A Literature Review on the Use of Aortic Allografts in Modern Cardiac Surgery for the Treatment of Infective Endocarditis: Is There Clear Evidence or Is It Merely a Perception?
Abstract
:1. Introduction
2. Methods
3. Results
3.1. Extent of Bacterium-Dependent Infective Endocarditis and Treatment Options for Native or Prosthetic Valvular Endocarditis
3.2. Clinical Use of Allograft
3.3. The Antibacterial Properties of Allograft
3.4. Alternative Valve Substitutes
4. Discussion
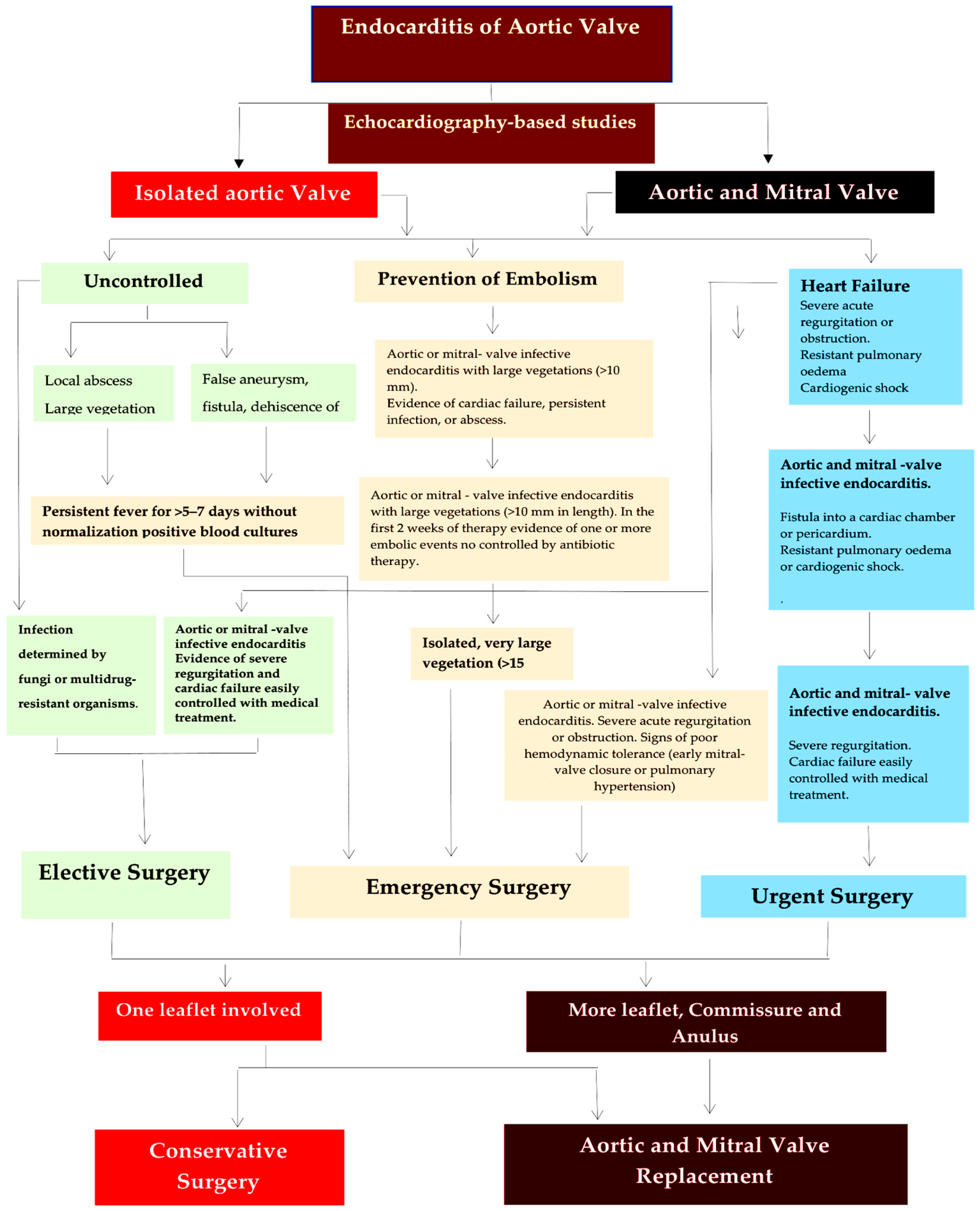
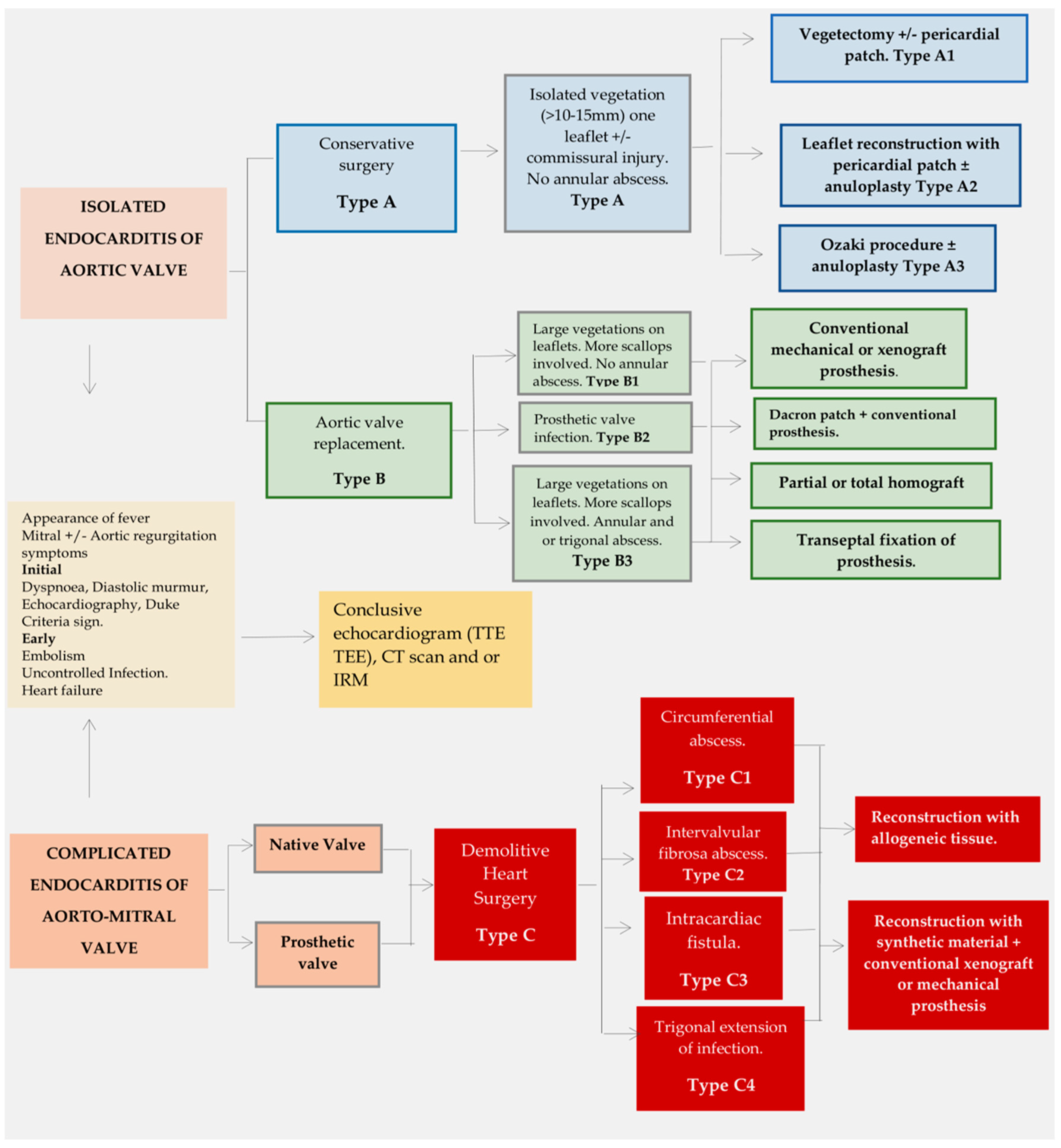
4.1. Systematic Approach to Treat Aortic Valve Endocarditis
4.2. Extended Infection: Replacement Surgery
4.3. Complicated Aorto-Mitral Endocarditis: Demolitive Surgery
5. Risk of Allograft Infection
6. Limitation
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chambers, H.F.; Bayer, A.S. Native-Valve Infective Endocarditis. N. Engl. J. Med. 2020, 383, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Cahill, T.J.; Prendergast, B.D. Infective endocarditis. Lancet 2016, 387, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Cahill, T.J.; Baddour, L.M.; Habib, G.; Hoen, B.; Salaun, E.; Pettersson, G.B.; Schäfers, H.J.; Prendergast, B.D. Challenges in Infective Endocarditis. J. Am. Coll. Cardiol. 2017, 69, 325–344. [Google Scholar] [CrossRef] [PubMed]
- Cresti, A.; Chiavarelli, M.; Scalese, M.; Nencioni, C.; Valentini, S.; Guerrini, F.; D’Aiello, I.; Picchi, A.; De Sensi, F.; Habib, G. Epidemiological and mortality trends in infective endocarditis, a 17-year population-based prospective study. Cardiovasc. Diagn. Ther. 2017, 7, 27–35. [Google Scholar] [CrossRef]
- El-Dalati, S.; Cronin, D.; Shea, M.; Weinberg, R.; James Riddell, I.V.; Washer, L.; Shuman, E.; Burke, J.; Murali, S.; Fagan, C.; et al. Clinical Practice Update on Infectious Endocarditis. Am. J. Med. 2019, 133, 44–49. [Google Scholar] [CrossRef]
- Correa de Sa, D.D.; Tleyjeh, I.M.; Anavekar, N.S.; Schultz, J.C.; Thomas, J.M.; Lahr, B.D.; Bachuwar, A.; Pazdernik, M.; Steckelberg, J.M.; Wilson, W.R.; et al. Epidemiological trends of infective endocarditis: A population-based study in Olmsted County, Minnesota. Mayo Clin. Proc. 2010, 85, 422–426. [Google Scholar] [CrossRef]
- Writing Committee Members; Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 77, 450–500. [Google Scholar] [CrossRef]
- Hussain, S.T.; Shrestha, N.K.; Gordon, S.M.; Houghtaling, P.L.; Blackstone, E.H.; Pettersson, G.B. Residual patient, anatomic, and surgical obstacles in treating active left-sided infective endocarditis. J. Thorac. Cardiovasc. Surg. 2014, 148, 981–988.e4. [Google Scholar] [CrossRef]
- Misfeld, M.; Girrbach, F.; Etz, C.D.; Binner, C.; Aspern, K.V.; Dohmen, P.M.; Davierwala, P.; Pfannmueller, B.; Borger, M.A.; Mohr, F.W. Surgery for infective endocarditis complicated by cerebral embolism: A consecutive series of 375 patients. J. Thorac. Cardiovasc. Surg. 2014, 147, 1837–1844. [Google Scholar] [CrossRef]
- Curlier, E.; Hoen, B.; Alla, F.; Selton-Suty, C.; Schubel, L.; Doco-Lecompte, T.; Minary, L.; Erpelding, M.L.; Duval, X.; Chirouze, C.; et al. Relationships between sex, early valve surgery and mortality in patients with left-sided infective endocarditis analysed in a population-based cohort study. Heart 2014, 100, 1173–1178. [Google Scholar] [CrossRef]
- David, T.E.; Gavra, G.; Feindel, C.M.; Regesta, T.; Armstrong, S.; Maganti, M.D. Surgical treatment of active infective endocarditis: A continued challenge. J. Thorac. Cardiovasc. Surg. 2007, 133, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Ejiofor, J.I.; Yammine, M.; Camuso, J.M.; Walsh, C.W.; Ando, M.; Melnitchouk, S.I.; Rawn, J.D.; Leacche, M.; MacGillivray, T.E.; et al. Are homografts superior to conventional prosthetic valves in the setting of infective endocarditis involving the aortic valve? J. Thorac. Cardiovasc. Surg. 2016, 151, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Spadaccio, C.; Acar, C. Use of allogeneic tissue to treat infective valvular disease: Has everything been said? J. Thorac. Cardiovasc. Surg. 2017, 153, 824–828. [Google Scholar] [CrossRef] [PubMed]
- David, T.E.; Regesta, T.; Gavra, G.; Armstrong, S.; Maganti, M.D. Surgical treatment of paravalvular abscess: Long-term results. Eur. J. Cardiothorac. Surg. 2007, 31, 43–48. [Google Scholar] [CrossRef]
- Sheikh, A.M.; Elhenawy, A.M.; Maganti, M.; Armstrong, S.; David, T.E.; Feindel, C.M. Outcomes of surgical intervention for isolated active mitral valve endocarditis. J. Thorac. Cardiovasc. Surg. 2009, 137, 110–116. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Eishi, K. Surgical treatment of active infective mitral valve endocarditis. Ann. Thorac. Cardiovasc. Surg. 2007, 13, 150–155. [Google Scholar]
- Feringa, H.H.; Shaw, L.J.; Poldermans, D.; Hoeks, S.; van der Wall, E.E.; Dion, R.A.; Bax, J.J. Mitral valve repair and replacement in endocarditis: A systematic review of literature. Ann. Thorac. Surg. 2007, 83, 564–570. [Google Scholar] [CrossRef]
- Manne, M.B.; Shrestha, N.K.; Lytle, B.W.; Nowicki, E.R.; Blackstone, E.; Gordon, S.M.; Pettersson, G.; Fraser, T.G. Outcomes after surgical treatment of native and prosthetic valve infective endocarditis. Ann. Thorac. Surg. 2012, 93, 489–493. [Google Scholar] [CrossRef]
- Pettersson, G.B.; Hussain, S.T.; Shrestha, N.K.; Gordon, S.; Fraser, T.G.; Ibrahim, K.S. Infective endocarditis: An atlas of disease progression for describing, staging, coding, and understanding the pathology. J. Thorac. Cardiovasc. Surg. 2014, 147, 1142–1149.e2. [Google Scholar] [CrossRef]
- Pettersson, G.B.; Coselli, J.S.; Hussain, S.T.; Griffin, B.; Blackstone, E.H.; Gordon, S.M.; LeMaire, S.A.; Woc-Colburn, L.E. 2016 American Association for Thoracic Surgery (AATS) consensus guide-lines: Surgical treatment of infective endocarditis. Executive summary. J. Thorac. Cardiovasc. Surg. 2017, 153, 1241–1258. [Google Scholar] [CrossRef]
- Fedeli, U.; Schievano, E.; Buonfrate, D.; Pellizzer, G.; Spolaore, P. Increasing incidence and mortality of infective endocarditis: A population-based study through a record-linkage system. BMC Infect. Dis. 2011, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Hoen, B.; Duval, X. Clinical practice. Infective endocarditis. N. Engl. J. Med. 2013, 368, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Isaksson, J.; Rasmussen, M.; Nilson, B.; Stadler, L.S.; Kurland, S.; Olaison, L.; Ek, E.; Herrmann, B. Comparison of species identification of endocarditis associated viridans streptococci using rnpB genotyping and 2 MALDI-TOF systems. Diagn. Microbiol. Infect. Dis. 2015, 81, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.E.; Weinstock, G.M. Enterococci: New aspects of an old organism. Proc. Assoc. Am. Physicians 1999, 111, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Richards, M.J.; Edwards, J.R.; Culver, D.H.; Gaynes, R.P. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect. Control Hosp. Epidemiol. 2000, 21, 510–515. [Google Scholar] [CrossRef]
- Megran, D.W. Enterococcal endocarditis. Clin. Infect. Dis. 1992, 15, 63–71. [Google Scholar] [CrossRef]
- Chirouze, C.; Athan, E.; Alla, F.; Chu, V.H.; Ralph Corey, G.; Selton-Suty, C.; Erpelding, M.L.; Miro, J.M.; Olaison, L.; Hoen, B.; et al. Enterococcal endocarditis in the beginning of the 21st century: Analysis from the International Collaboration on Endocarditis-Prospective Cohort Study. Clin. Microbiol. Infect. 2013, 19, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, D.R.; Corey, G.R.; Hoen, B.; Miró, J.M.; Fowler, V.G., Jr.; Bayer, A.S.; Karchmer, A.W.; Olaison, L.; Pappas, P.A.; Moreillon, P.; et al. and the International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: The International Collaboration on Endocarditis-Prospective Cohort Study. Arch. Intern. Med. 2009, 169, 463–473. [Google Scholar]
- Nappi, F.; Martuscelli, G.; Bellomo, F.; Avtaar Singh, S.S.; Moon, M.R. Infective Endocarditis in High-Income Countries. Metabolites 2022, 12, 682. [Google Scholar] [CrossRef]
- Moorjani, N.; Saad, R.; Gallagher, P.; Livesey, S. Endocarditis of the mitral valve posteromedial papillary muscle. J. Card. Surg. 2014, 29, 213–215. [Google Scholar] [CrossRef]
- Caspar, T.; Delabranche, X.; Mazzucotelli, J.P.; Samet, H.; Morel, O.; Ohlmann, P. Left ventricular rupture after embolic myocardial infarction due to mitral valve endocarditis. Echocardiography 2014, 31, E104–E106. [Google Scholar] [CrossRef]
- Tayama, E.; Chihara, S.; Fukunaga, S.; Akashi, H.; Aoyagi, S.; Mizoguchi, K. Embolic myocardial infarction and left ventricular rupture due to mitral valve endocarditis. Ann. Thorac. Cardiovasc. Surg. 2007, 13, 206–208. [Google Scholar] [PubMed]
- Thuny, F.; Avierinos, J.F.; Tribouilloy, C.; Giorgi, R.; Casalta, J.P.; Milandre, L.; Brahim, A.; Nadji, G.; Riberi, A.; Collart, F.; et al. Impact of cerebrovascular complications on mortality and neurologic outcome during infective endocarditis: A prospective multicentre study. Eur. Heart J. 2007, 28, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.N. Homograft replacement of the aortic valve. Lancet 1962, 2, 487. [Google Scholar] [CrossRef]
- Ross, D.; Yacoub, M.H. Homograft replacement of the aortic valve: A critical review. Prog. Cardiovasc. Dis. 1969, 11, 275–293. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.F.; Harrocks, S.; Stafford, E.G.; Gardner, M.A.; Pohlner, P.G.; Tesar, P.J.; Stephens, F. The homograft aortic valve: A 29-year, 99.3% follow up of 1022 valve replacements. J. Heart Valve Dis. 2001, 10, 334–344. [Google Scholar] [PubMed]
- Haydock, D.; Barratt-Boyes, B.; Macedo, T.; Kirklin, J.W.; Blackstone, E. Aortic valve replacement for active infectious endocarditis in 108 patients. A comparison of freehand allograft valves with mechanical prostheses and bioprostheses. J. Thorac. Cardiovasc. Surg. 1992, 103, 130–139. [Google Scholar] [CrossRef]
- McGiffin, D.C.; Galbraith, A.J.; McLachlan, G.J.; Stower, R.E.; Wong, M.L.; Stafford, E.G.; Gardner, M.A.; Pohlner, P.G.; O’Brien, M.F. Aortic valve infection. Risk factors for death and recurrent endocarditis after aortic valve replacement. J. Thorac. Cardiovasc. Surg. 1992, 104, 511–520. [Google Scholar] [CrossRef]
- Nappi, F.; Nenna, A.; Petitti, T.; Spadaccio, C.; Gambardella, I.; Lusini, M.; Chello, M.; Acar, C. Long-term outcome of cryopreserved allograft for aortic valve replacement. J. Thorac. Cardiovasc. Surg. 2018, 156, 1357–1365.e6. [Google Scholar] [CrossRef]
- Jassar, A.S.; Bavaria, J.E.; Szeto, W.Y.; Moeller, P.J.; Maniaci, J.; Milewski, R.K.; Gorman, J.H., 3rd; Desai, N.D.; Gorman, R.C.; Pochettino, A. Graft selection for aortic root replacement in complex active endocarditis: Does it matter? Ann. Thorac. Surg. 2012, 93, 480–487. [Google Scholar] [CrossRef]
- Musci, M.; Weng, Y.; Hubler, M.; Amiri, A.; Pasic, M.; Kosky, S.; Stein, J.; Siniawski, H.; Hetzer, R. Homograft aortic root replacement in native or prosthetic active infective endocarditis: Twenty-year single-center experience. J. Thorac. Cardiovasc. Surg. 2010, 139, 665–673. [Google Scholar] [CrossRef]
- Yankah, A.C.; Pasic, M.; Klose, H.; Siniawski, H.; Weng, Y.; Hetzer, R. Homograft reconstruction of the aortic root for endocarditis with periannular abscess: A 17-year study. Eur. J. Cardiothorac. Surg. 2005, 28, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Witten, J.C.; Durbak, E.; Houghtaling, P.L.; Unai, S.; Roselli, E.E.; Bakaeen, F.G.; Johnston, D.R.; Svensson, L.G.; Jaber, W.; Blackstone, E.H.; et al. Performance and durability of cryopreserved allograft aortic valve replacements. Ann. Thorac. Surg. 2021, 111, 1893–1900. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.R.; Miller, D.C.; Moore, K.A.; Oyer, P.E.; Mitchell, R.S.; Robbins, R.C.; Stinson, E.B.; Shumway, N.E.; Reitz, B.A. Treatment of endocarditis with valve replacement: The question of tissue versus mechanical prosthesis. Ann. Thorac. Surg. 2001, 71, 1164–1171. [Google Scholar] [CrossRef]
- Ratschiller, T.; Sames-Dolzer, E.; Paulus, P.; Schimetta, W.; Muller, H.; Zierer, A.F.; Mair, R. Long-term Evaluation of the Ross Procedure in Acute Infective Endocarditis. Semin. Thorac. Cardiovasc. Surg. 2017, 29, 494–501. [Google Scholar] [CrossRef]
- Johnston, D.R.; Soltesz, E.G.; Vakil, N.; Rajeswaran, J.; Roselli, E.E.; Sabik, J.F.; IIISmedira, N.G.; Svensson, L.G.; Lytle, B.W.; Blackstone, E.H. Long-term durability of bioprosthetic aortic valves: Implications from 12,569 implants. Ann. Thorac. Surg. 2015, 99, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Glaser, N.; Jackson, V.; Holzmann, M.J.; Franco-Cereceda, A.; Sartipy, U. Prosthetic valve endocarditis after surgical aortic valve replacement. Circulation 2017, 136, 329–331. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, N.; Itagaki, S.; Tannous, H.; Egorova, N.N.; Chikwe, J. Bioprosthetic versus mechanical valve replacement for infective endocarditis: Focus on recurrence rates. Ann. Thorac. Surg. 2018, 106, 99–106. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Delahaye, F.; Obadia, J.F.; Duval, X.; Selton-Suty, C.; Carteaux, J.P.; Hoen, B.; Alla, F.; AEPEI study group. Aortic valve replacement for active infective endocarditis: 5-year survival comparison of bioprostheses, homografts and mechanical prostheses. Eur. J. Cardiothorac. Surg. 2010, 37, 1025–1032. [Google Scholar] [CrossRef]
- Ostergaard, L.; Valeur, N.; Ihlemann, N.; Smerup, M.H.; Bundgaard, H.; Gislason, G.; Torp-Pedersen, C.; Bruun, N.E.; Køber, L.; Fosbøl, E.L. Incidence and factors associated with infective endocarditis in patients undergoing left-sided heart valve replacement. Eur. Heart J. 2018, 39, 2668–2675. [Google Scholar] [CrossRef]
- Danneels, P.; Chabrun, F.; Picard, L.; Martinet, P.; Rezig, S.; Lorleac’h, A.; Buzelé, R.; Beaudron, A.; Kempf, M.; Le Moal, G.; et al. Enterococcus faecalis endocarditis risk assessment in patients with bacteremia: External validation of the DENOVA score. J Infect. 2023, 6, S0163-4453. [Google Scholar] [CrossRef] [PubMed]
- Giessel, B.E.; Koenig, C.J.; Blake, R.L., Jr. Management of bacterial endocarditis. Am. Fam. Physician 2000, 61, 1725–1732. [Google Scholar] [PubMed]
- Fridkin, S.K.; Gaynes, R.P. Antimicrobial resistance in intensive care units. Clin. Chest Med. 1999, 20, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Ammerlaan, H.S.M.; Harbarth, S.; Buiting, A.G.M.; Crook, D.W.; Fitzpatrick, F.; Hanberger, H.; Herwaldt, L.A.; van Keulen, P.H.; Kluytmans, J.A.; Kola, A.; et al. Secular trends in nosocomial bloodstream infections: Antibiotic-resistant bacteria increase the total burden of infection. Clin. Infect. Dis. 2013, 56, 798–805. [Google Scholar] [CrossRef]
- Allegranzi, B.; Bagheri Nejad, S.; Combescure, C.; Graafmans, W.; Attar, H.; Donaldson, L.; Pittet, D. Burden of endemic health-care-associated infection in developing countries: Systematic review and metaanalysis. Lancet 2011, 377, 228–241. [Google Scholar] [CrossRef]
- Molton, J.S.; Tambyah, P.A.; Ang, B.S.P.; Ling, M.L.; Fisher, D.A. The global spread of healthcare-associated multidrug-resistant bacteria:a perspective from Asia. Clin. Infect. Dis. 2013, 56, 1310–1318. [Google Scholar]
- Novick, R.P. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol. Microbiol. 2003, 48, 1429–1449. [Google Scholar] [CrossRef]
- Cunningham, M.W. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 2000, 13, 470–511. [Google Scholar] [CrossRef]
- Pillar, C.M.; Gilmore, M.S. Enterococcal virulence-pathogenicity island of E. faecalis. Front. Biosci. 2004, 9, 2335–2346. [Google Scholar] [CrossRef]
- Gilmore, M.S. The Enterococci: Pathogenesis, Molecular Biology, and Antibiotic Resistance; ASM Press: Washington, DC, USA, 2002; 439p. [Google Scholar]
- Rivas, J.M.; Speziale, P.; Patti, J.M.; Hook, M. MSCRAMM-targeted vaccines and immunotherapy for staphylococcal infection. Curr. Opin. Drug Discov. Devel. 2004, 7, 223–227. [Google Scholar]
- Yanagawa, R.; Honda, E. Presence of pili in species of human and animal parasites and pathogens of the genus Corynebacterium. Infect. Immun. 1976, 13, 1293–1295. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.K. Actinomyces: Surface macromolecules and bacteria–host interactions. In Gram-Positive Pathogens; Fischetti, V.A., Novick, R.P., Ferretti, J.J., Portnoy, D.A., Rood, J.I., Eds.; American Society for Microbiology: Washington, DC, USA, 2000; pp. 583–593. [Google Scholar]
- Wu, H.; Fives-Taylor, P.M. Molecular strategies for fimbrial expression and assembly. Crit. Rev. Oral Biol. Med. 2001, 12, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Ton-That, H.; Schneewind, O. Assembly of pili on the surface of C. diphtheriae. Mol. Microbiol. 2003, 50, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Sillanpaa, J.; Xu, Y.; Nallapareddy, S.R.; Murray, B.E.; Hook, M. A family of putative MSCRAMMs from Enterococcusfaecalis. Microbiology 2004, 150, 2069–2078. [Google Scholar] [CrossRef]
- Nappi, F.; Spadaccio, C.; Dreyfus, J.; Attias, D.; Acar, C.; Bando, K. Mitral endocarditis: A new management framework. J. Thorac. Cardiovasc. Surg. 2018, 156, 1486–1495.e4. [Google Scholar] [CrossRef]
- Arabkhani, B.; Bekkers, J.A.; Andrinopoulou, E.-R.; Roos-Hesselink, J.W.; Takkenberg, J.J.M.; Bogers, A.J.J.C. Allografts in aortic position: Insights from a 27-year, single-center prospective study. J. Thorac. Cardiovasc. Surg. 2016, 152, 1572–1579.e3. [Google Scholar] [CrossRef]
- Fukushima, S.; Tesar, P.J.; Pearse, B.; Jalali, H.; Sparks, L.; Fraser, J.F.; Pohlner, P.G. Long-term clinical outcomes after aortic valve replacement using cryopreserved aortic allograft. J. Thorac. Cardiovasc. Surg. 2014, 148, 65–72.e2. [Google Scholar] [CrossRef]
- Kowert, A.; Vogt, F.; Beiras-Fernandez, A.; Reichart, B.; Kilian, E. Outcome after homograft redo operation in aortic position. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio Thorac. Surg. 2012, 41, 404–408. [Google Scholar] [CrossRef]
- Joudinaud, T.M.; Baron, F.; Raffoul, R.; Pagis, B.; Vergnat, M.; Parisot, C.; Hvass, U.; Nataf, P.R. Redo aortic root surgery for failure of an aortic homograft is a major technical challenge. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio Thorac. Surg. 2008, 33, 989–994. [Google Scholar] [CrossRef]
- El-Hamamsy, I.; Eryigit, Z.; Stevens, L.M.; Sarang, Z.; George, R.; Clark, L.; Melina, G.; Takkenberg, J.J.; Yacoub, M.H. Long-term outcomes after autograft versus homograft aortic root replacement in adults with aortic valve disease: A randomized controlled trial. Lancet 2010, 376, 524–531. [Google Scholar] [CrossRef]
- Dignan, R.; O’Brien, M.; Hogan, P.; Passage, J.; Stephens, F.; Thornton, A.; Harrocks, S. Influence of HLA matching and associated factors on aortic valve homograft function. J. Heart Valve Dis. 2000, 9, 504–511. [Google Scholar] [PubMed]
- Brennan, J.M.; Edwards, F.H.; Zhao, Y.; O’Brien, S.; Booth, M.E.; Dokholyan, R.S.; Douglas, P.S.; Peterson, E.D.; DEcIDE AVR (Developing Evidence to Inform Decisions about Effectiveness–Aortic Valve Replacement) Research Team. Long-term safety and effectiveness of mechanical versus biologic aortic valve prostheses in older patients: Results from the Society of Thoracic Surgeons adult cardiac surgery national database. Circulation 2013, 127, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, S.; Jeppsson, A.; Frojd, V.; Svensson, G. Surgical Treatment of Aortic Prosthetic Valve Endocarditis: A 20-Year Single-Center Experience. Ann. Thorac. Surg. 2016, 101, 1426–1432. [Google Scholar] [CrossRef] [PubMed]
- Sabik, J.F.; Lytle, B.W.; Blackstone, E.H.; Marullo, A.G.; Pettersson, G.B.; Cosgrove, D.M. Aortic root replacement with cryopreserved allograft for prosthetic valve endocarditis. Ann. Thorac. Surg. 2002, 74, 650–659. [Google Scholar] [CrossRef]
- Klieverik, L.M.A.; Yacoub, M.H.; Edwards, S.; Bekkers, J.A.; Roos-Hesselink, J.W.; Kappetein, A.P.; Takkenberg, J.J.; Bogers, A.J. Surgical Treatment of Active Native Aortic Valve Endocarditis With Allografts and Mechanical Prostheses. Ann. Thorac. Surg. 2009, 88, 1814–1821. [Google Scholar] [CrossRef]
- Bekkers, J.A.; Klieverik, L.M.; Raap, G.B.; Takkenberg, J.J.; Bogers, A.J. Re-operations for aortic allograft root failure: Experience from a 21-year single-center prospective follow-up study. Eur. J. Cardiothorac. Surg. 2011, 40, 35–42. [Google Scholar] [CrossRef]
- Nappi, F.; Acar, C. Monobloc or Separate Aortic and Mitral Homografts for Endocarditis of the Intervalvular Fibrosa? Ann. Thorac. Surg. 2021, 112, 1382–1383. [Google Scholar] [CrossRef]
- Lalani, T.; Chu, V.H.; Park, L.P.; Cecchi, E.; Corey, G.R.; Durante-Mangoni, E.; Fowler, V.G., Jr.; Gordon, D.; Grossi, P.; Hannan, M.; et al. In-hospital and 1-year mortality in patients undergoing early surgery for prosthetic valve endocarditis. JAMA Intern. Med. 2013, 173, 1495–1504. [Google Scholar] [CrossRef]
- Steffen, V.; Marsch, G.; Burgwitz, K.; Kuehn, C.; Teebken, O.E. Resistance to infection of long-term cryopreserved human aortic valve allografts. J. Thorac. Cardiovasc. Surg. 2016, 151, 1251–1259. [Google Scholar] [CrossRef]
- Kuehn, C.; Graf, K.; Mashaqi, B.; Pichlmaier, M.; Heuer, W.; Hilfiker, A.; Stiesch, M.; Chaberny, I.F.; Haverich, A. Prevention of early vascular graft infection using regional antibiotic release. J. Surg. Res. 2010, 164, 185–191. [Google Scholar] [CrossRef]
- Zander, J.; Maier, B.; Zoller, M.; Dobbeler, G.; Frey, L.; Teupser, D.; Vogeser, M. Effects of biobanking conditions on six antibiotic substances in human serum assessed by a novel evaluation protocol. Clin. Chem. Lab. Med. 2016, 54, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Reece, T.B.; Welke, K.F.; O’Brien, S.; Grau-Sepulveda, M.V.; Grover, F.L.; Gammie, J.S. Rethinking the Ross procedure in adults. Ann. Thorac. Surg. 2014, 97, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Mazine, A.; El-Hamamsy, I.; Verma, S.; Peterson, M.D.; Bonow, R.O.; Yacoub, M.H.; David, T.E.; Bhatt, D.L. Ross Procedure in Adults for Cardiologists and Cardiac Surgeons: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 2761–2777. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Spadaccio, C.; Acar, C.; El-Hamamsy, I. Lights and Shadows on the Ross Procedure: Biological Solutions for Biological Problems. Semin. Thorac. Cardiovasc. Surg. 2020, 32, 815–822. [Google Scholar] [CrossRef]
- Nappi, F.; Avtaar Singh, S.S.; Spadaccio, C.; Acar, C. Ross operation 23 years after surgery: It should not be a “forgotten” option. J. Card. Surg. 2020, 35, 952–956. [Google Scholar] [CrossRef]
- Wang, M.; Furnary, A.P.; Li, H.F.; Grunkemeier, G.L. Bioprosthetic aortic valve durability: A meta-regression of published studies. Ann. Thorac. Surg. 2017, 104, 1080–1087. [Google Scholar] [CrossRef]
- Foroutan, F.; Guyatt, G.H.; O’Brien, K.; Bain, E.; Stein, M.; Bhagra, S.; Sit, D.; Kamran, R.; Chang, Y.; Devji, T.; et al. Prognosis after surgical replacement with a bioprosthetic aortic valve in patients with severe symptomatic aortic stenosis: Systematic review of observational studies. BMJ 2016, 354, i5065. [Google Scholar] [CrossRef]
- David, T.E.; Feindel, C.M.; Bos, J.; Ivanov, J.; Armstrong, S. Aortic valve replacement with Toronto SPV bioprosthesis: Optimal patient survival but suboptimal valve durability. J. Thorac. Cardiovasc. Surg. 2008, 135, 19–24. [Google Scholar] [CrossRef]
- Garrido-Olivares, L.; Maganti, M.; Armstrong, S.; David, T. Aortic valve replacement with Hancock II bioprothesis with and without replacement of the ascending aorta. Ann. Thorac. Surg. 2011, 92, 541–547. [Google Scholar] [CrossRef]
- Bourguignon, T.; Bouquiaux-Stablo, A.L.; Candolfi, P.; Mirza, A.; Loardi, C.; May, M.A.; El-Khoury, R.; Marchand, M.; Aupart, M. Very Long-Term Outcomes of the Carpentier-Edwards Perimount Valve in Aortic Position. Ann. Thorac. Surg. 2015, 99, 831–837. [Google Scholar] [CrossRef]
- Bourguignon, T.; El Khoury, G.; Candolfi, P.; Loardi, C.; Mirza, A.; Boulanger-Lothion, J.; Bouquiaux-Stablo-Duncan, A.L.; Espitalier, F.; Marchand, M.; Aupart, M. Very Long-Term Outcomes of the Carpentier-Edwards Perimount Aortic Valve in Patients Aged 60 or Younger. Ann. Thorac. Surg. 2015, 100, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Sénage, T.; Le Tourneau, T.; Foucher, Y.; Pattier, S.; Cueff, C.; Michel, M.; Serfaty, J.M.; Mugniot, A.; Périgaud, C.; Carton, H.F.; et al. Early structural valve deterioration of Mitroflow aortic bioprosthesis: Mode, incidence, and impact on outcome in a large cohort of patients. Circulation 2014, 130, 2012–2020. [Google Scholar] [CrossRef] [PubMed]
- Flameng, W.; Daenen, W.; Jashari, R.; Herijgers, P.; Meuris, B. Durability of homografts used to treat complex aortic valve endocarditis. Ann. Thorac. Surg. 2015, 99, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Byrne, J.G.; Rezai, K.; Sanchez, J.A.; Bernstein, R.A.; Okum, E.; Leacche, M.; Balaguer, J.M.; Prabhakaran, S.; Bridges, C.R.; Higgins, R.S. Surgical management of endocarditis: The society of thoracic surgeons clinical practice guideline. Ann. Thorac. Surg. 2011, 91, 2012–2019. [Google Scholar] [CrossRef] [PubMed]
- Sonneville, R.; Mirabel, M.; Hajage, D.; Tubach, F.; Vignon, P.; Perez, P.; Lavoué, S.; Kouatchet, A.; Pajot, O.; Mekontso Dessap, A.; et al. Neurologic complications and outcomes of infective endocarditis in critically ill patients: The ENDOcardite en REAnimation prospective multicenter study. Crit. Care Med. 2011, 39, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
- Mirabel, M.; Sonneville, R.; Hajage, D.; Novy, E.; Tubach, F.; Vignon, P.; Perez, P.; Lavoué, S.; Kouatchet, A.; Pajot, O.; et al. Long-term outcomes and cardiac surgery in critically ill patients with infective endocarditis. Eur. Heart J. 2014, 35, 1195–1204. [Google Scholar] [CrossRef]
- Toyoda, N.; Itagaki, S.; Egorova, N.N.; Tannous, H.; Anyanwu, A.C.; El-Eshmawi, A.; Adams, D.H.; Chikwe, J. Real-world outcomes of surgery for native mitral valve endocarditis. J. Thorac. Cardiovasc. Surg. 2017, 154, 1906–1912.e9. [Google Scholar] [CrossRef]
- Kang, D.H.; Kim, Y.J.; Kim, S.H.; Sun, B.J.; Kim, D.H.; Yun, S.C.; Song, J.M.; Choo, S.J.; Chung, C.H.; Song, J.K.; et al. Early surgery versus conventional treatment for infective endocarditis. N. Engl. J. Med. 2012, 366, 2466–2473. [Google Scholar] [CrossRef]
- Nappi, F.; Spadaccio, C. keep fumbling around in the dark when it comes to infective endocarditis, or produce new, reliable data to redesign the guidelines? J. Thorac. Cardiovasc. Surg. 2018, 155, 75–76. [Google Scholar] [CrossRef]
- Liang, F.; Song, B.; Liu, R.; Yang, L.; Tang, H.; Li, Y. Optimal timing for early surgery in infective endocarditis: A meta-analysis. Interact. Cardiovasc. Thorac. Surg. 2016, 22, 336–345. [Google Scholar] [CrossRef]
- Okita, Y.; Minakata, K.; Yasuno, S.; Uozumi, R.; Sato, T.; Ueshima, K.; Konishi, H.; Morita, N.; Harada, M.; Kobayashi, J.; et al. Optimal timing of surgery for active infective endocarditis with cerebral complications: A Japanese multicentre study. Eur. J. Cardiothorac. Surg. 2016, 50, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Spadaccio, C. Simplest solutions are not always the cleverest: Can we stitch in an infected annulus? Should we rethink the current guidelines? J. Thorac. Cardiovasc. Surg. 2017, 154, 1899–1900. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, S.; Kawase, I.; Yamashita, H.; Uchida, S.; Takatoh, M.; Kiyohara, N. Midterm outcomes after aortic valve neocuspidization with glutaraldehyde-treated autologous pericardium. J. Thorac. Cardiovasc. Surg. 2018, 155, 2379–2387. [Google Scholar] [CrossRef]
- Youssefi, P.; Zacek, P.; Debauchez, M.; Lansac, E. Valve-Sparing Aortic Root Replacement Using the Remodeling Technique With Aortic Annuloplasty: Bicuspid Valves With Repair of Specific Lesion Sets: How I Teach It. Ann. Thorac. Surg. 2019, 108, 324–333. [Google Scholar] [CrossRef]
- de Kerchove, L.; Vanoverschelde, J.L.; Poncelet, A.; Glineur, D.; Rubay, J.; Zech, F.; Noirhomme, P.; El Khoury, G. Reconstructive surgery in active mitral valve endocarditis: Feasibility, safety and durability. Eur. J. Cardiothorac. Surg. 2007, 31, 592–599. [Google Scholar] [CrossRef]
- Mestres, C.A.; Castella, M.; Moreno, A.; Pare, J.C.; del Rio, A.; Azqueta, M.; Fernández, C.; Miró, J.M.; Pomar, J.L.; Hospital Clínico Endocarditis Study Group. Cryopreserved mitral homograft in the tricuspid position for infective endocarditis: A valve that can be repaired in the long-term (13 years). J. Heart Valve Dis. 2006, 15, 389–391. [Google Scholar] [PubMed]
- Acar, C.; Farge, A.; Ramsheyi, A.; Chachques, J.C.; Mihaileanu, S.; Gouezo, R.; Gerota, J.; Carpentier, A.F. Mitral valve replacement using a cryopreserved mitral homograft. Ann. Thorac. Surg. 1994, 57, 746–748. [Google Scholar] [CrossRef]
- Elgharably, H.; Hakim, A.H.; Unai, S.; Hussain, S.T.; Shrestha, N.K.; Gordon, S.; Rodriguez, L.; Gillinov, A.M.; Svensson, L.G.; Navia, J.L. The incorporated aortomitral homograft for double-valve endocarditis: The “hemi-Commando” procedure. Early and mid-term outcomes. Eur. J. Cardiothorac. Surg. 2018, 53, 1055–1061. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, B. Are valve repairs associated with better outcomes than replacements in patients with native active valve endocarditis? Interact. Cardiovasc. Thorac. Surg. 2014, 19, 1036–1039. [Google Scholar] [CrossRef]
- Bando, K. Does type of prosthesis affect long-term outcomes after aortic valve replacement for infective endocarditis? How should we properly answer this question? J. Thorac. Cardiovasc. Surg. 2017, 153, 829–830. [Google Scholar] [CrossRef]
- Greason, K.L.; Thomas, M.; Steckelberg, J.M.; Daly, R.C.; Schaff, H.V.; Li, Z.; Dearani, J.A. Outcomes of surgery in the treatment of isolated nonnative mitral valve infective endocarditis. J. Thorac. Cardiovasc. Surg. 2014, 147, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Yun, K.L.; Sintek, C.F.; Miller, D.C.; Pfeffer, T.A.; Kochamba, G.S.; Khonsari, S.; Zile, M.R. Randomized trial comparing partial versus complete chordal-sparing mitral valve replacement: Effects on left ventricular volume and function. J. Thorac. Cardiovasc. Surg. 2002, 123, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Savage, E.B.; Saha-Chaudhuri, P.; Asher, C.R.; Brennan, J.M.; Gammie, J.S. Outcomes and prosthesis choice for active aortic valve infective endocarditis: Analysis of the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann. Thorac. Surg. 2014, 98, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Acar, C.; Tolan, M.; Berrebi, A.; Gaer, J.; Gouezo, R.; Marchix, T.; Gerota, J.; Chauvaud, S.; Fabiani, J.N.; Deloche, A.; et al. Homograft replacement of the mitral valve. Graft selection, technique of implantation, and results in forty-three patients. J. Thorac. Cardiovasc. Surg. 1996, 111, 367–378, discussion 78–80. [Google Scholar] [CrossRef]
- Ali, M.; Iung, B.; Lansac, E.; Bruneval, P.; Acar, C. Homograft replacement of the mitral valve: Eight-year results. J. Thorac. Cardiovasc. Surg. 2004, 128, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Doty, D.B.; Acar, C. Mitral valve replacement with homograft. Ann. Thorac. Surg. 1998, 66, 2127–2131. [Google Scholar] [CrossRef]
- Kumar, A.S.; Choudhary, S.K.; Mathur, A.; Saxena, A.; Roy, R.; Chopra, P. Homograft mitral valve replacement: Five years’ results. J. Thorac. Cardiovasc. Surg. 2000, 120, 450–458. [Google Scholar] [CrossRef]
- Yankah, A.C.; Sievers, H.H.; Lange, P.E.; Bernhard, A. Clinical report on stentless mitral allografts. J. Heart Valve Dis. 1995, 4, 40–44. [Google Scholar]
- Olivito, S.; Lalande, S.; Nappi, F.; Hammoudi, N.; D’Alessandro, C.; Fouret, P.; Acar, C. Structural deterioration of the cryopreserved mitral homograft valve. J. Thorac. Cardiovasc. Surg. 2012, 144, 313–320.e1. [Google Scholar] [CrossRef]
- Gould, F.K.; Denning, D.W.; Elliott, T.S.J.; Foweraker, J.; Perry, J.D.; Prendergast, B.D.; Sandoe, J.A.; Spry, M.J.; Watkin, R.W. Working Party of the British Society for Antimicrobial Chemotherapy. Guidelines for the diagnosis and antibiotic treatment of endocarditis in adults: A report of the Working Party of the British Society for Antimicrobial Chemotherapy. J. Antimicrob. Chemother. 2012, 67, 269–289. [Google Scholar] [CrossRef]
- Nappi, F. The Cryopreserved Mitral Homograft Valve: 19 Years’ Experience. JACC Cardiovasc. Interv. 2014, 7, S58. [Google Scholar] [CrossRef]
- Sadler, L.; McCowan, L.; White, H.; Stewart, A.; Bracken, M.; North, R. Pregnancy outcomes and cardiac complications in women with mechanical, bioprosthetic and homograft valves. BJOG Int. J. Obstet. Gynaecol. 2000, 107, 245–253. [Google Scholar] [CrossRef]
- Nappi, F.; Spadaccio, C.; Chello, M.; Lusini, M.; Acar, C. Impact of structural valve deterioration on outcomes in the cryopreserved mitral homograft valve. J. Card. Surg. 2014, 29, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Obadia, J.F.; Henaine, R.; Bergerot, C.; Ginon, I.; Nataf, P.; Chavanis, N.; Robin, J.; André-Fouët, X.; Ninet, J.; Raisky, O. Monobloc aorto-mitral homograft or mechanical valve replacement: A new surgical option for extensive bivalvular endocarditis. J. Thorac. Cardiovasc. Surg. 2006, 131, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Butt, J.H.; Ihlemann, N.; De Backer, O.; Sondergaard, L.; Havers-Borgersen, E.; Gislason, G.H.; Torp-Pedersen, C.; Køber, L.; Fosbøl, E.L. Long-term risk of infective endocarditis after transcatheter aortic valve replacement. J. Am. Coll. Cardiol. 2019, 73, 1646–1655. [Google Scholar] [CrossRef]
- Nappi, F.; Nenna, A.; Spadaccio, C.; Avtaar Singh, S.S.; Almazil, A.; Acar, C. The Use of the Cryopreserved Aortic Homograft for Aortic Valve Replacement: Is It Still an Option? Cardiovasc. Dev. Dis. 2023, 10, 248. [Google Scholar] [CrossRef]
- Bonn, D. “Serious concerns” over CryoLife heart valves. Lancet Infect. Dis. 2002, 2, 587. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Candida albicans endocarditis associated with a contaminated aortic valve allograft–California, 1996. MMWR Morb Mortal Wkly Rep. 1997, 46, 261–263. [Google Scholar]
- d’Udekem, Y.; David, T.E.; Feindel, C.M.; Armstrong, S.; Sun, Z. Long-term results of operation for paravalvular abscess. Ann. Thorac. Surg. 1996, 62, 48–53. [Google Scholar] [CrossRef]
- Solari, S.; Mastrobuoni, S.; De Kerchove, L.; Navarra, E.; Astarci, P.; Noirhomme, P.; Rubay, J.; El Khoury, G. Over 20 years experience with aortic homograft in aortic valve replacement during acute infective endocarditis. Eur. J. Cardiothorac. Surg. 2016, 50, 1158–1164. [Google Scholar] [CrossRef]
- Svensson, L.G.; Pillai, S.T.; Rajeswaran, J.; Desai, M.Y.; Griffin, B.; Grimm, R.; Hammer, D.F.; Thamilarasan, M.; Roselli, E.E.; Pettersson, G.B.; et al. Long-term survival, valve durability, and reoperation for 4 aortic root procedures combined with ascending aorta replacement. J. Thorac. Cardiovasc. Surg. 2016, 151, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Navia, J.L.; Elgharably, H.; Hakim, A.H.; Witten, J.C.; Haupt, M.J.; Germano, E.; Houghtaling, P.L.; Bakaeen, F.G.; Pettersson, G.B.; Lytle, B.W.; et al. Long-term Outcomes of Surgery for Invasive Valvular Endocarditis Involving the Aortomitral Fibrosa. Ann. Thorac. Surg. 2019, 108, 1314–1323. [Google Scholar] [CrossRef] [PubMed]

| Items | Specification |
|---|---|
| Date of Search (specified to date, month, and year) | From February 2023 to June 2023 |
| Databases and other sources searched | MEDLINE, Embase, and the Cochrane Library |
| Search terms used (including MeSH and free text search terms and filters) | “endocarditis” or “infective endocarditis” together with “allograft”, “homograft”, “allograft endocarditis”, “pathogenesis”, “manifestations”, “treatment”, and “surgery” |
| Timeframe | Up to June 2023 |
| Inclusion and exclusion criteria (study type, language restrictions etc.) | English language |
| Selection process | Two authors independently selected articles after screening for duplicates |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nappi, F.; Schoell, T.; Spadaccio, C.; Acar, C.; da Costa, F.D.A. A Literature Review on the Use of Aortic Allografts in Modern Cardiac Surgery for the Treatment of Infective Endocarditis: Is There Clear Evidence or Is It Merely a Perception? Life 2023, 13, 1980. https://doi.org/10.3390/life13101980
Nappi F, Schoell T, Spadaccio C, Acar C, da Costa FDA. A Literature Review on the Use of Aortic Allografts in Modern Cardiac Surgery for the Treatment of Infective Endocarditis: Is There Clear Evidence or Is It Merely a Perception? Life. 2023; 13(10):1980. https://doi.org/10.3390/life13101980
Chicago/Turabian StyleNappi, Francesco, Thibaut Schoell, Cristiano Spadaccio, Christophe Acar, and Francisco Diniz Affonso da Costa. 2023. "A Literature Review on the Use of Aortic Allografts in Modern Cardiac Surgery for the Treatment of Infective Endocarditis: Is There Clear Evidence or Is It Merely a Perception?" Life 13, no. 10: 1980. https://doi.org/10.3390/life13101980






