors [41]. At the molecular level, SGLT-2i lead to transcriptional reprogramming of cardiomyocytes that closely resembles that observed during nutrient deprivation. This alteration in signaling triggers the housekeeping pathway of autophagy, which clears the cytosol of threatening cytosolic constituents that cause cellular stress, thereby decreasing the risk of cardiomyopathy development [42]. Remarkably, similar alterations in cellular signaling and autophagic flux have also been observed in neurohormonal inhibitors.
4. Conclusions
Most symptomatic HF phenotypes converge on the NHOS which is an important outcome predictor and one of the rare rewarding treatment targets in HF. As the NHOS is present throughout the HF spectrum of phenotypes, the LVEF-based HF classification and treatment guidelines should be abandoned as this strategy has deprived HF patients of years of potentially lifesaving treatments. As HF mortality remains high despite the advances in therapeutics, machine learning approaches could better assess the multiple and higher-dimension interactions leading to HF syndrome and define clusters of HF treatment efficacy. This strategy has already been successfully used in HF both for more accurate phenotyping [43] and the implementation of individualized treatment [44].
Author Contributions
Conceptualization, A.X., J.S., F.T.; data curation, A.X., J.S., F.T.; writing—original draft preparation, A.X., F.T.; writing—review and editing, J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef] [PubMed]
- Triposkiadis, F.; Butler, J.; Abboud, F.M.; Armstrong, P.W.; Adamopoulos, S.; Atherton, J.J.; Backs, J.; Bauersachs, J.; Burkhoff, D.; Bonow, R.O.; et al. The continuous heart failure spectrum: Moving beyond an ejection fraction classification. Eur. Heart J. 2019, 40, 2155–2163. [Google Scholar] [CrossRef] [PubMed]
- Triposkiadis, F.; Giamouzis, G.; Kitai, T.; Skoularigis, J.; Starling, R.C.; Xanthopoulos, A. A Holistic View of Advanced Heart Failure. Life 2022, 12, 1298. [Google Scholar] [CrossRef]
- Triposkiadis, F.; Starling, R.C. Chronic Heart Failure: Diagnosis and Management beyond LVEF Classification. J. Clin. Med. 2022, 11, 1718. [Google Scholar] [CrossRef] [PubMed]
- Triposkiadis, F.; Xanthopoulos, A.; Starling, R.C. Medical Treatment of Heart Failure: Ignore the Ejection Fraction and Treat All? J. Card. Fail. 2021, 27, 907–909. [Google Scholar] [CrossRef] [PubMed]
- Xanthopoulos, A.; Giamouzis, G.; Skoularigis, J.; Triposkiadis, F. Heart failure with reduced, mildly reduced, or preserved left ventricular ejection fraction: Has reasoning been lost? World J. Cardiol. 2022, 14, 438–445. [Google Scholar] [CrossRef]
- Chioncel, O.; Lainscak, M.; Seferovic, P.M.; Anker, S.D.; Crespo-Leiro, M.G.; Harjola, V.-P.; Parissis, J.; Laroche, C.; Piepoli, M.F.; Fonseca, C.; et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: An analysis of the ESC Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2017, 19, 1574–1585. [Google Scholar] [CrossRef]
- Vergaro, G.; Aimo, A.; Prontera, C.; Ghionzoli, N.; Arzilli, C.; Zyw, L.; Taddei, C.; Gabutti, A.; Poletti, R.; Giannoni, A.; et al. Sympathetic and renin-angiotensin-aldosterone system activation in heart failure with preserved, mid-range and reduced ejection fraction. Int. J. Cardiol. 2019, 296, 91–97. [Google Scholar] [CrossRef]
- Triposkiadis, F.; Giamouzis, G.; Parissis, J.; Starling, R.C.; Boudoulas, H.; Skoularigis, J.; Butler, J.; Filippatos, G. Reframing the association and significance of co-morbidities in heart failure. Eur. J. Heart Fail. 2016, 18, 744–758. [Google Scholar] [CrossRef]
- Triposkiadis, F.; Xanthopoulos, A.; Butler, J. Cardiovascular Aging and Heart Failure: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 74, 804–813. [Google Scholar] [CrossRef]
- Verbrugge, F.H.; Guazzi, M.; Testani, J.M.; Borlaug, B.A. Altered Hemodynamics and End-Organ Damage in Heart Failure: Impact on the Lung and Kidney. Circulation 2020, 142, 998–1012. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.N.V.; Melenovsky, V.; Redfield, M.M.; Nishimura, R.A.; Borlaug, B.A. High-Output Heart Failure: A 15-Year Experience. J. Am. Coll. Cardiol. 2016, 68, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Badrov, M.B.; Mak, S.; Floras, J.S. Cardiovascular Autonomic Disturbances in Heart Failure with Preserved Ejection Fraction. Can. J. Cardiol. 2021, 37, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Kaye, D.M.; Nanayakkara, S.; Wang, B.; Shihata, W.; Marques, F.Z.; Esler, M.; Lambert, G.; Mariani, J. Characterization of Cardiac Sympathetic Nervous System and Inflammatory Activation in HFpEF Patients. JACC Basic Transl. Sci. 2022, 7, 116–127. [Google Scholar] [CrossRef]
- Anand, I.S. High-Output Heart Failure Revisited. J. Am. Coll. Cardiol. 2016, 68, 483–486. [Google Scholar] [CrossRef]
- Adamo, L.; Rocha-Resende, C.; Prabhu, S.D.; Mann, D.L. Reappraising the role of inflammation in heart failure. Nat. Rev. Cardiol. 2020, 17, 269–285. [Google Scholar] [CrossRef]
- Paulus, W.J. Cytokines and heart failure. Heart Fail. Monit. 2000, 1, 50–56. [Google Scholar]
- Schwinger, R.H.G. Pathophysiology of heart failure. Cardiovasc. Diagn. Ther. 2021, 11, 263–276. [Google Scholar] [CrossRef]
- Miller, W.L. Fluid Volume Overload and Congestion in Heart Failure: Time to Reconsider Pathophysiology and How Volume Is Assessed. Circ. Heart Fail. 2016, 9, e002922. [Google Scholar] [CrossRef]
- Fudim, M.; Hernandez, A.F.; Felker, G.M. Role of Volume Redistribution in the Congestion of Heart Failure. J. Am. Heart Assoc. 2017, 6, e006817. [Google Scholar] [CrossRef]
- Kuwahara, K. The natriuretic peptide system in heart failure: Diagnostic and therapeutic implications. Pharmacol. Ther. 2021, 227, 107863. [Google Scholar] [CrossRef] [PubMed]
- Baba, M.; Yoshida, K.; Ieda, M. Clinical Applications of Natriuretic Peptides in Heart Failure and Atrial Fibrillation. Int. J. Mol. Sci. 2019, 20, 2824. [Google Scholar] [CrossRef] [PubMed]
- Diez, J. Chronic heart failure as a state of reduced effectiveness of the natriuretic peptide system: Implications for therapy. Eur. J. Heart Fail. 2017, 19, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Havakuk, O.; King, K.S.; Grazette, L.; Yoon, A.J.; Fong, M.; Bregman, N.; Elkayam, U.; Kloner, R.A. Heart Failure-Induced Brain Injury. J. Am. Coll. Cardiol. 2017, 69, 1609–1616. [Google Scholar] [CrossRef]
- Rangaswami, J.; Bhalla, V.; Blair, J.E.; Chang, T.I.; Costa, S.; Lentine, K.L.; Lerma, E.V.; Mezue, K.; Molitch, M.; Mullens, W.; et al. Cardiorenal Syndrome: Classification, Pathophysiology, Diagnosis, and Treatment Strategies: A Scientific Statement from the American Heart Association. Circulation 2019, 139, e840–e878. [Google Scholar] [CrossRef]
- Xanthopoulos, A.; Starling, R.C.; Kitai, T.; Triposkiadis, F. Heart Failure and Liver Disease: Cardiohepatic Interactions. JACC Heart Fail. 2019, 7, 87–97. [Google Scholar] [CrossRef]
- Cho, J.H. Sudden Death and Ventricular Arrhythmias in Heart Failure with Preserved Ejection Fraction. Korean Circ. J. 2022, 52, 251–264. [Google Scholar] [CrossRef]
- Huang, W.A.; Boyle, N.G.; Vaseghi, M. Cardiac Innervation and the Autonomic Nervous System in Sudden Cardiac Death. Card. Electrophysiol. Clin. 2017, 9, 665–679. [Google Scholar] [CrossRef]
- Yang, K.C.; Kyle, J.W.; Makielski, J.C.; Dudley, S.C., Jr. Mechanisms of sudden cardiac death: Oxidants and metabolism. Circ. Res. 2015, 116, 1937–1955. [Google Scholar] [CrossRef]
- Shen, L.; Jhund, P.S.; Petrie, M.C.; Claggett, B.L.; Barlera, S.; Cleland, J.G.; Dargie, H.J.; Granger, C.B.; Kjekshus, J.; Køber, L.; et al. Declining Risk of Sudden Death in Heart Failure. N. Engl. J. Med. 2017, 377, 41–51. [Google Scholar] [CrossRef]
- Li, H.L.; Lip, G.Y.H.; Feng, Q.; Fei, Y.; Tse, Y.K.; Wu, M.Z.; Ren, Q.W.; Tse, H.F.; Cheung, B.Y.; You, K.H. Sodium-glucose cotransporter 2 inhibitors (SGLT2i) and cardiac arrhythmias: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2021, 20, 100. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.C.; Fernandes, A.; Cardoso, R.; Penalver, J.; Knijnik, L.; Mitrani, R.D.; Myerburg, R.J.; Goldberger, J.J. Association of SGLT2 inhibitors with arrhythmias and sudden cardiac death in patients with type 2 diabetes or heart failure: A meta-analysis of 34 randomized controlled trials. Heart Rhythm. 2021, 18, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Sfairopoulos, D.; Zhang, N.; Wang, Y.; Chen, Z.; Letsas, K.P.; Tse, G.; Li, G.; Lip, G.Y.H.; Liu, T.; Korantzopoulos, P. Association between sodium-glucose cotransporter-2 inhibitors and risk of sudden cardiac death or ventricular arrhythmias: A meta-analysis of randomized controlled trials. Europace 2022, 24, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zheng, H.; Guo, Z. Effect of Sodium-Glucose Co-transporter Protein 2 Inhibitors on Arrhythmia in Heart Failure Patients with or without Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials. Front. Cardiovasc. Med. 2022, 9, 902923. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J. Dissecting the reduction in cardiovascular death with SGLT2 inhibitors: Potential contribution of effects on ventricular arrhythmias and sudden cardiac death? Diabetes Epidemiol. Manag. 2022, 8, 100107. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, 1757–1780. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Claggett, B.; Assmann, S.F.; Boineau, R.; Anand, I.S.; Clausell, N.; Desai, A.S.; Diaz, R.; Fleg, J.L.; Gordeev, I.; et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015, 131, 34–42. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Anand, I.S.; Ge, J.; Lam, C.S.P.; Maggioni, A.P.; Martinez, F.; Packer, M.; Pfeffer, M.A.; Pieske, B.; et al. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2019, 381, 1609–1620. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Leoncini, G.; Russo, E.; Bussalino, E.; Barnini, C.; Viazzi, F.; Pontremoli, R. SGLT2is and Renal Protection: From Biological Mechanisms to Real-World Clinical Benefits. Int. J. Mol. Sci. 2021, 22, 4441. [Google Scholar] [CrossRef]
- Packer, M. Molecular, Cellular, and Clinical Evidence That Sodium-Glucose Cotransporter 2 Inhibitors Act as Neurohormonal Antagonists When Used for the Treatment of Chronic Heart Failure. J. Am. Heart Assoc. 2020, 9, e016270. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Critical Reanalysis of the Mechanisms Underlying the Cardiorenal Benefits of SGLT2 Inhibitors and Reaffirmation of the Nutrient Deprivation Signaling/Autophagy Hypothesis. Circulation 2022, 146, 1383–1405. [Google Scholar] [CrossRef] [PubMed]
- Verdonschot, J.A.J.; Merlo, M.; Dominguez, F.; Wang, P.; Henkens, M.T.H.M.; Adriaens, M.E.; Hazebroek, M.R.; Masè, M.; Escobar, L.E.; Cobas-Paz, R.; et al. Phenotypic clustering of dilated cardiomyopathy patients highlights important pathophysiological differences. Eur. Heart J. 2021, 42, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Karwath, A.; Bunting, K.V.; Gill, S.K.; Tica, O.; Pendleton, S.; Aziz, F.; Barsky, A.D.; Chernbumroong, S.; Duan, J.; Mobley, A.R.; et al. Redefining beta-blocker response in heart failure patients with sinus rhythm and atrial fibrillation: A machine learning cluster analysis. Lancet 2021, 398, 1427–1435. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).