Mealworm Ethanol Extract Enhances Myogenic Differentiation and Alleviates Dexamethasone-Induced Muscle Atrophy in C2C12 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Mealworm Ethanol Extract
2.2. Cell Culture and Cell Differentiation
2.3. Cell Viability
2.4. Western Blot Analysis
2.5. RNA Isolation and Quantitative Real-Time PCR Analysis
2.6. Statistical Analysis
3. Results
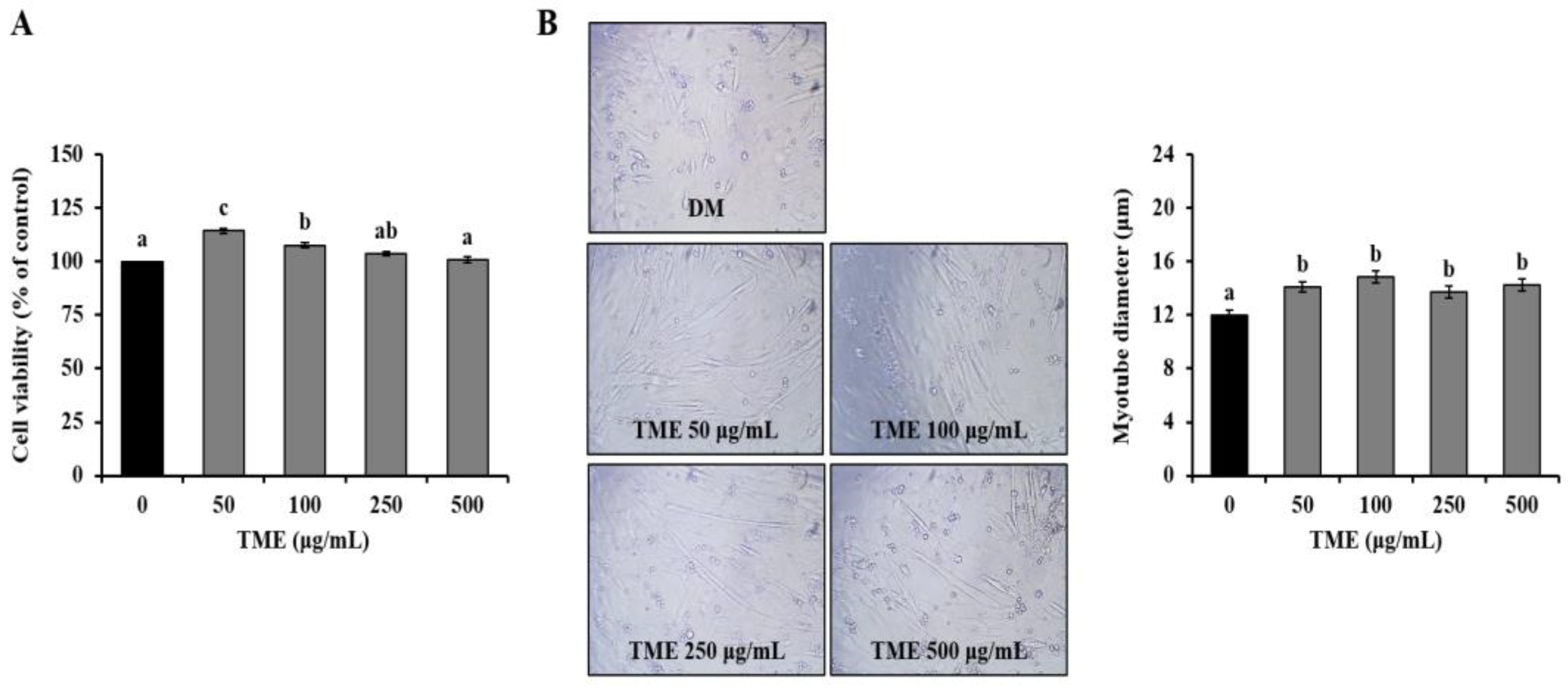
3.1. Effects of TME on Cell Viability of C2C12 Myoblast, and Morphology of C2C12 Myotubes
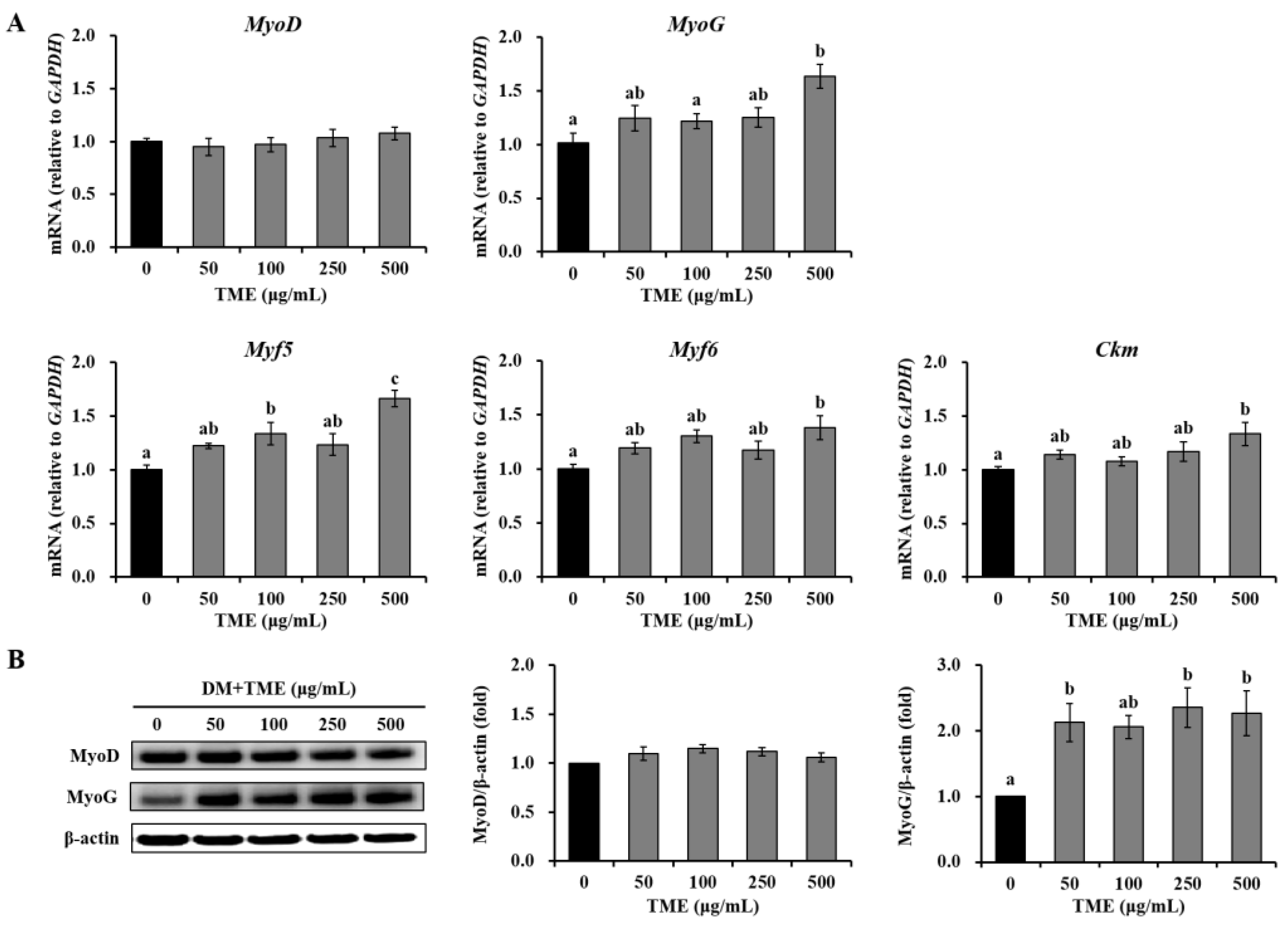
3.2. Effects of TME on the Expression of Myogenic Differentiation-Related Factors in C2C12 Myotubes
3.3. Effects of TME on the mTOR Pathway in C2C12 Myotubes
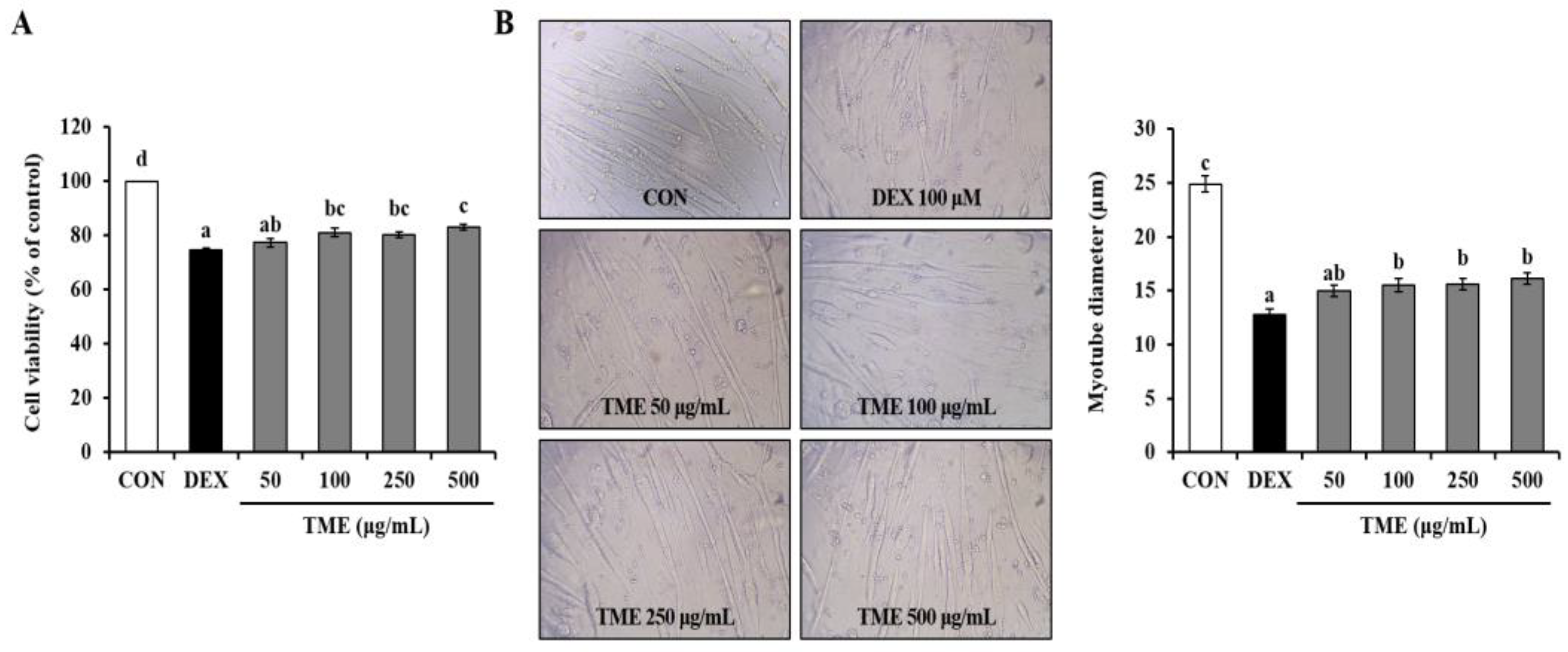
3.4. Effects of TME on Dexamethasone-Induced Cell Viability Decrease and Morphological Changes
3.5. Effects of TME on Dexamethasone-Induced Muscle Atrophy-Related Gene Expression
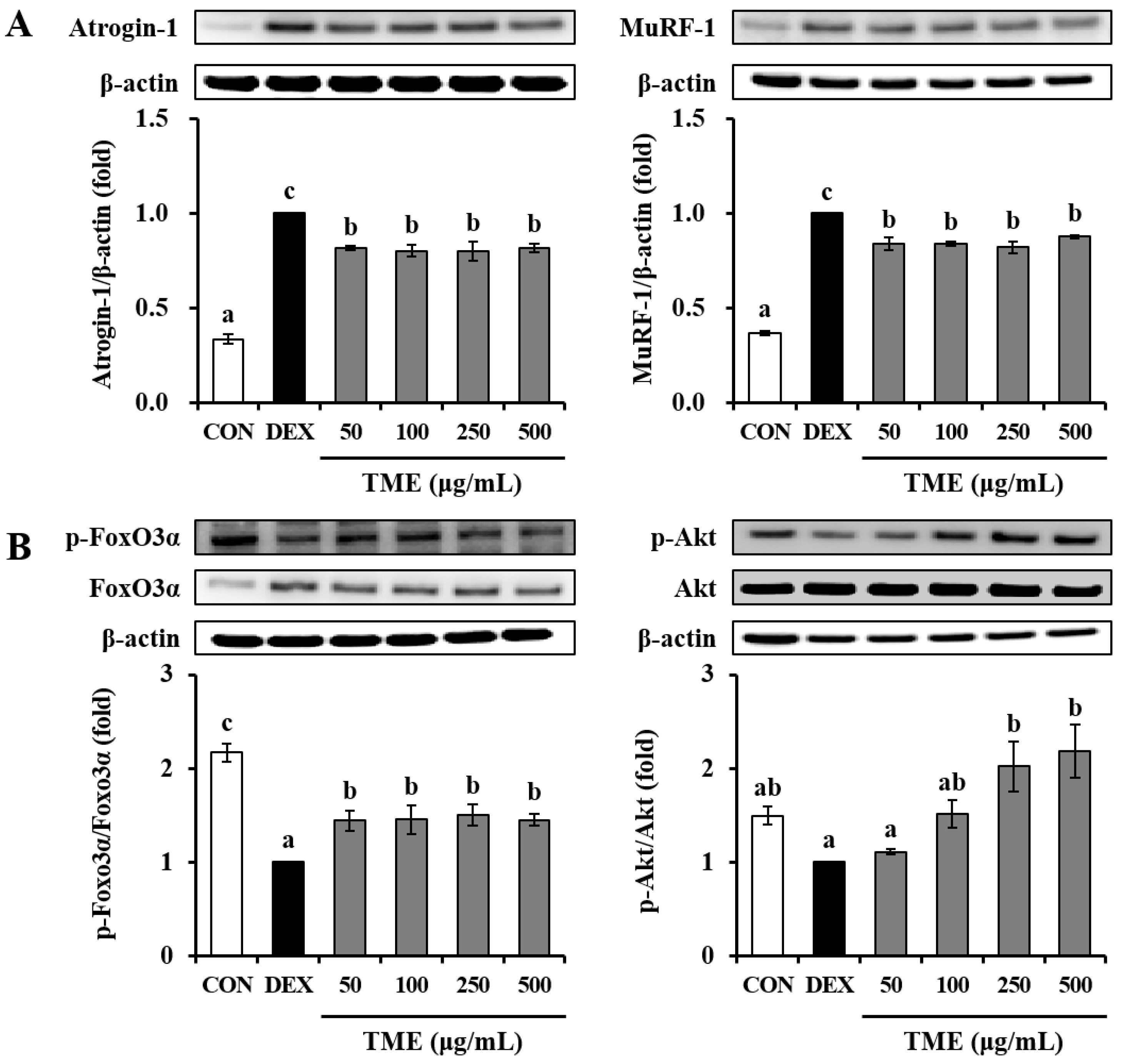
3.6. Effects of TME on Dexamethasone-Induced Muscle Atrophy-Related Protein Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walston, J.D. Sarcopenia in older adults. Curr. Opin. Rheumatol. 2012, 24, 623–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, S.; Nathan, J.A.; Goldberg, A.L. Muscle wasting in disease: Molecular mechanisms and promising therapies. Nat. Rev. Drug Discov. 2015, 14, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.H.; Lee, S.J.; Lee, K.B.; Lee, J.H.; Jeong, E.M.; Chung, S.G.; Park, S.C.; Kim, I.G. Dexamethasone downregulates caveolin-1 causing muscle atrophy via inhibited insulin signaling. J. Endocrinol. 2015, 225, 27–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasselgren, P. Glucocorticoids and muscle catabolism. Curr. Opin. Clin. Nutr. Metab. Care 1999, 2, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Lecker, S.H.; Goldberg, A.L.; Mitch, W.E. Protein degradation by the ubiquitin–proteasome pathway in normal and disease states. J. Am. Soc. Nephrol. 2006, 17, 1807–1819. [Google Scholar] [CrossRef] [PubMed]
- Gumucio, J.P.; Mendias, C.L. Atrogin-1, MuRF-1, and sarcopenia. Endocrine 2013, 43, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Clarke, B.A.; Drujan, D.; Willis, M.S.; Murphy, L.O.; Corpina, R.A.; Burova, E.; Rakhilin, S.V.; Stitt, T.N.; Patterson, C.; Latres, E.; et al. The E3 Ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab. 2007, 6, 376–385. [Google Scholar] [CrossRef] [Green Version]
- Yoon, M.S. mTOR as a key regulator in maintaining skeletal muscle mass. Front. Physiol. 2017, 8, 788. [Google Scholar] [CrossRef] [Green Version]
- Feng, S. Tenebrio molitor L., entomophagy and processing into ready to use therapeutic ingredients: A review. J. Nutr. Health Food Eng. 2018, 8, 280–285. [Google Scholar] [CrossRef]
- Kim, Y.; Yoon, Y.; Oh, E. Effect on myogenesis and anti-inflammation of mealworm (Tenebrio molitor) larvae protein hydrolysate. Curr. Dev. Nutr. 2020, 4, 40. [Google Scholar] [CrossRef]
- Lee, J.B.; Kwon, D.K.; Jeon, Y.J.; Song, Y.J. Mealworm (Tenebrio molitor)-derived protein supplementation attenuates skeletal muscle atrophy in hindlimb casting immobilized rats. Chin. J. Physiol. 2021, 64, 211–217. [Google Scholar] [PubMed]
- Pajalunga, D.; Crescenzi, M. Restoring the cell cycle and proliferation competence in terminally differentiated skeletal muscle myotubes. Cells 2021, 10, 2753. [Google Scholar] [CrossRef] [PubMed]
- Berkes, C.A.; Tapscott, S.J. MyoD and the transcriptional control of myogenesis. Semin. Cell Dev. Biol. 2005, 16, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Sung, B.; Kang, Y.J.; Kim, D.H.; Lee, Y.; Hwang, S.Y.; Yoon, J.H.; Yoo, M.A.; Kim, C.M.; Chung, H.Y.; et al. The combination of ursolic acid and leucine potentiates the differentiation of C2C12 murine myoblasts through the mTOR signaling pathway. Int. J. Mol. Med. 2015, 35, 755–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mielcarek, M.; Isalan, M. Kinetin stimulates differentiation of C2C12 myoblasts. PLoS ONE 2021, 16, e0258419. [Google Scholar] [CrossRef] [PubMed]
- Andrés, V.; Walsh, K. Myogenin expression, cell cycle withdrawal, and phenotypic differentiation are temporally separable events that precede cell fusion upon myogenesis. J. Cell Biol. 1996, 132, 657–666. [Google Scholar] [CrossRef] [Green Version]
- Dedieu, S.; Mazeres, G.; Cottin, P.; Brustis, J. Involvement of myogenic regulator factors during fusion in the cell line C2C12. Int. J. Dev. Biol. 2002, 46, 235–241. [Google Scholar]
- Sumitani, S.; Goya, K.; Testa, J.R.; Kouhara, H.; Kasayama, S. Akt1 and Akt2 differently regulate muscle creatine kinase and myogenin gene transcription in insulin-induced differentiation of C2C12 myoblasts. Endocrinology 2002, 143, 820–828. [Google Scholar] [CrossRef]
- Ma, X.M.; Blenis, J. Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol. 2009, 10, 307–318. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Ali, S.M.; Sabatini, D.M. Growing roles for the mTOR pathway. Curr. Opin. Cell Biol. 2005, 17, 596–603. [Google Scholar] [CrossRef]
- Yang, H.Y.; Xue, L.Y.; Xing, L.X.; Wang, J.; Wang, J.L.; Yan, X.; Zhang, X.H. Putative role of the mTOR/4E-BP1 signaling pathway in the carcinogenesis and progression of gastric cardiac adenocarcinoma. Mol. Med. Rep. 2013, 7, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Wang, M.; Shi, L.; Zhou, J.; Ma, R.; Feng, K.; Chen, X.; Xu, X.; Li, X.; Li, T.; et al. Panax ginseng total protein facilitates recovery from dexamethasone-induced muscle atrophy through the activation of glucose consumption in C2C12 myotubes. Biomed. Res. Int. 2019, 2019, 3719643. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.; Du, R.; Yang, Y.; Zhang, H.; Li, Q.; Liu, L.; Guan, H.; Hou, J.; An, X. Dexamethasone-induced skeletal muscle atrophy was associated with upregulation of myostatin promoter activity. Res. Vet. Sci. 2013, 94, 84–89. [Google Scholar] [CrossRef]
- Clavel, S.; Siffroi-Fernandez, S.; Coldefy, A.S.; Boulukos, K.; Pisani, D.F.; Dérijard, B. Regulation of the intracellular localization of Foxo3a by stress-activated protein kinase signaling pathways in skeletal muscle cells. Mol. Cell. Biol. 2010, 30, 470–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandri, M.; Sandri, C.; Gilbert, A.; Skurk, C.; Calabria, E.; Picard, A.; Walsh, K.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 2004, 117, 399–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodine, S.C.; Latres, E.; Baumhueter, S.; Lai, V.K.; Nunez, L.; Clarke, B.A.; Poueymirou, W.T.; Panaro, F.J.; Na, E.; Dharmarajan, K.; et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 2001, 294, 1704–1708. [Google Scholar] [CrossRef]
- Jin, K.N.; Jeong, E.J.; Kim, Y.S. Antioxidant components and antioxidant activities of mealworm (Tenebrio molitor larvae). J. Food Hyg. Saf. 2021, 36, 86–92. [Google Scholar] [CrossRef]
- Errico, S.; Spagnoletta, A.; Verardi, A.; Moliterni, S.; Dimatteo, S.; Sangiorgio, P. Tenebrio molitor as a source of interesting natural compounds, their recovery processes, biological effects, and safety aspects. Compr. Rev. Food Sci. Food Saf. 2022, 21, 148–197. [Google Scholar] [CrossRef]


| Gene | Full Name | Forward/Reverse(5′–3′) |
|---|---|---|
| Atrogin-1 | F-box protein 32 | TCATGCAGAGGCTGAGTGAC/ TCAAACGCTTGCGAATCTGC |
| Ckm | Muscle creatine kinase | CTTCCTGTTTGACAAGCCCG/ CTCCTCGTTCACCCACACAA |
| Foxo3α | Forkhead box O3 | TGAAGGGAAGGAGCCGAGGTA/ GCTCTCTCCTCTCGAGCCCA |
| Gapdh | Glyceraldehyde-3-phosphate dehydrogenase | GGAGAGTGTTTCCTCGTCCC/ ATGAAGGGGTCGTTGATGGC |
| MuRF-1 | Muscle RING finger protein-1 | ACACATAGCAGAGGCCTTGAG/ TCTTTACCCTCTGTGGTCACG |
| Myf5 | Myogenic factor 5 | ACCCTAACCAGAGACTCCCC/ GTCCCGGCAGGCTGTAATAG |
| Myf6 | Myogenic factor 6 | CCACAGATCGTCGGAAAGCA/ CAGTCTCTGGTTGGGGTTGG |
| MyoD | Myogenic differentiation 1 | TACAGTGGCGACTCAGATGC/ CACTGTAGTAGGCGGTGTCG |
| MyoG | Myogenin | CCCTACAGACGCCCACAATC/ AGTTGGGCATGGTTTCGTCT |
| Myostatin | Myostatin | TGCTGTAACCTTCCCAGGAC/ TCAAGCCCAAAGTCTCTCCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, R.-Y.; Kim, B.S.; Ban, E.-J.; Seo, M.; Lee, J.H.; Kim, I.-W. Mealworm Ethanol Extract Enhances Myogenic Differentiation and Alleviates Dexamethasone-Induced Muscle Atrophy in C2C12 Cells. Life 2023, 13, 58. https://doi.org/10.3390/life13010058
Choi R-Y, Kim BS, Ban E-J, Seo M, Lee JH, Kim I-W. Mealworm Ethanol Extract Enhances Myogenic Differentiation and Alleviates Dexamethasone-Induced Muscle Atrophy in C2C12 Cells. Life. 2023; 13(1):58. https://doi.org/10.3390/life13010058
Chicago/Turabian StyleChoi, Ra-Yeong, Bong Sun Kim, Eu-Jin Ban, Minchul Seo, Joon Ha Lee, and In-Woo Kim. 2023. "Mealworm Ethanol Extract Enhances Myogenic Differentiation and Alleviates Dexamethasone-Induced Muscle Atrophy in C2C12 Cells" Life 13, no. 1: 58. https://doi.org/10.3390/life13010058





