Molecular Mechanisms in the Vascular and Nervous Systems following Traumatic Spinal Cord Injury
Abstract
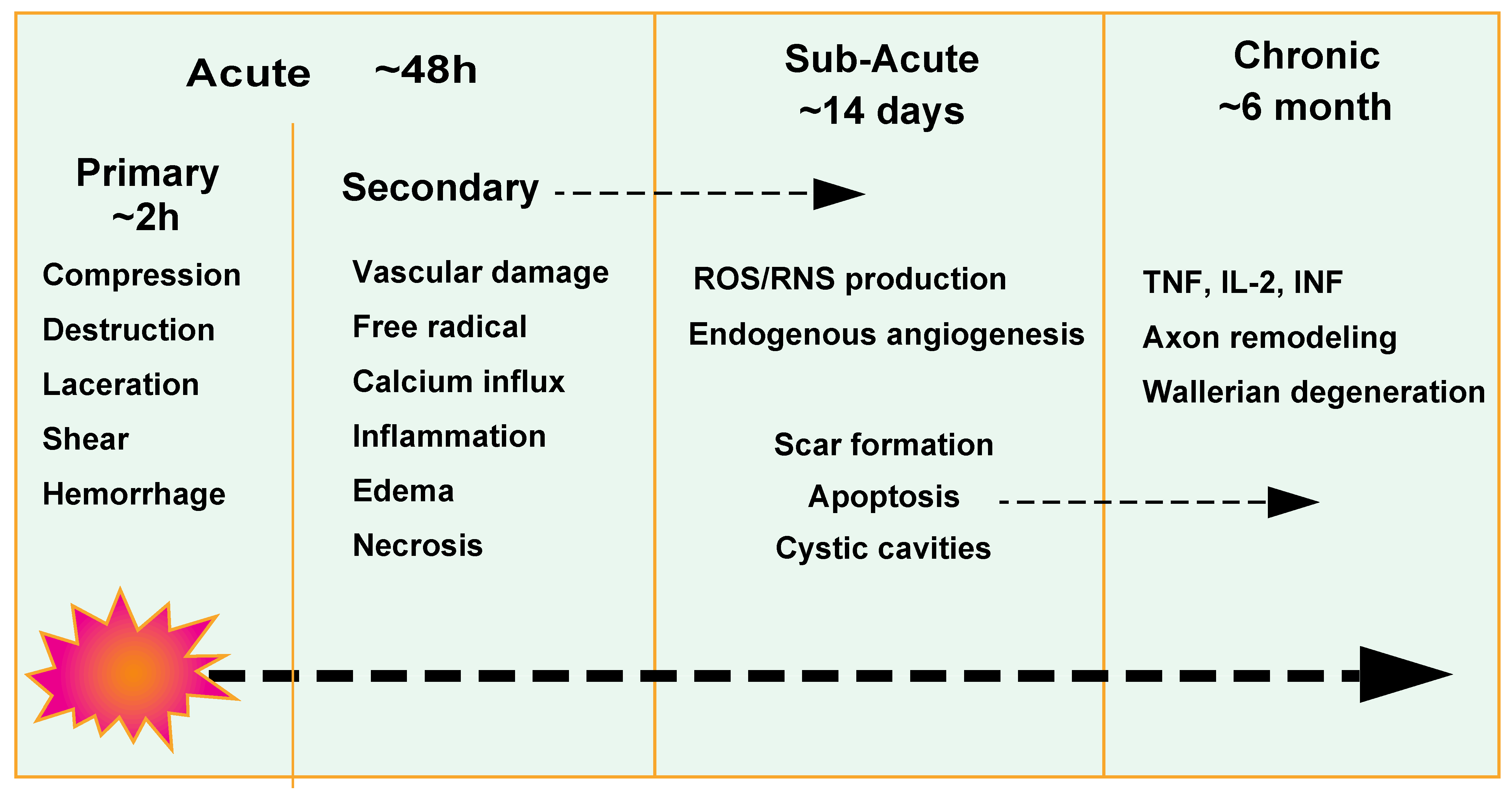
:1. Introduction
2. Molecular Mechanisms in the Nervous System
2.1. Neurotrophic Factors
2.2. Growth Factors
2.3. Cytokines
2.4. Regulatory-Related Ions
3. Molecular Mechanisms in the Vascular System
3.1. Vascular Responses after Spinal Cord Injury
3.2. Blood Vessel Loss
3.3. Blood Spinal Cord Barrier Breakdown
3.4. Endogenous Angiogenesis
3.5. Spinal Cord Recovery
- (i)
- Administration of proangiogenic factors: VEGF has a considerable impact on EC migration and proliferation and on blood vessel development [43]. In various trials, alone or in combination, treatment with VEGF and its isoform (VEGF-A165, 121, 189) significantly improved neuroprotection and post-traumatic recovery. Furthermore, the administration of modified zinc protein transcription factor (ZFP) activates all VEGF-A isoforms, whereas VEGF combined with PDGF, bFGF, and Ang1 increases blood vessel density, decreases BSCB permeability, and increases blood supply [116]. Several hormones, enzymes, or chemicals, including melatonin and estrogen, influence angiogenesis in the treatment of SCI. Chondroitinase ABC (ChABC) promotes axonal remodeling and regeneration by inducing revascularization. ChABC causes the degradation of extracellular chondroitin sulfate proteoglycans (CSPG), promoting angiogenesis and protecting BM vessels. Furthermore, the presence of MMPs, flufenamic acid (FFA) or MMP-8 inhibitors, and granulocyte colony-stimulating factor (G-CSF) increases local revascularization and prevents BSCB disruption [117].
- (ii)
- Gene modulation: Many studies have found evidence of neuroprotection and functional recovery via gene manipulation. Kumar et al. investigated transient potential channel protein (TRPV4) function following SCI and discovered that TRPV4 activation has negative effects on endothelial cell damage, the progression of inflammation, and rehabilitation and functional recovery [118]. The decrease in ubiquitously transcribed tetratricopeptide repeat on chromosome X (UTX) levels, a histone H3K27 demethylase that is substantially increased after SCI, promotes EC migration and tubule/tube formation/genesis via the miR-24 pathway, and epigenetically stimulates vascular remodeling and functional retrieval [119].
- (iii)
- Cell-based therapy approaches: Stem cell transplantation has emerged as a convincing therapeutic strategy in both degenerative and traumatic illnesses because of the inherent differentiation variety and favorable treatment possibilities provided by stem cells. Mesenchymal stem cells derived from the bone marrow, umbilical cord, adipose tissue, and amnion encourage BSCB repair and improve revascularization at the location of the lesion [116].
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hutson, T.H.; Di Giovanni, S. The translational landscape in spinal cord injury: Focus on neuroplasticity and regeneration. Nat. Rev. Neurol. 2019, 15, 732–745. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.A.; Sousa, N.; Reis, R.L.; Salgado, A.J. From basics to clinical: A comprehensive review on spinal cord injury. Prog. Neurobiol. 2014, 114, 25–57. [Google Scholar] [CrossRef] [PubMed]
- Anjum, A.; Yazid, M.D.; Daud, M.F.; Idris, J.; Ng, A.M.H.; Naicker, A.S.; Ismail, O.H.R.; Kumar, R.K.A.; Lokanathan, Y. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int. J. Mol. Sci. 2020, 21, 7533. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Primer. 2017, 3, 17018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pineau, I.; Lacroix, S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: Multiphasic expression pattern and identification of the cell types involved. J. Comp. Neurol. 2007, 500, 267–285. [Google Scholar] [CrossRef]
- Choo, A.M.; Liu, J.; Lam, C.K.; Dvorak, M.; Tetzlaff, W.; Oxland, T.R. Contusion, dislocation, and distraction: Primary hemorrhage and membrane permeability in distinct mechanisms of spinal cord injury. J. Neurosurg. Spine 2007, 6, 255–266. [Google Scholar] [CrossRef]
- Oyinbo, C.A. Secondary injury mechanisms in traumatic spinal cord injury: A nugget of this multiply cascade. Acta Neurobiol. Exp. 2011, 71, 281–299. [Google Scholar]
- Fehlings, M.G.; Vaccaro, A.R.; Boakye, M. Essentials of Spinal Cord Injury: Basic Research to Clinical Practice; Thieme: New York, NY, USA, 2012. [Google Scholar]
- von Leden, R.E.; Yauger, Y.J.; Khayrullina, G.; Byrnes, K.R. Central Nervous System Injury and Nicotinamide Adenine Dinucleotide Phosphate Oxidase: Oxidative Stress and Therapeutic Targets. J. Neurotrauma. 2017, 34, 755–764. [Google Scholar] [CrossRef] [Green Version]
- Silver, J. The glial scar is more than just astrocytes. Exp. Neurol. 2016, 286, 147–149. [Google Scholar] [CrossRef]
- Kroner, A.; Almanza, J.R. Role of microglia in spinal cord injury. Neurosci. Lett. 2019, 709, 134370. [Google Scholar] [CrossRef]
- Hackett, A.R.; Lee, J.K. Understanding the NG2 Glial Scar after Spinal Cord Injury. Front. Neurol. 2016, 7, 199. [Google Scholar] [CrossRef]
- Silver, J.; Schwab, M.E.; Popovich, P.G. Central Nervous System Regenerative Failure: Role of Oligodendrocytes, Astrocytes, and Microglia. Cold Spring Harb. Perspect. Biol. 2015, 7, a020602. [Google Scholar] [CrossRef] [Green Version]
- Evans, T.A.; Barkauskas, D.S.; Myers, J.T.; Hare, E.G.; You, J.Q.; Ransohoff, R.M.; Huang, A.Y.; Silver, J. High-resolution intravital imaging reveals that blood-derived macrophages but not resident microglia facilitate secondary axonal dieback in traumatic spinal cord injury. Exp. Neurol. 2014, 254, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Soderblom, C.; Luo, X.; Blumenthal, E.; Bray, E.; Lyapichev, K.; Ramos, J.; Krishnan, V.; Lai-Hsu, C.; Park, K.K.; Tsoulfas, P.; et al. Perivascular Fibroblasts Form the Fibrotic Scar after Contusive Spinal Cord Injury. J. Neurosci. 2013, 33, 13882–13887. [Google Scholar] [CrossRef] [Green Version]
- Rolls, A.; Shechter, R.; Schwartz, M. The bright side of the glial scar in CNS repair. Nat. Rev. Neurosci. 2009, 10, 235–241. [Google Scholar] [CrossRef]
- Anderson, M.A.; Burda, J.E.; Ren, Y.; Ao, Y.; O’Shea, T.M.; Kawaguchi, R.; Coppola, G.; Khakh, B.S.; Deming, T.J.; Sofroniew, M.V. Astrocyte scar formation aids central nervous system axon regeneration. Nature 2016, 532, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Geoffroy, C.G.; Zheng, B. Myelin-associated inhibitors in axonal growth after CNS injury. Curr. Opin. Neurobiol. 2014, 27, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Fleming, J.C.; Norenberg, M.D.; Ramsay, D.A.; Dekaban, G.A.; Marcillo, A.E.; Saenz, A.D.; Pasquale-Styles, M.; Dietrich, W.D.; Weaver, L.C. The cellular inflammatory response in human spinal cords after injury. Brain 2006, 129, 3249–3269. [Google Scholar] [CrossRef] [Green Version]
- Garcia, E.; Aguilar-Cevallos, J.; Silva-Garcia, R.; Ibarra, A. Cytokine and Growth Factor Activation In Vivo and In Vitro after Spinal Cord Injury. Mediat. Inflamm. 2016, 2016, 9476020. [Google Scholar] [CrossRef] [Green Version]
- Fassbender, J.M.; Whittemore, S.R.; Hagg, T. Targeting microvasculature for neuroprotection after SCI. Neurother. J. Am. Soc. Exp. Neurother. 2011, 8, 240–251. [Google Scholar] [CrossRef] [Green Version]
- Means, E.D.; Anderson, D.K.; Nicolosi, G.; Gaudsmith, J. Microvascular perfusion experimental spinal cord injury. Surg. Neurol. 1978, 9, 353–360. [Google Scholar] [PubMed]
- Wolman, L. The disturbance of circulation in traumatic paraplegia in acute and late stages: A pathological study. Spinal Cord 1965, 2, 213–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartanusz, V.; Jezova, D.; Alajajian, B.; Digicaylioglu, M. The blood-spinal cord barrier: Morphology and Clinical Implications. Ann. Neurol. 2011, 70, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Haggerty, A.E.; Maldonado-Lasunción, I.; Oudega, M. Biomaterials for revascularization and immunomodulation after spinal cord injury. Biomed. Mater. 2018, 13, 044105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, M.T.L.; Stammers, A.T.; Kwon, B.K. Vascular Disruption and the Role of Angiogenic Proteins After Spinal Cord Injury. Transl. Stroke Res. 2011, 2, 474–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in Neuronal Development and Function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef] [Green Version]
- Keefe, K.M.; Sheikh, I.S.; Smith, G.M. Targeting Neurotrophins to Specific Populations of Neurons: NGF, BDNF, and NT-3 and Their Relevance for Treatment of Spinal Cord Injury. Int. J. Mol. Sci. 2017, 18, 548. [Google Scholar] [CrossRef] [Green Version]
- Levi-Montalcini, R.; Hamburger, V. Selective growth stimulating effects of mouse sarcoma on the sensory and sympathetic nervous system of the chick embryo. J. Exp. Zool. 1951, 116, 321–361. [Google Scholar] [CrossRef]
- Romero, M.I.; Rangappa, N.; Li, L.; Lightfoot, E.; Garry, M.G.; Smith, G.M. Extensive Sprouting of Sensory Afferents and Hyperalgesia Induced by Conditional Expression of Nerve Growth Factor in the Adult Spinal Cord. J. Neurosci. 2000, 20, 4435–4445. [Google Scholar] [CrossRef] [Green Version]
- Lu, P.; Blesch, A.; Graham, L.; Wang, Y.; Samara, R.; Banos, K.; Haringer, V.; Havton, L.; Weishaupt, N.; Bennett, D.; et al. Motor Axonal Regeneration after Partial and Complete Spinal Cord Transection. J. Neurosci. 2012, 32, 8208–8218. [Google Scholar] [CrossRef] [Green Version]
- Tuinstra, H.M.; Aviles, M.O.; Shin, S.; Holland, S.J.; Zelivyanskaya, M.L.; Fast, A.G.; Ko, S.Y.; Margul, D.J.; Bartels, A.K.; Boehler, R.M.; et al. Multifunctional, multichannel bridges that deliver neurotrophin encoding lentivirus for regeneration following spinal cord injury. Biomaterials 2012, 33, 1618–1626. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Ajiki, T.; Inoue, H.; Kikuchi, M.; Yashiro, T.; Nakama, S.; Hoshino, Y.; Murakami, T.; Kobayashi, E. Early exercise in spinal cord injured rats induces allodynia through TrkB signaling. Biochem. Biophys. Res. Commun. 2009, 381, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Constandil, L.; Aguilera, R.; Goich, M.; Hernández, A.; Alvarez, P.; Infante, C.; Pelissier, T. Involvement of spinal cord BDNF in the generation and maintenance of chronic neuropathic pain in rats. Brain Res. Bull. 2011, 86, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.K.; Teng, K.; Lee, R.; Wright, S.; Tevar, S.; Almeida, R.; Kermani, P.; Torkin, R.; Chen, Z.-Y.; Lee, F.S.; et al. ProBDNF Induces Neuronal Apoptosis via Activation of a Receptor Complex of p75NTR and Sortilin. J. Neurosci. 2005, 25, 5455–5463. [Google Scholar] [CrossRef] [PubMed]
- Wong, I.; Liao, H.; Bai, X.; Zaknic, A.; Zhong, J.; Guan, Y.; Li, H.-Y.; Wang, Y.-J.; Zhou, X.-F. ProBDNF inhibits infiltration of ED1+ macrophages after spinal cord injury. Brain Behav. Immun. 2010, 24, 585–597. [Google Scholar] [CrossRef]
- Schnell, L.; Schneider, R.; Kolbeck, R.; Barde, Y.; Schwab, M.E. Neurotrophin-3 enhances sprouting of corticospinal tract during development and after adult spinal cord lesion. Nature 1994, 367, 170–173. [Google Scholar] [CrossRef]
- Hajebrahimi, Z.; Mowla, S.J.; Movahedin, M.; Tavallaei, M. Gene expression alterations of neurotrophins, their receptors and prohormone convertases in a rat model of spinal cord contusion. Neurosci. Lett. 2008, 441, 261–266. [Google Scholar] [CrossRef]
- Lamballe, F.; Klein, R.; Barbacid, M. trkC, a new member of the trk family of tyrosine protein kinases, is a receptor for neurotrophin-3. Cell 1991, 66, 967–979. [Google Scholar] [CrossRef]
- Sieck, G.C.; Mantilla, C. Role of neurotrophins in recovery of phrenic motor function following spinal cord injury. Respir. Physiol. Neurobiol. 2009, 169, 218–225. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.-C.; Shen, L.-F.; Kuo, H.-S.; Cheng, H.; Chak, K.-F. Involvement of Acidic Fibroblast Growth Factor in Spinal Cord Injury Repair Processes Revealed by a Proteomics Approach. Mol. Cell. Proteom. 2008, 7, 1668–1687. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Li, C.; Dong, Z.; Liu, J.; Li, W.; Liu, Y.; Xue, H.; Chen, D. Co-transplantation of bFGF-expressing amniotic epithelial cells and neural stem cells promotes functional recovery in spinal cord-injured rats. Cell Biol. Int. 2008, 32, 1546–1558. [Google Scholar] [CrossRef]
- Hwang, D.H.; Kim, H.M.; Kang, Y.M.; Joo, I.S.; Cho, C.-S.; Yoon, B.-W.; Kim, S.U.; Kim, B.G. Combination of Multifaceted Strategies to Maximize the Therapeutic Benefits of Neural Stem Cell Transplantation for Spinal Cord Repair. Cell Transplant. 2011, 20, 1361–1380. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.N.; Oh, S.H.; Lee, K.H.; Yoon, D.H. Effect of human mesenchymal stem cell transplantation combined with growth factor infusion in the repair of injured spinal cord. In Advances in Functional and Reparative Neurosurgery; Chang, J.W., Katayama, Y., Yamamoto, T., Eds.; Springer: Vienna, Austria, 2006; pp. 133–136. [Google Scholar]
- Goldshmit, Y.; Frisca, F.; Pinto, A.R.; Pébay, A.; Tang, J.K.K.; Siegel, A.L.; Kaslin, J.; Currie, P.D. Fgf2 improves functional recovery—Decreasing gliosis and increasing radial glia and neural progenitor cells after spinal cord injury. Brain Behav. 2014, 4, 187–200. [Google Scholar] [CrossRef]
- Duarte Azevedo, M.; Sander, S.; Tenenbaum, L. GDNF, A Neuron-Derived Factor Upregulated in Glial Cells during Disease. J. Clin. Med. 2020, 9, E456. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.-X.; Deng, P.; Ruan, Y.; Xu, Z.C.; Liu, N.-K.; Wen, X.; Smith, G.M.; Xu, X.-M. A Novel Growth-Promoting Pathway Formed by GDNF-Overexpressing Schwann Cells Promotes Propriospinal Axonal Regeneration, Synapse Formation, and Partial Recovery of Function after Spinal Cord Injury. J. Neurosci. 2013, 33, 5655–5667. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.-X.; Hu, J.; Liu, N.; Wang, X.; Smith, G.M.; Wen, X.; Xu, X.-M. GDNF modifies reactive astrogliosis allowing robust axonal regeneration through Schwann cell-seeded guidance channels after spinal cord injury. Exp. Neurol. 2011, 229, 238–250. [Google Scholar] [CrossRef] [Green Version]
- Ansorena, E.; De Berdt, P.; Ucakar, B.; Simón-Yarza, T.; Jacobs, D.; Schakman, O.; Jankovski, A.; Deumens, R.; Blanco-Prieto, M.J.; Préat, V.; et al. Injectable alginate hydrogel loaded with GDNF promotes functional recovery in a hemisection model of spinal cord injury. Int. J. Pharm. 2013, 455, 148–158. [Google Scholar] [CrossRef] [Green Version]
- Tiraihi, T.; Abdanipour, A.; Taheri, T. Intraspinal transplantation of motoneuron-like cell combined with delivery of polymer-based glial cell line-derived neurotrophic factor for repair of spinal cord contusion injury. Neural Regen. Res. 2014, 9, 1003–1013. [Google Scholar] [CrossRef]
- Cao, L.; Liu, L.; Chen, Z.; Wang, L.; Ye, J.; Qiu, H.; Lu, C.; He, C. Olfactory ensheathing cells genetically modified to secrete GDNF to promote spinal cord repair. Brain 2003, 127, 535–549. [Google Scholar] [CrossRef] [Green Version]
- Gates, M.; Ph, O.; Jd, M. Faculty Opinions recommendation of IGF-I specifically enhances axon outgrowth of corticospinal motor neurons. Nat. Neurosci. 2006, 9, 1371–1381. [Google Scholar] [CrossRef]
- Hung, K.-S.; Lin, J.-W. Gene transfer of insulin-like growth factor–I providing neuroprotection after spinal cord injury in rats. J. Neurosurg. 2007, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.; Tatara, A.; Shiu, A.; Sakiyama-Elbert, S.E. Controlled Release of Neurotrophin-3 and Platelet-Derived Growth Factor from Fibrin Scaffolds Containing Neural Progenitor Cells Enhances Survival and Differentiation into Neurons in a Subacute Model of SCI. Cell Transplant. 2010, 19, 89–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvey, A.R.; Hu, Y.; Leaver, S.G.; Mellough, C.B.; Park, K.; Verhaagen, J.; Plant, G.W.; Cui, Q. Gene therapy and transplantation in CNS repair: The visual system. Prog. Retin. Eye Res. 2006, 25, 449–489. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.; Leibinger, M. Promoting optic nerve regeneration. Prog. Retin. Eye Res. 2012, 31, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shinoda, Y.; Ogawa, S.; Ikegaya, S.; Li, S.; Matsuyama, Y.; Sato, K.; Yamagishi, S. Expression of FLRT2 in Postnatal Central Nervous System Development and After Spinal Cord Injury. Front. Mol. Neurosci. 2021, 14, 756264. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Hampel, F.; Hata, K.; del Toro, D.; Schwark, M.; Kvachnina, E.; Bastmeyer, M.; Yamashita, T.; Tarabykin, V.; Klein, R.; et al. FLRT2 and FLRT3 act as repulsive guidance cues for Unc5-positive neurons. EMBO J. 2011, 30, 2920–2933. [Google Scholar] [CrossRef] [Green Version]
- Behzadi, P.; Sameer, A.S.; Nissar, S.; Banday, M.Z.; Gajdács, M.; García-Perdomo, H.A.; Akhtar, K.; Pinheiro, M.; Magnusson, P.; Sarshar, M.; et al. The Interleukin-1 (IL-1) Superfamily Cytokines and Their Single Nucleotide Polymorphisms (SNPs). J. Immunol. Res. 2022, 2022, 2054431. [Google Scholar] [CrossRef]
- Taylor, M.W. Interferons. In Viruses and Man: A History of Interactions; Springer International Publishing: Cham, Switzerland, 2014; pp. 101–119. [Google Scholar]
- Pestka, S.; Krause, C.D.; Walter, M.R. Interferons, interferon-like cytokines, and their receptors. Immunol. Rev. 2004, 202, 8–32. [Google Scholar] [CrossRef]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef]
- Gok, B.; Okutan, O.; Beskonakli, E.; Palaoglu, S.; Erdamar, H.; Sargon, M.F. Effect of Immunomodulation With Human Interferon-β on Early Functional Recovery From Experimental Spinal Cord Injury. Spine 2007, 32, 873–880. [Google Scholar] [CrossRef]
- Fujiyoshi, T.; Kubo, T.; Chan, C.C.; Koda, M.; Okawa, A.; Takahashi, K.; Yamazaki, M. Interferon-γ Decreases Chondroitin Sulfate Proteoglycan Expression and Enhances Hindlimb Function after Spinal Cord Injury in Mice. J. Neurotrauma 2010, 27, 2283–2294. [Google Scholar] [CrossRef]
- Nishimura, Y.; Natsume, A.; Ito, M.; Hara, M.; Motomura, K.; Fukuyama, R.; Sumiyoshi, N.; Aoki, I.; Saga, T.; Lee, H.J.; et al. Interferon-β Delivery via Human Neural Stem Cell Abates Glial Scar Formation in Spinal Cord Injury. Cell Transplant. 2013, 22, 2187–2201. [Google Scholar] [CrossRef]
- Roselli, F.; Chandrasekar, A.; Morganti-Kossmann, M.C. Interferons in Traumatic Brain and Spinal Cord Injury: Current Evidence for Translational Application. Front. Neurol. 2018, 9, 458. [Google Scholar] [CrossRef] [Green Version]
- Mukaino, M.; Nakamura, M.; Yamada, O.; Okada, S.; Morikawa, S.; Renault-Mihara, F.; Iwanami, A.; Ikegami, T.; Ohsugi, Y.; Tsuji, O.; et al. Anti-IL-6-receptor antibody promotes repair of spinal cord injury by inducing microglia-dominant inflammation. Exp. Neurol. 2010, 224, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Funk, L.H.; Hackett, A.R.; Bunge, M.B.; Lee, J.K. Tumor necrosis factor superfamily member APRIL contributes to fibrotic scar formation after spinal cord injury. J. Neuroinflammation 2016, 13, 87. [Google Scholar] [CrossRef] [Green Version]
- McCormick, S.M.; Heller, N.M. Commentary: IL-4 and IL-13 receptors and signaling. Cytokine 2015, 75, 38–50. [Google Scholar] [CrossRef] [Green Version]
- Junttila, I.S. Tuning the Cytokine Responses: An Update on Interleukin (IL)-4 and IL-13 Receptor Complexes. Front. Immunol. 2018, 9, 888. [Google Scholar] [CrossRef] [Green Version]
- Thompson, C.D.; Zurko, J.C.; Hanna, B.F.; Hellenbrand, D.J.; Hanna, A. The Therapeutic Role of Interleukin-10 after Spinal Cord Injury. J. Neurotrauma 2013, 30, 1311–1324. [Google Scholar] [CrossRef]
- Hellenbrand, D.J.; Reichl, K.A.; Travis, B.J.; Filipp, M.; Khalil, A.S.; Pulito, D.J.; Gavigan, A.V.; Maginot, E.R.; Arnold, M.T.; Adler, A.G.; et al. Sustained interleukin-10 delivery reduces inflammation and improves motor function after spinal cord injury. J. Neuroinflammation 2019, 16, 93. [Google Scholar] [CrossRef]
- Beladi, R.N.; Varkoly, K.S.; Schutz, L.; Zhang, L.; Yaron, J.R.; Guo, Q.; Burgin, M.; Hogue, I.; Tierney, W.; Dobrowski, W.; et al. Serine Proteases and Chemokines in Neurotrauma: New Targets for Immune Modulating Therapeutics in Spinal Cord Injury. Curr. Neuropharmacol. 2021, 19, 1835–1854. [Google Scholar] [CrossRef]
- Lenz, K.M.; Nelson, L. Microglia and Beyond: Innate Immune Cells As Regulators of Brain Development and Behavioral Function. Front. Immunol. 2018, 9, 698. [Google Scholar] [CrossRef] [PubMed]
- Smith-Thomas, L.; Fok-Seang, J.; Stevens, J.; Du, J.; Muir, E.; Faissner, A.; Geller, H.; Rogers, J.; Fawcett, J. An inhibitor of neurite outgrowth produced by astrocytes. J. Cell Sci. 1994, 107, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, J.; Sun, D.; Alcock, N.W.; Wen, J. Spinal cord injury increases iron levels: Catalytic production of hydroxyl radicals. Free Radic. Biol. Med. 2003, 34, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-M.; Wu, J.-Y.; Li, F.-C.; Chen, Q.-X. Ion channel blockers and spinal cord injury. J. Neurosci. Res. 2011, 89, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Black, J.A.; Cummins, T.R.; Yoshimura, N.; de Groat, W.C.; Waxman, S.G. Tetrodotoxin-resistant sodium channels Nav1.8/SNS and Nav1.9/NaN in afferent neurons innervating urinary bladder in control and spinal cord injured rats. Brain Res. 2003, 963, 132–138. [Google Scholar] [CrossRef]
- Kaptanoglu, E.; Solaroglu, I.; Surucu, H.S.; Akbiyik, F.; Beskonakli, E. Blockade of sodium channels by phenytoin protects ultrastructure and attenuates lipid peroxidation in experimental spinal cord injury. Acta Neurochir. 2005, 147, 405–412. [Google Scholar] [CrossRef]
- Rosenberg, L.J.; Teng, Y.D.; Wrathall, J.R. Effects of the Sodium Channel Blocker Tetrodotoxin on Acute White Matter Pathology After Experimental Contusive Spinal Cord Injury. J. Neurosci. 1999, 19, 6122–6133. [Google Scholar] [CrossRef] [Green Version]
- Satkunendrarajah, K.; Nassiri, F.; Karadimas, S.; Lip, A.; Yao, G.; Fehlings, M. Riluzole promotes motor and respiratory recovery associated with enhanced neuronal survival and function following high cervical spinal hemisection. Exp. Neurol. 2016, 276, 59–71. [Google Scholar] [CrossRef] [Green Version]
- Bacia, A.; Wollmann, R.; Soliven, B. K+ channel blockade impairs remyelination in the cuprizone model. Glia 2004, 48, 156–165. [Google Scholar] [CrossRef]
- Hayes, K.C. Fampridine-SR for multiple sclerosis and spinal cord injury. Expert Rev. Neurother. 2007, 7, 453–461. [Google Scholar] [CrossRef]
- Fan, B.; Wei, Z.; Yao, X.; Shi, G.; Cheng, X.; Zhou, X.; Zhou, H.; Ning, G.; Kong, X.; Feng, S. Microenvironment Imbalance of Spinal Cord Injury. Cell Transplant. 2018, 27, 853–866. [Google Scholar] [CrossRef]
- Hu, R.; Duan, B.; Wang, D.; Yu, Y.; Li, W.; Luo, H.; Lu, P.; Lin, J.; Zhu, G.; Wan, Q.; et al. Role of Acid-Sensing Ion Channel 1a in the Secondary Damage of Traumatic Spinal Cord Injury. Ann. Surg. 2011, 254, 353–362. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, S.; Li, J.; Li, Z.; Quan, J.; Liu, X.; Tang, Y.; Liu, B. The Latest View on the Mechanism of Ferroptosis and Its Research Progress in Spinal Cord Injury. Oxidative Med. Cell. Longev. 2020, 2020, 6375938. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, Y.; Hao, J.; Duan, H.-Q.; Zhao, C.-X.; Sun, C.; Li, B.; Fan, B.-Y.; Li, W.-X.; Fu, X.-H.; et al. Deferoxamine promotes recovery of traumatic spinal cord injury by inhibiting ferroptosis. Neural Regen. Res. 2019, 14, 532–541. [Google Scholar] [CrossRef]
- Feng, Z.; Min, L.; Chen, H.; Deng, W.; Tan, M.; Liu, H.; Hou, J. Iron overload in the motor cortex induces neuronal ferroptosis following spinal cord injury. Redox Biol. 2021, 43, 101984. [Google Scholar] [CrossRef]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic spinal cord injury: An overview of pathophysiology, models and acute injury mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef] [Green Version]
- Tran, A.P.; Warren, P.M.; Silver, J. The Biology of Regeneration Failure and Success After Spinal Cord Injury. Physiol. Rev. 2018, 98, 881–917. [Google Scholar] [CrossRef]
- Matsushita, T.; Lankford, K.L.; Arroyo, E.J.; Sasaki, M.; Neyazi, M.; Radtke, C.; Kocsis, J.D. Diffuse and persistent blood–spinal cord barrier disruption after contusive spinal cord injury rapidly recovers following intravenous infusion of bone marrow mesenchymal stem cells. Exp. Neurol. 2015, 267, 152–164. [Google Scholar] [CrossRef]
- Takigawa, T.; Yonezawa, T.; Yoshitaka, T.; Minaguchi, J.; Kurosaki, M.; Tanaka, M.; Sado, Y.; Ohtsuka, A.; Ozaki, T.; Ninomiya, Y. Separation of the Perivascular Basement Membrane Provides a Conduit for Inflammatory Cells in a Mouse Spinal Cord Injury Model. J. Neurotrauma 2010, 27, 739–751. [Google Scholar] [CrossRef]
- Figley, S.A.; Khosravi, R.; Legasto, J.M.; Tseng, Y.-F.; Fehlings, M.G. Characterization of Vascular Disruption and Blood–Spinal Cord Barrier Permeability following Traumatic Spinal Cord Injury. J. Neurotrauma 2014, 31, 541–552. [Google Scholar] [CrossRef] [Green Version]
- Losey, P.; Young, C.; Krimholtz, E.; Bordet, R.; Anthony, D.C. The role of hemorrhage following spinal-cord injury. Brain Res. 2014, 1569, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Tator, C.H.; Koyanagi, I. Vascular mechanisms in the pathophysiology of human spinal cord injury. J. Neurosurg. 1997, 86, 483–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, H.S. Pathophysiology of Blood-Spinal Cord Barrier in Traumatic Injury and Repair. Curr. Pharm. Des. 2005, 11, 1353–1389. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Huang, J.; Hu, J.; Zhu, H. Advance in spinal cord ischemia reperfusion injury: Blood–spinal cord barrier and remote ischemic preconditioning. Life Sci. 2016, 154, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Oudega, M. Molecular and cellular mechanisms underlying the role of blood vessels in spinal cord injury and repair. Cell Tissue Res. 2012, 349, 269–288. [Google Scholar] [CrossRef]
- Jin, L.-Y.; Li, J.; Wang, K.-F.; Xia, W.-W.; Zhu, Z.-Q.; Wang, C.-R.; Li, X.-F.; Liu, H.-Y. Blood–Spinal Cord Barrier in Spinal Cord Injury: A Review. J. Neurotrauma 2021, 38, 1203–1224. [Google Scholar] [CrossRef]
- Donnelly, D.J.; Popovich, P.G. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp. Neurol. 2008, 209, 378–388. [Google Scholar] [CrossRef] [Green Version]
- Yao, C.; Cao, X.; Yu, B. Revascularization After Traumatic Spinal Cord Injury. Front. Physiol. 2021, 12, 631500. [Google Scholar] [CrossRef]
- Makinde, T.; Agrawal, D. Intra and extravascular transmembrane signalling of angiopoietin-1-Tie2 receptor in health and disease. J. Cell. Mol. Med. 2008, 12, 810–828. [Google Scholar] [CrossRef]
- Adams, R.H.; Eichmann, A. Axon Guidance Molecules in Vascular Patterning. Cold Spring Harb. Perspect. Biol. 2010, 2, a001875. [Google Scholar] [CrossRef] [Green Version]
- Evans, C.E.; Iruela-Arispe, M.L.; Zhao, Y.-Y. Mechanisms of Endothelial Regeneration and Vascular Repair and Their Application to Regenerative Medicine. Am. J. Pathol. 2021, 191, 52–65. [Google Scholar] [CrossRef]
- Tsuji-Tamura, K.; Ogawa, M. Morphology regulation in vascular endothelial cells. Inflamm. Regen. 2018, 38, 25. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Liu, N.; Yang, C.; Liao, S.; Guo, H.; Zhao, K.; Li, X.; Liu, S.; Guan, L.; Liu, C.; et al. HDAC Inhibitor L-Carnitine and Proteasome Inhibitor Bortezomib Synergistically Exert Anti-Tumor Activity In Vitro and In Vivo. PLoS ONE 2012, 7, e52576. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, X.; Zhao, D.X.; Yin, J.; Hu, G.; Evans, C.; Zhao, Y.-Y. Endothelial Hypoxia-Inducible Factor-1α Is Required for Vascular Repair and Resolution of Inflammatory Lung Injury through Forkhead Box Protein M1. Am. J. Pathol. 2019, 189, 1664–1679. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, L.; Marsboom, G.; Jambusaria, A.; Xiong, S.; Toth, P.; Benevolenskaya, E.V.; Rehman, J.; Malik, A.B. Sox17 is required for endothelial regeneration following inflammation-induced vascular injury. Nat. Commun. 2019, 10, 2126. [Google Scholar] [CrossRef] [Green Version]
- McDonald, A.I.; Shirali, A.S.; Aragón, R.; Ma, F.; Hernandez, G.; Vaughn, D.A.; Mack, J.J.; Lim, T.Y.; Sunshine, H.; Zhao, P.; et al. Endothelial regeneration of large vessels is a biphasic process driven by local cells with distinct proliferative capacities. Cell Stem Cell 2018, 23, 210–225. [Google Scholar] [CrossRef] [Green Version]
- Wilhelm, K.; Happel, K.; Eelen, G.; Schoors, S.; Oellerich, M.F.; Lim, R.; Zimmermann, B.; Aspalter, I.M.; Franco, C.A.; Boettger, T.; et al. FOXO1 couples metabolic activity and growth state in the vascular endothelium. Nature 2016, 529, 216–220. [Google Scholar] [CrossRef] [Green Version]
- Sacilotto, N.; Chouliaras, K.M.; Nikitenko, L.L.; Lu, Y.W.; Fritzsche, M.; Wallace, M.D.; Nornes, S.; García-Moreno, F.; Payne, S.; Bridges, E.; et al. MEF2 transcription factors are key regulators of sprouting angiogenesis. Genes Dev. 2016, 30, 2297–2309. [Google Scholar] [CrossRef] [Green Version]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef] [Green Version]
- Tsuji-Tamura, K.; Ogawa, M. Inhibition of the PI3K/Akt and mTORC1 signaling pathways promotes the elongation of vascular endothelial cells. J. Cell Sci. 2016, 129, 1165–1178. [Google Scholar] [CrossRef] [Green Version]
- Loy, D.N.; Crawford, C.H.; Darnall, J.B.; Burke, D.A.; Onifer, S.M.; Whittemore, S.R. Temporal progression of angiogenesis and basal lamina deposition after contusive spinal cord injury in the adult rat. J. Comp. Neurol. 2002, 445, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Segura, I.; De Smet, F.; Hohensinner, P.J.; de Almodovar, C.R.; Carmeliet, P. The neurovascular link in health and disease: An update. Trends Mol. Med. 2009, 15, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Tsivelekas, K.K.; Evangelopoulos, D.S.; Pallis, D.; Benetos, I.S.; Papadakis, S.A.; Vlamis, J.; Pneumaticos, S.G. Angiogenesis in Spinal Cord Injury: Progress and Treatment. Cureus 2022, 14, e25475. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Jo, M.-J.; Choi, H.; Muttigi, M.S.; Shon, S.; Kim, B.-J.; Lee, S.-H.; Han, I.-B. Matrix Metalloproteinase-8 Inhibition Prevents Disruption of Blood–Spinal Cord Barrier and Attenuates Inflammation in Rat Model of Spinal Cord Injury. Mol. Neurobiol. 2018, 55, 2577–2590. [Google Scholar] [CrossRef]
- Kumar, H.; Lim, C.S.; Choi, H.; Joshi, H.P.; Kim, K.-T.; Kim, Y.H.; Park, C.-K.; Kim, H.M.; Han, I.-B. Elevated TRPV4 Levels Contribute to Endothelial Damage and Scarring in Experimental Spinal Cord Injury. J. Neurosci. 2020, 40, 1943–1955. [Google Scholar] [CrossRef]
- Ni, S.; Luo, Z.; Jiang, L.; Guo, Z.; Li, P.; Xu, X.; Cao, Y.; Duan, C.; Wu, T.; Li, C.; et al. UTX/KDM6A Deletion Promotes Recovery of Spinal Cord Injury by Epigenetically Regulating Vascular Regeneration. Mol. Ther. 2019, 27, 2134–2146. [Google Scholar] [CrossRef] [Green Version]
- Halder, S.K.; Kant, R.; Milner, R. Chronic mild hypoxia promotes profound vascular remodeling in spinal cord blood vessels, preferentially in white matter, via an α5β1 integrin-mediated mechanism. Angiogenesis 2018, 21, 251–266. [Google Scholar] [CrossRef]
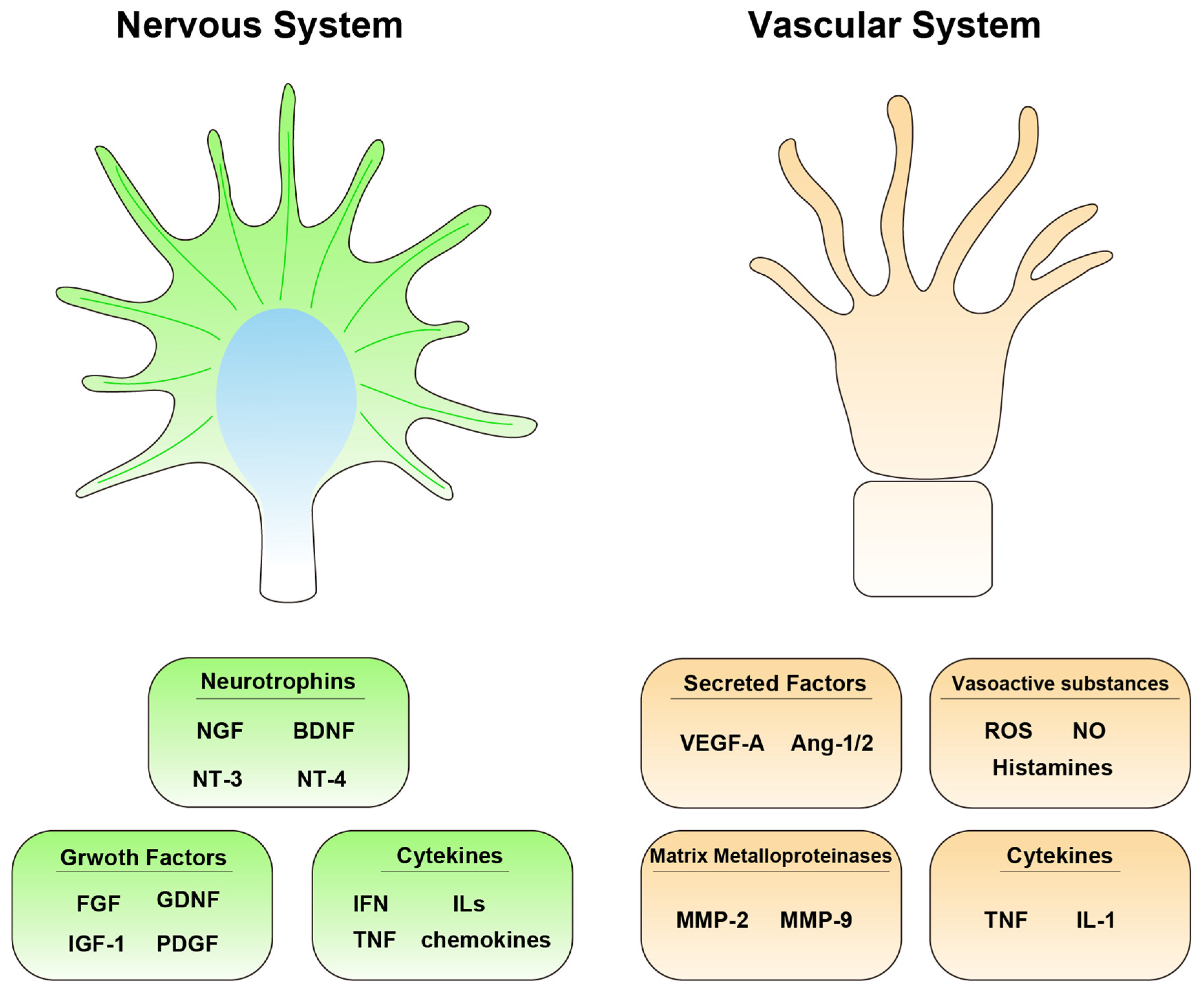
| Molecules in the Nervous System | Molecules in the Vascular System |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Dinh, H.T.P.; Matsuyama, Y.; Sato, K.; Yamagishi, S. Molecular Mechanisms in the Vascular and Nervous Systems following Traumatic Spinal Cord Injury. Life 2023, 13, 9. https://doi.org/10.3390/life13010009
Li S, Dinh HTP, Matsuyama Y, Sato K, Yamagishi S. Molecular Mechanisms in the Vascular and Nervous Systems following Traumatic Spinal Cord Injury. Life. 2023; 13(1):9. https://doi.org/10.3390/life13010009
Chicago/Turabian StyleLi, Shuo, Hoai Thi Phuong Dinh, Yukihiro Matsuyama, Kohji Sato, and Satoru Yamagishi. 2023. "Molecular Mechanisms in the Vascular and Nervous Systems following Traumatic Spinal Cord Injury" Life 13, no. 1: 9. https://doi.org/10.3390/life13010009
APA StyleLi, S., Dinh, H. T. P., Matsuyama, Y., Sato, K., & Yamagishi, S. (2023). Molecular Mechanisms in the Vascular and Nervous Systems following Traumatic Spinal Cord Injury. Life, 13(1), 9. https://doi.org/10.3390/life13010009






