Chronic Hepatitis C Virus Infection: An Ongoing Challenge in Screening and Treatment
Abstract
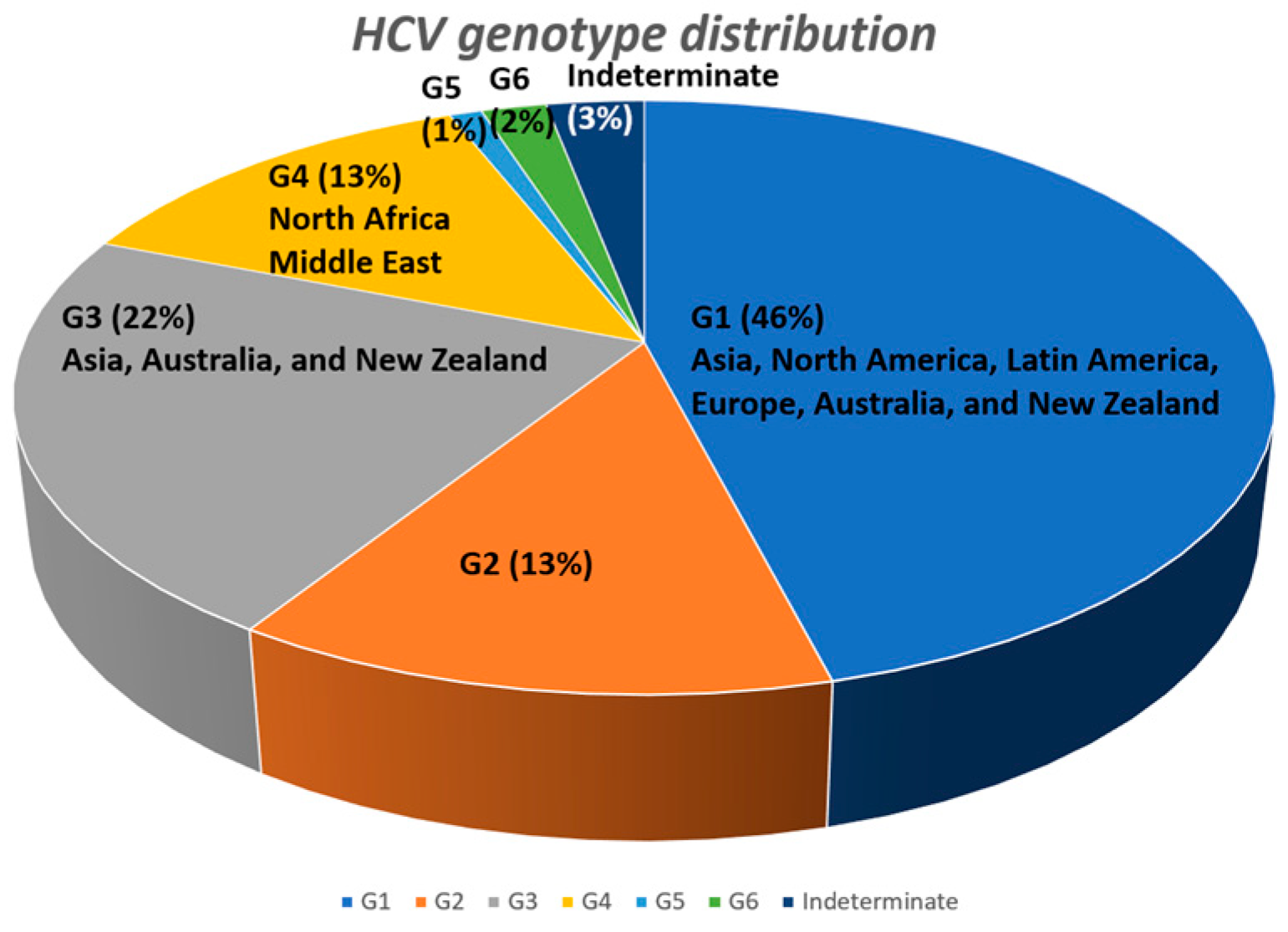
:1. Introduction
2. Screening in Different Populations
2.1. Screening of Patients with Undiagnosed/Untreated Hepatitis C Virus Infection
2.2. Hepatitis C Virus Screening in High-Risk Patients
3. Hepatitis C Virus Infection Treatment in Special Populations
3.1. Hepatitis C Virus Infection in Patients with Hepatitis B Virus Coinfection
3.2. Hepatitis C Virus Infection in Patients with Decompensated Cirrhosis
3.3. Hepatitis C Virus Infection after Liver Transplantation
4. Post-Sustained Virological Response Conditions
4.1. Hepatitis C Virus Reinfection
4.2. Hepatocellular Carcinoma Surveillance after Sustained Virological Response to Hepatitis C Virus Infection
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Polaris Observatory HCVC. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: A modelling study. Lancet Gastroenterol. Hepatol. 2022, 7, 396–415. [Google Scholar] [CrossRef] [PubMed]
- Gower, E.; Estes, C.; Blach, S.; Razavi-Shearer, K.; Razavi, H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J. Hepatol. 2014, 61 (Suppl. S1), S45–S57. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, C. Treatment failure with DAA therapy: Importance of resistance. J. Hepatol. 2021, 74, 1472–1482. [Google Scholar] [CrossRef] [PubMed]
- Papatheodoridis, G.V.; Hatzakis, A.; Cholongitas, E.; Baptista-Leite, R.; Baskozos, I.; Chhatwal, J.; Colombo, M.; Cortez-Pinto, H.; Craxi, A.; Goldberg, D.; et al. Hepatitis C: The beginning of the end-key elements for successful European and national strategies to eliminate HCV in Europe. J. Viral Hepat. 2018, 25 (Suppl. S1), 6–17. [Google Scholar] [CrossRef] [PubMed]
- Polaris Observatory HCVC. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: A modelling study. Lancet Gastroenterol. Hepatol. 2017, 2, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections, 2021. 2021. Available online: https://www.who.int/publications/i/item/9789240027077 (accessed on 20 January 2023).
- Global Health Sector Strategies on, Respectively, HIV, Viral Hepatitis and Sexually Transmitted Infections for the Period 2022–2030. 2022. Available online: https://www.who.int/publications/i/item/9789240053779 (accessed on 20 January 2023).
- Global Health Sector Strategy on Viral Hepatitis 2016–2021. Towards Ending Viral Hepatitis. 2016. Available online: https://www.who.int/publications/i/item/WHO-HIV-2016.06 (accessed on 20 January 2023).
- Combating Hepatitis B and C to Reach Elimination by 2030. 2016. Available online: https://www.who.int/publications/i/item/combating-hepatitis-b-and-c-to-reach-elimination-by-2030 (accessed on 20 January 2023).
- Veeramachaneni, H.; Park, B.; Blakely, D.; Palacio, A.; Darby, R.; Fluker, S.; Lyles, R.H.; Miller, L.S. Differences in inpatient and outpatient hepatitis C virus prevalence and linkage to care rates in a safety net hospital hepatitis C screening program. J. Gastroenterol. Hepatol. 2021, 36, 2285–2291. [Google Scholar] [CrossRef]
- Hayes, C.N.; Imamura, M.; Tanaka, J.; Chayama, K. Road to elimination of HCV: Clinical challenges in HCV management. Liver Int. 2022, 42, 1935–1944. [Google Scholar] [CrossRef]
- Ghany, M.G.; Morgan, T.R.; AASLD-IDSA Hepatitis C Guidance Panel. Hepatitis C Guidance 2019 Update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Hepatology 2020, 71, 686–721. [Google Scholar] [CrossRef]
- Liu, L.; Xu, H.; Hu, Y.; Shang, J.; Jiang, J.; Yu, L.; Zhao, C.; Zhang, D.; Zhang, X.; Li, J.; et al. Hepatitis C screening in hospitals: Find the missing patients. Virol. J. 2019, 16, 47. [Google Scholar] [CrossRef]
- Turhanoglu, M.; Onur, A.; Bilman, F.B.; Ayaydin, Z.; Aktar, G.S. Eight-year seroprevalence of HBV, HCV and HIV in Diyarbakir training and research hospital. Int. J. Med. Sci. 2013, 10, 1595–1601. [Google Scholar] [CrossRef]
- Rosato, V.; Kondili, L.A.; Nevola, R.; Perillo, P.; Mastrocinque, D.; Aghemo, A.; Claar, E. Elimination of hepatitis C in southern Italy: A model of HCV screening and linkage to care among hospitalized patients at different hospital divisions. Viruses 2022, 14, 1096. [Google Scholar] [CrossRef] [PubMed]
- Nevola, R.; Messina, V.; Marrone, A.; Coppola, N.; Rescigno, C.; Esposito, V.; Sangiovanni, V.; Claar, E.; Pisaturo, M.; Fusco, F.M.; et al. Epidemiology of HCV and HBV in a High Endemic Area of Southern Italy: Oppor-tunities from the COVID-19 Pandemic-Standardized National Screening or One Tailored to Local Epidemiology? Biology 2022, 11, 609. [Google Scholar] [CrossRef] [PubMed]
- Piazzolla, A.V.; Paroni, G.; Bazzocchi, F.; Cassese, M.; Cisternino, A.; Ciuffreda, L.; Gorgoglione, F.; Gorgoglione, L.; Palazzo, V.; Sciannamè, N.; et al. High rates of hidden HCV infections among hospitalized patients aged 55–85. Pathogens 2021, 10, 695. [Google Scholar] [CrossRef]
- Mazzeo, C.; Azzaroli, F.; Giovanelli, S.; Dormi, A.; Festi, D.; Colecchia, A.; Miracolo, A.; Natale, P.; Nigro, G.; Alberti, A.; et al. Ten year incidence of HCV infection in northern Italy and frequency of spontaneous viral clearance. Gut 2003, 52, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Cornett, J.K.; Bodiwala, V.; Razuk, V.; Shukla, D.; Narayanan, N. Results of a hepatitis C virus screening program of the 1945–1965 birth cohort in a large emergency department in New Jersey. Open Forum Infect. Dis. 2018, 5, ofy065. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, F.D.; Li, L.Q.; Chen, E.Q. Management of in- and out-of-hospital screening for hepatitis C. Front. Public Health 2022, 10, 984810. [Google Scholar] [CrossRef]
- Oze, T.; Hiramatsu, N.; Yakushijin, T.; Mochizuki, K.; Oshita, M.; Hagiwara, H.; Mita, E.; Ito, T.; Fukui, H.; Inui, Y.; et al. Indications and limitations for aged patients with chronic hepatitis C in pegylated interferon alfa-2b plus ribavirin combination therapy. J. Hepatol. 2011, 54, 604–611. [Google Scholar] [CrossRef]
- Huang, C.; Yang, J.; Dai, C.; Huang, J.; Hou, N.; Lin, Z.; Chen, S.; Hsieh, M.; Wang, L.; Chang, W.; et al. Efficacy and safety of pegylated interferon combined with ribavirin for the treatment of older patients with chronic hepatitis C. J. Infect. Dis. 2010, 201, 751–759. [Google Scholar] [CrossRef]
- van Dijk, M.; Drenth, J.P.H.; HepNed Study Group; Arends, J.E.; Brakenhoff, S.M.; Isfordink, C.J.; de Knegt, R.; van der Valk, M. Loss to follow-up in the hepatitis C care cascade: A substantial problem but opportunity for micro-elimination. J. Viral Hepat. 2020, 27, 1270–1283. [Google Scholar] [CrossRef]
- Kracht, P.A.M.; Arends, J.E.; van Erpecum, K.J.; Thijsen, S.F.T.; Vlaminckx, B.J.M.; Weersink, A.J.L.; Wensing, A.M.J.; Deege, M.P.H.; Dimmendaal, M.; Stadhouders, P.H.G.M.; et al. Retrieval and cure of chronic hepatitis C (REACH): Results of micro-elimination in the Utrecht province. Liver Int. 2019, 39, 455–462. [Google Scholar] [CrossRef]
- Vargas-Accarino, E.; Martínez-Campreciós, J.; Domínguez-Hernández, R.; Rando-Segura, A.; Riveiro-Barciela, M.; Rodríguez-Frías, F.; Barreira, A.; Palom, A.; Casado, M.Á.; Esteban, R.; et al. Cost-effectiveness analysis of an active search to retrieve HCV patients lost to follow-up (RELINK-C strategy) and the impact of COVID-19. J. Viral Hepat. 2022, 29, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Rich, N.E.; Jain, M.K.; Blackwell, J.-M.; Murphy, C.C.; Perryman, P.; McBryde, J.; Quirk, L.; Clark, C.; Villarreal, D.; et al. Randomized Clinical trial of inreach with or without mailed outreach to promote hepatitis C screening in a difficult-to-reach patient population. Am. J. Gastroenterol. 2021, 116, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Martró, E.; Ouaarab, H.; Saludes, V.; Buti, M.; Treviño, B.; Roade, L.; Egea-Cortés, L.; Reyes-Ureña, J.; Not, A.; Majó, X.; et al. Pilot hepatitis C micro-elimination strategy in Pakistani migrants in Catalonia through a community intervention. Liver Int. 2022, 42, 1751–1761. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chang, T.S.; Chang, S.Z.; Chen, C.H.; Chen, M.Y. Challenges of transferring rural adults with chronic HCV infection for further HCV RNA confirmation and free DAAs treatment: A success story of the interdisciplinary collaboration approach. BMC Infect. Dis. 2020, 20, 737. [Google Scholar] [CrossRef] [PubMed]
- Tien, H.M.; Cheng, T.C.; Lien, H.C.; Yang, K.F.; Shy, C.G.; Chen, Y.L.; Hsu, N.T.; Lu, S.N.; Wang, J.H. Liver disease screening and hepatitis C virus elimination in Taiwan rural idigenous townships: Village-by-village screening and linking to outreach hpatology Care. Int. J. Environ. Res. Public Health 2022, 19, 3269. [Google Scholar] [CrossRef]
- Mane, A.; Sacks, J.; Sharma, S.; Singh, H.; Tejada-Strop, A.; Kamili, S.; Kacholia, K.; Gautam, R.; Thakar, M.; Gupta, R.S.; et al. Evaluation of five rapid diagnostic tests for detection of antibodies to hepatitis C virus (HCV): A step towards scale-up of HCV screening efforts in India. PLoS ONE 2019, 14, e0210556. [Google Scholar] [CrossRef]
- Gupta, V.; Kumar, A.; Sharma, P.; Arora, A. Newer direct-acting antivirals for hepatitis C virus infection: Perspectives for India. Indian. J. Med. Res. 2017, 146, 23–33. [Google Scholar]
- Viejo, L.G.-E.; Herola, A.G.; Lloret, I.S.; Ruano, F.S.; Paulino, I.C.; Ivorra, C.Q.; Saavedra, I.A.; Pérez, D.M.; de la Osa, J.V. Screening of hepatitis C virus infection in adult general population in Spain. Eur. J. Gastroenterol. Hepatol. 2018, 30, 1077–1081. [Google Scholar] [CrossRef]
- Scheibe, A.; Young, K.; Versfeld, A.; Spearman, C.W.; Sonderup, M.W.; Prabdial-Sing, N.; Puren, A.; Hausler, H. Hepatitis B, hepatitis C and HIV prevalence and related sexual and substance use risk practices among key populations who access HIV prevention, treatment and related services in South Africa: Findings from a seven-city cross-sectional survey (2017). BMC Infect. Dis. 2020, 20, 655. [Google Scholar] [CrossRef]
- Edmunds, B.L.; Miller, E.R.; Tsourtos, G. The distribution and socioeconomic burden of Hepatitis C virus in South Australia: A cross-sectional study 2010–2016. BMC Public Health 2019, 19, 527. [Google Scholar] [CrossRef]
- Shehata, N.; Austin, T.; Ha, S.; Timmerman, K. Barriers to and facilitators of hepatitis C virus screening and testing: A scoping review. Can. Commun. Dis. Rep. 2018, 44, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Fiore, V.; Rastrelli, E.; Madeddu, G.; Ranieri, R.; De Vito, A.; Giuliani, R.; Di Mizio, G.; Bolcato, M.; De Matteis, G.; Ialungo, A.M.; et al. HCV spread among female incarcerated population and treatment pathways to viral elimination in Italian prison settings: Clinical perspectives and medico legal aspects. BMC Infect. Dis. 2022, 22, 601. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-H.; Fang, Y.-J.; Hsu, S.-J.; Lee, J.-Y.; Chiu, M.-C.; Yu, J.-J.; Kuo, C.-C.; Chen, C.-H. Microelimination of chronic hepatitis C by universal screening plus direct-acting antivirals for incarcerated persons in Taiwan. Open Forum Infect. Dis. 2020, 7, ofaa301. [Google Scholar] [CrossRef] [PubMed]
- Samso Jofra, L.; Puig, T.; Sola, I.; Trujols, J. Interim opioid agonist treatment for opioid addiction: A systematic review. Harm Reduct. J. 2022, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Bregenzer, A.; Krismer, C.; Wendel, S.; Roser, P.; Fux, C.A. HCV elimination in a Swiss opioid agonist therapy programme—A cohort study. Swiss Med. Wkly. 2022, 152, 40009. [Google Scholar] [CrossRef]
- Mirzazadeh, A.; Hosseini-Hooshyar, S.; Shahesmaeili, A.; Sharafi, H.; Shafiei, M.; Zarei, J.; Mousavian, G.; Tavakoli, F.; Ghalekhani, N.; Shokoohi, M.; et al. An on-site community-based model for hepatitis C screening, diagnosis, and treatment among people who inject drugs in Kerman, Iran: The Rostam study. Int. J. Drug Policy 2022, 102, 103580. [Google Scholar] [CrossRef]
- Tofighi, B.; Lee, J.D.; Sindhu, S.S.; Chemi, C.; Leonard, N.R. Engagement in the hepatitis C care continuum among people who use drugs. J. Subst. Use 2020, 25, 343–349. [Google Scholar] [CrossRef]
- Wondmagegn, M.; Wondimeneh, Y.; Getaneh, A.; Ayalew, G. Seroprevalence of hepatitis B virus, hepatitis C virus, syphilis and associated factors among female sex workers in Gondar Town, Northwest Ethiopia. Infect. Drug Resist. 2022, 15, 5915–5927. [Google Scholar] [CrossRef]
- Lapadula, G.; Soria, A.; Modesti, M.; Vecchi, A.; Sabbatini, F.; Monopoli, A.; Squillace, N.; Lungu, E.; Coloma, J.; Columpsi, P.; et al. Behavioural survey and street-based HIV and HCV rapid testing programme among transgender sex workers. Sex. Transm. Infect. 2022, 99, 41–46. [Google Scholar] [CrossRef]
- Wang, Z.; Mo, P.K.H.; Fang, Y.; Ip, M.; Lau, J.T.F. Factors predicting first-time hepatitis C virus testing uptake among men who have sex with men in China: An observational prospective cohort study. Sex. Transm. Infect. 2020, 96, 258–264. [Google Scholar] [CrossRef]
- Vaux, S.; Chevaliez, S.; Saboni, L.; Sauvage, C.; Sommen, C.; Barin, F.; Alexandre, A.; Jauffret-Roustide, M.; Lot, F.; Velter, A.; et al. Prevalence of hepatitis C infection, screening and associated factors among men who have sex with men attending gay venues: A cross-sectional survey (PREVAGAY), France, 2015. BMC Infect. Dis. 2019, 19, 315. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.K. Management of hepatitis C in special populations: HIV coinfection, renal disease, and decompensated cirrhosis. Clin. Liver Dis. 2020, 16, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, D.; Deutsch, M. The spectrum of HBV/HCV coinfection: Epidemiology, clinical characteristics, viralinteractions and management. Ann. Gastroenterol. 2015, 28, 221–228. [Google Scholar] [PubMed]
- Marot, A.; Belaid, A.; Orlent, H.; Sersté, T.; Michielsen, P.; Colle, I.; Laleman, W.; de Galocsy, C.; Reynaert, H.; D’heygere, F.; et al. Characteristics of patients with hepatitis B virus and hepatitis C virus dual infection in a Western European country: Comparison with monoinfected patients. Clin. Res. Hepatol. Gastroenterol. 2017, 41, 656–663. [Google Scholar] [CrossRef]
- Pol, S.; Haour, G.; Fontaine, H.; Dorival, C.; Petrov-Sanchez, V.; Bourliere, M.; Capeau, J.; Carrieri, P.; Larrey, D.; Larsen, C.; et al. The negative impact of HBV/HCV coinfection on cirrhosis and its consequences. Aliment. Pharmacol. Ther. 2017, 46, 1054–1060. [Google Scholar] [CrossRef]
- Takayama, H.; Sato, T.; Ikeda, F.; Fujiki, S. Reactivation of hepatitis B virus during interferon-free therapy with daclatasvir and asunaprevir in patient with hepatitis B virus/hepatitis C virus co-infection. Hepatol. Res. 2016, 46, 489–491. [Google Scholar] [CrossRef]
- Ou, P.; Fang, Z.; Chen, J. Hepatitis B reactivation in a chronic hepatitis C patient treated with ledipasvir and sofosbuvir: A case report. Clin. Res. Hepatol. Gastroenterol. 2017, 41, e17–e18. [Google Scholar] [CrossRef]
- Liu, C.-J.; Chuang, W.-L.; Sheen, I.-S.; Wang, H.-Y.; Chen, C.-Y.; Tseng, K.-C.; Chang, T.-T.; Massetto, B.; Yang, J.C.; Yun, C.; et al. Efficacy of Ledipasvir and sofosbuvir treatment of HCV infection in patients coinfected with HBV. Gastroenterology 2018, 154, 989–997. [Google Scholar] [CrossRef]
- Yeh, M.L.; Huang, C.F.; Huang, C.I.; Holmes, J.A.; Hsieh, M.H.; Tsai, Y.S.; Liang, P.C.; Tsai, P.C.; Hsieh, M.Y.; Lin, Z.Y.; et al. Hepatitis B-related outcomes following direct-acting antiviral therapy in Taiwanese patients with chronic HBV/HCV co-infection. J. Hepatol. 2020, 73, 62–71. [Google Scholar] [CrossRef]
- Cheng, P.-N.; Liu, C.-J.; Chen, C.-Y.; Tseng, K.-C.; Lo, C.-C.; Peng, C.-Y.; Lin, C.-L.; Chiu, H.-C.; Chiu, Y.-C.; Chen, P.-J. Entecavir prevents HBV reactivation during direct acting antivirals for HCV/HBV dual infection: A Randomized Trial. Clin. Gastroenterol. Hepatol. 2022, 20, 2800–2808. [Google Scholar] [CrossRef]
- Charlton, M.; Everson, G.T.; Flamm, S.L.; Kumar, P.; Landis, C.; Brown, R.S.; Jr Fried, M.W.; Terrault, N.A.; O’Leary, J.G.; Vargas, H.E.; et al. Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology 2015, 149, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Curry, M.P.; O’leary, J.G.; Bzowej, N.; Muir, A.J.; Korenblat, K.M.; Fenkel, J.M.; Reddy, K.R.; Lawitz, E.; Flamm, S.L.; Schiano, T.; et al. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N. Engl. J. Med. 2015, 373, 2618–2628. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.S.; Adams, N.; Vachharajani, N.; Dageforde, L.; Wellen, J.; Shenoy, S.; Crippin, J.S.; Doyle, M.B.; Chapman, W.C. Liver transplantation for hepatitis C patients in the era of direct-acting antiviral treatment: A retrospective cohort study. Int. J. Surg. 2020, 75, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Bunchorntavakul, C.; Reddy, R.K. HCV therapy in decompensated cirrhosis before or after liver transplantation: A paradoxical quandary. Am. J. Gastroenterol. 2018, 113, 449–452. [Google Scholar] [CrossRef]
- Verna, E.C. The dynamic landscape of liver transplant in the era of effective hepatitis C virus therapy. Hepatology 2017, 65, 763–766. [Google Scholar] [CrossRef]
- Belli, L.S.; Duvoux, C.; Berenguer, M.; Berg, T.; Coilly, A.; Colle, I.; Fagiuoli, S.; Khoo, S.; Pageaux, G.P.; Puoti, M.; et al. ELITA consensus statements on the use of DAAs in liver transplant candidates and recipients. J. Hepatol. 2017, 67, 585–602. [Google Scholar] [CrossRef]
- Garcia-Retortillo, M.; Forns, X.; Feliu, A.; Moitinho, E.; Costa, J.; Navasa, M.; Rimola, A.; Rodes, J. Hepatitis C virus kinetics during and immediately after liver transplantation. Hepatology 2002, 35, 680–687. [Google Scholar] [CrossRef]
- Yilmaz, N.; Shiffman, M.L.; Stravitz, R.T.; Sterling, R.K.; Luketic, V.A.; Sanyal, A.J.; Mills, A.S.; Contos, M.J.; Coterell, A.; Maluf, D.; et al. A prospective evaluation of fibrosis progression in patients with recurrent hepatitis C virus following liver transplantation. Liver Transplant. 2007, 13, 975–983. [Google Scholar] [CrossRef]
- Gane, E.J. The natural history of recurrent hepatitis C and what influences this. Liver Transplant. 2008, 14 (Suppl. S2), S36–S44. [Google Scholar] [CrossRef]
- Cotter, T.G.; Paul, S.; Sandıkçı, B.; Couri, T.; Bodzin, A.S.; Little, E.C.; Sundaram, V.; Charlton, M. Improved graft survival after liver transplantation for recipients with hepatitis C virus in the direct-acting antiviral era. Liver Transplant. 2019, 25, 598–609. [Google Scholar] [CrossRef]
- Gambato, M.; Lens, S.; Navasa, M.; Forns, X. Treatment options in patients with decompensated cirrhosis, pre- and post-transplantation. J. Hepatol. 2014, 61, S120–S131. [Google Scholar] [CrossRef] [PubMed]
- Charlton, M.; Gane, E.; Manns, M.P.; Brown, R.S.; Curry, M.P.; Kwo, P.Y.; Fontana, R.J.; Gilroy, R.; Teperman, L.; Muir, A.J.; et al. Sofosbuvir and ribavirin for treatment of compensated recurrent hepatitis C virus infection after liver transplantation. Gastroenterology 2015, 148, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Blackard, J.T.; Sherman, K.E. Hepatitis C virus coinfection and superinfection. J. Infect. Dis. 2007, 195, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.; Krajden, M.; Shoveller, J.; Gustafson, P.; Gilbert, M.; Wong, J.; Tyndall, M.W.; Janjua, N.Z.; Yu, A.; Kuo, M.; et al. Hepatitis C cross-genotype immunity and implications for vaccine development. Sci. Rep. 2017, 7, 12326. [Google Scholar] [CrossRef]
- Midgard, H.; Weir, A.; Palmateer, N.; Re, V.L.; Pineda, J.A.; Macías, J.; Dalgard, O. HCV epidemiology in high-risk groups and the risk of reinfection. J. Hepatol. 2016, 65, S33–S45. [Google Scholar] [CrossRef]
- Falade-Nwulia, O.; Sulkowski, M.S.; Merkow, A.; Latkin, C.; Mehta, S.H. Understanding and addressing hepatitis C reinfection in the oral direct-acting antiviral era. J. Viral Hepat. 2018, 25, 220–227. [Google Scholar] [CrossRef]
- Rossi, C.; Butt, Z.A.; Wong, S.; Buxton, J.A.; Islam, N.; Yu, A.; Darvishian, M.; Gilbert, M.; Wong, J.; Chapinal, N.; et al. Hepatitis C virus reinfection after successful treatment with direct-acting antiviral therapy in a large population-based cohort. J. Hepatol. 2018, 69, 1007–1014. [Google Scholar] [CrossRef]
- Peckham, A.M.; Young, E.H. Opportunities to offer harm reduction to people who inject drugs during infectious disease encounters: Narrative Review. Open Forum Infect. Dis. 2020, 7, ofaa503. [Google Scholar] [CrossRef]
- Platt, L.; Minozzi, S.; Reed, J.; Vickerman, P.; Hagan, H.; French, C.; Jordan, A.; Degenhardt, L.; Hope, V.; Hutchinson, S.; et al. Needle syringe programmes and opioid substitution therapy for preventing hepatitis C transmission in people who inject drugs. Cochrane Database Syst. Rev. 2017, 9, CD012021. [Google Scholar] [CrossRef]
- Hajarizadeh, B.; Cunningham, E.B.; Valerio, H.; Martinello, M.; Law, M.; Janjua, N.Z.; Midgard, H.; Dalgard, O.; Dillon, J.; Hickman, M.; et al. Hepatitis C reinfection after successful antiviral treatment among people who inject drugs: A meta-analysis. J. Hepatol. 2020, 72, 643–657. [Google Scholar] [CrossRef]
- Ingiliz, P.; Martin, T.C.; Rodger, A.; Stellbrink, H.-J.; Mauss, S.; Boesecke, C.; Mandorfer, M.; Bottero, J.; Baumgarten, A.; Bhagani, S.; et al. HCV reinfection incidence and spontaneous clearance rates in HIV-positive men who have sex with men in Western Europe. J. Hepatol. 2017, 66, 282–287. [Google Scholar] [CrossRef]
- Chaillon, A.; Sun, X.; Cachay, E.R.; Looney, D.; Wyles, D.; Garfein, R.S.; Martin, T.C.S.; Jain, S.; Mehta, S.R.; Smith, D.M.; et al. Primary incidence of hepatitis C virus infection among HIV-infected men who have sex with men in San Diego, 2000–2015. Open Forum Infect. Dis. 2019, 6, ofz160. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, J.; Gil-Martin, Á.; Jarrin, I.; Montes, M.L.; Domínguez, L.; Aldámiz-Echevarría, T.; Téllez, M.J.; Santos, I.; Troya, J.; Losa, J.E.; et al. Reinfection by hepatitis C virus following effective all-oral direct-acting antiviral drug therapy in HIV/hepatitis C virus coinfected individuals. AIDS 2019, 33, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Ingiliz, P.; Wehmeyer, M.H.; Boesecke, C.; Wiesch, J.S.Z.; Schewe, K.; Lutz, T.; Baumgarten, A.; Simon, K.-G.; Hueppe, D.; Rockstroh, J.K.; et al. Reinfection with the hepatitis C virus in men who have sex with men after successful treatment with direct-acting antivirals in Germany: Current incidence rates, compared with rates during the interferon era. Clin. Infect. Dis. 2020, 71, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Martinello, M.; Yee, J.; Bartlett, S.R.; Read, P.; Baker, D.; Post, J.J.; Finlayson, R.; Bloch, M.; Doyle, J.; Shaw, D.; et al. Moving towards hepatitis C microelimination among people living with human immunodeficiency virus in Australia: The CEASE study. Clin. Infect. Dis. 2020, 71, 1502–1510. [Google Scholar] [CrossRef]
- Adu, P.A.; Rossi, C.; Binka, M.; Wong, S.; Wilton, J.; Wong, J.; Butt, Z.A.; Bartlett, S.; Jeong, D.; Pearce, M.; et al. HCV reinfection rates after cure or spontaneous clearance among HIV-infected and uninfected men who have sex with men. Liver Int. 2021, 41, 482–493. [Google Scholar] [CrossRef]
- Nakagawa, S.; Wei, L.; Song, W.M.; Higashi, T.; Ghoshal, S.; Kim, R.S.; Bian, C.B.; Yamada, S.; Sun, X.; Venkatesh, A.; et al. Molecular liver cancer prevention in cirrhosis by organ transcriptome analysis and lysophosphatidic acid pathway inhibition. Cancer Cell 2016, 30, 879–890. [Google Scholar] [CrossRef]
- Bandiera, S.; Billie Bian, C.; Hoshida, Y.; Baumert, T.F.; Zeisel, M.B. Chronic hepatitis C virus infection and pathogenesis of hepatocellular carcinoma. Curr. Opin. Virol. 2016, 20, 99–105. [Google Scholar] [CrossRef]
- Rockey, D.C. Fibrosis reversal after hepatitis C virus elimination. Curr. Opin. Gastroenterol. 2019, 35, 137–144. [Google Scholar] [CrossRef]
- Ioannou, G.N. HCC surveillance after SVR in patients with F3/F4 fibrosis. J. Hepatol. 2021, 74, 458–465. [Google Scholar] [CrossRef]
- Hamdane, N.; Jühling, F.; Crouchet, E.; El Saghire, H.; Thumann, C.; Oudot, M.A.; Bandiera, S.; Saviano, A.; Ponsolles, C.; Roca Suarez, A.A.R.; et al. HCV-induced epigenetic changes associated with liver cancer risk persist after sustained virologic response. Gastroenterology 2019, 156, 2313–2329.e7. [Google Scholar] [CrossRef] [PubMed]
- Semmler, G.; Meyer, E.L.; Kozbial, K.; Schwabl, P.; Hametner-Schreil, S.; Zanetto, A.; Bauer, D.; Chromy, D.; Simbrunner, B.; Scheiner, B.; et al. HCC risk stratification after cure of hepatitis C in patients with compensated advanced chronic liver disease. J. Hepatol. 2022, 76, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Papatheodoridis, G.; Sun, J.; Innes, H.; Toyoda, H.; Xie, Q.; Mo, S.; Sypsa, V.; Guha, I.N.; Kumada, T.; et al. aMAP risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis. J. Hepatol. 2020, 73, 1368–1378. [Google Scholar] [CrossRef]
- Nahon, P.; Bourcier, V.; Layese, R.; Audureau, E.; Cagnot, C.; Marcellin, P.; Guyader, D.; Fontaine, H.; Larrey, D.; De Lédinghen, V.; et al. Eradication of hepatitis C virus infection in patients with cirrhosis reduces risk of liver and non-liver complications. Gastroenterology 2017, 152, 142–156.e2. [Google Scholar] [CrossRef] [PubMed]
- Carrat, F.; Fontaine, H.; Dorival, C.; Simony, M.; Diallo, A.; Hezode, C.; De Ledinghen, V.; Larrey, D.; Haour, G.; Bronowicki, J.-P.; et al. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: A prospective cohort study. Lancet 2019, 393, 1453–1464. [Google Scholar] [CrossRef]
- Tani, J.; Morishita, A.; Sakamoto, T.; Takuma, K.; Nakahara, M.; Fujita, K.; Oura, K.; Tadokoro, T.; Mimura, S.; Nomura, T.; et al. Simple scoring system for prediction of hepatocellular carcinoma occurrence after hepatitis C virus eradication by direct-acting antiviral treatment: All Kagawa Liver Disease Group Study. Oncol. Lett. 2020, 19, 2205–2212. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Tang, W.; Beste, L.A.; Tincopa, M.A.; Su, G.L.; Van, T.; Tapper, E.B.; Singal, A.G.; Zhu, J.; Waljee, A.K. Assessment of a deep learning model to predict hepatocellular carcinoma in patients with hepatitis C cirrhosis. JAMA Netw. Open 2020, 3, e2015626. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Green, P.K.; Beste, L.A.; Mun, E.J.; Kerr, K.F.; Berry, K. Development of models estimating the risk of hepatocellular carcinoma after antiviral treatment for hepatitis C. J. Hepatol. 2018, 69, 1088–1098. [Google Scholar] [CrossRef]
- Trevisani, F.; Cantarini, M.C.; Wands, J.R.; Bernardi, M. Recent advances in the natural history of hepatocellular carcinoma. Carcinogenesis 2008, 29, 1299–1305. [Google Scholar] [CrossRef]
- Tzartzeva, K.; Obi, J.; Rich, N.E.; Parikh, N.D.; Marrero, J.A.; Yopp, A.; Waljee, A.K.; Singal, A.G. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: A meta-analysis. Gastroenterology 2018, 154, 1706–1718.e1. [Google Scholar] [CrossRef]

| 1. Lack of effective HCV screening strategies in rural areas and in low-economic countries |
| 2. Poor linkage to care in special populations, such as drug abusers, sex workers, and MSM |
| 3. Need for re-linkage to care for already known but untreated HCV-infected subjects |
| 4. Strategies needed to reduce or prevent reinfection in high-risk populations |
| 5. Poor health knowledge of HCV infection and its management that lead to low screening rate in the general population |
| 6. Variations of medical resources in different countries to engage in HCV elimination program |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, W.-C.; Chiang, H.-C.; Chiu, Y.-C.; Chien, S.-C.; Cheng, P.-N.; Chiu, H.-C. Chronic Hepatitis C Virus Infection: An Ongoing Challenge in Screening and Treatment. Life 2023, 13, 1964. https://doi.org/10.3390/life13101964
Tsai W-C, Chiang H-C, Chiu Y-C, Chien S-C, Cheng P-N, Chiu H-C. Chronic Hepatitis C Virus Infection: An Ongoing Challenge in Screening and Treatment. Life. 2023; 13(10):1964. https://doi.org/10.3390/life13101964
Chicago/Turabian StyleTsai, Wei-Chu, Hsueh-Chien Chiang, Yen-Cheng Chiu, Shih-Chieh Chien, Pin-Nan Cheng, and Hung-Chih Chiu. 2023. "Chronic Hepatitis C Virus Infection: An Ongoing Challenge in Screening and Treatment" Life 13, no. 10: 1964. https://doi.org/10.3390/life13101964
APA StyleTsai, W.-C., Chiang, H.-C., Chiu, Y.-C., Chien, S.-C., Cheng, P.-N., & Chiu, H.-C. (2023). Chronic Hepatitis C Virus Infection: An Ongoing Challenge in Screening and Treatment. Life, 13(10), 1964. https://doi.org/10.3390/life13101964





