Identification of Glycoxidative Lesion in Isolated Low-Density Lipoproteins from Diabetes Mellitus Subjects
Abstract
:1. Introduction
2. Results
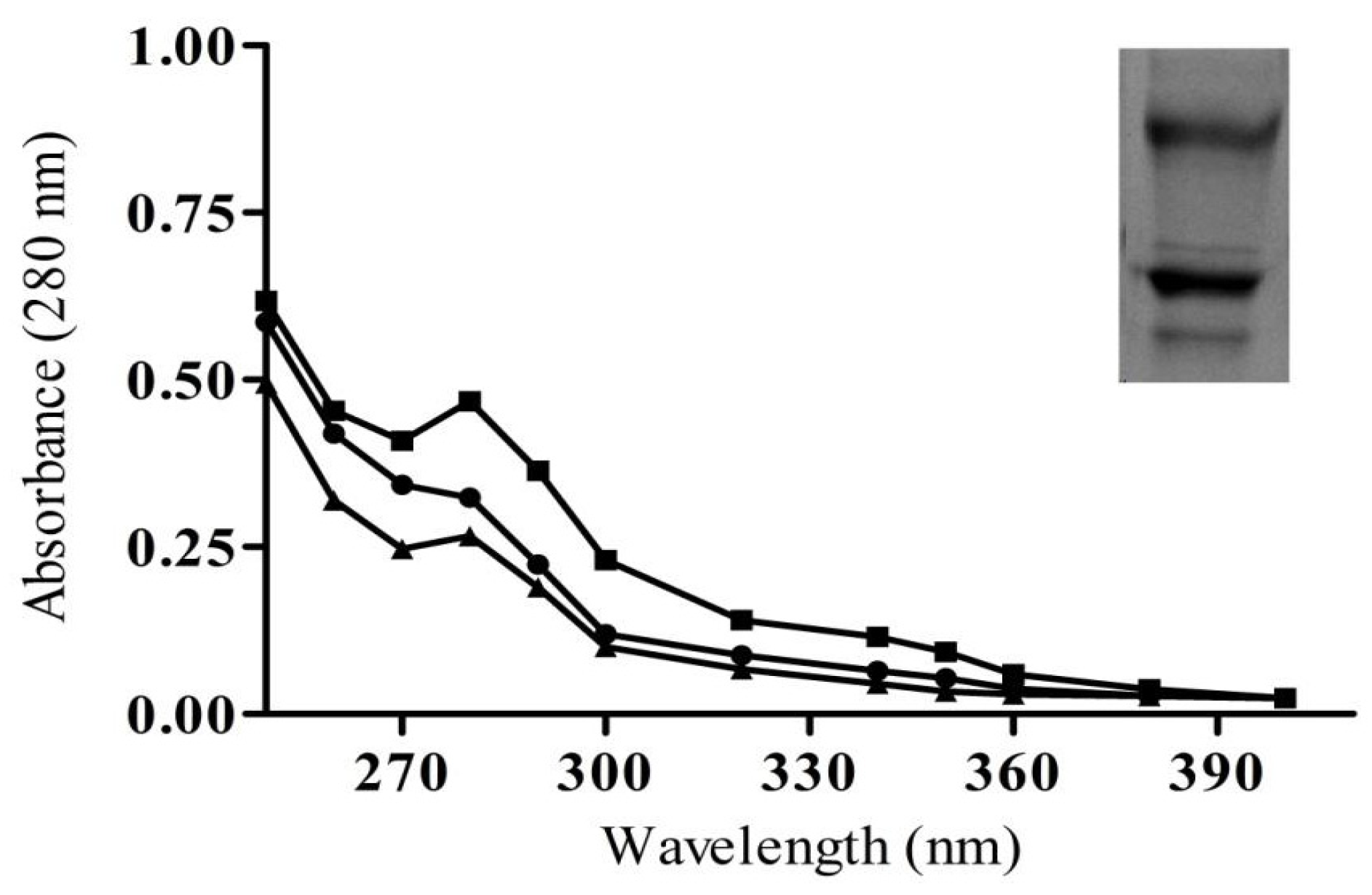
2.1. Modification Detection in Glycated LDL
2.2. Biochemical Estimation-Ketoamines, HMF, Protein-Carbonyl, and CML in Sera Samples
2.3. Characterization of Purified IgG from Immunized Animals
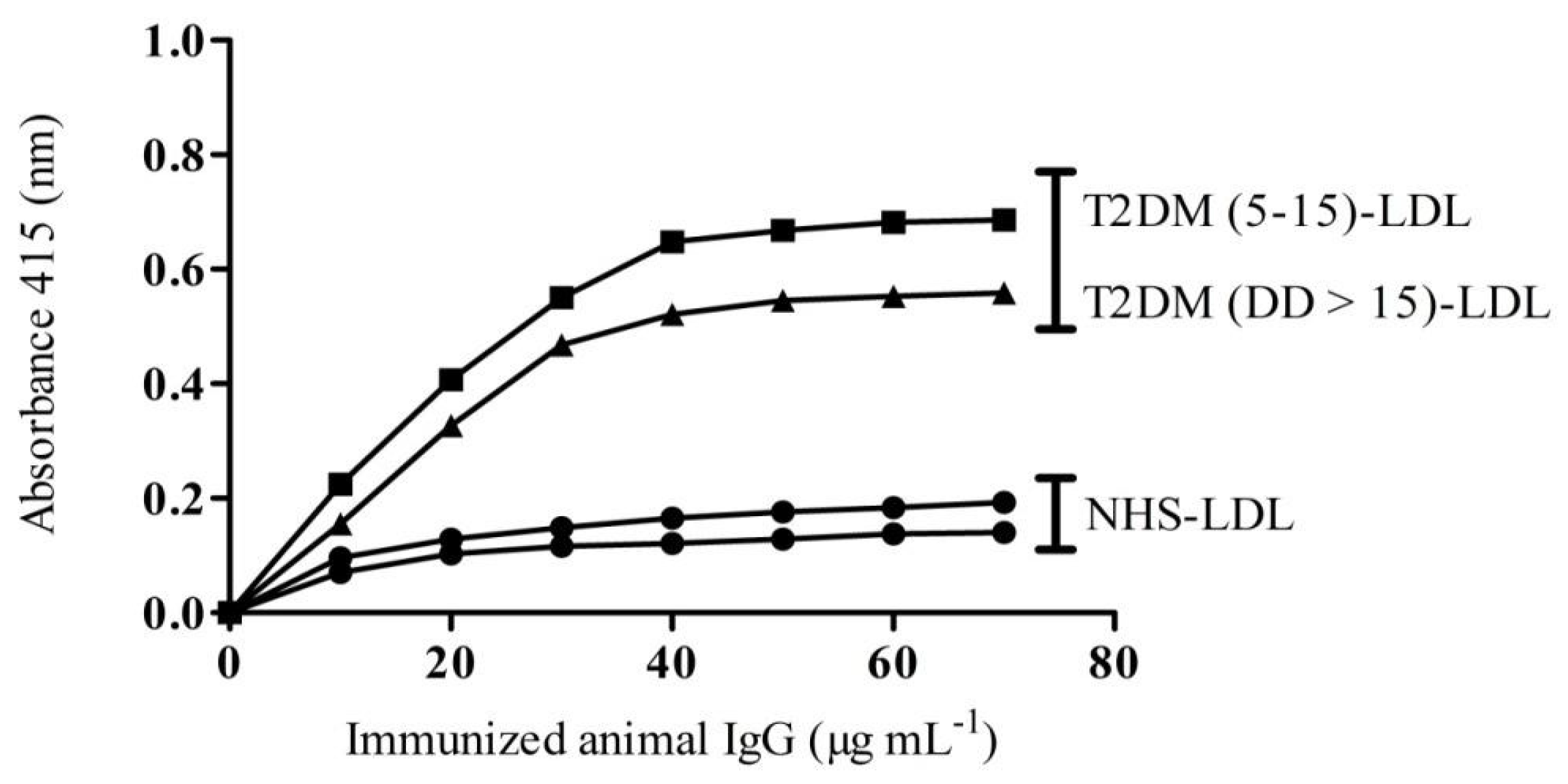
2.4. Binding Affinity of IgG from Experimental Animals towards Patients LDL
2.5. Glycoxidative lesion Detection by Inhibition ELISA
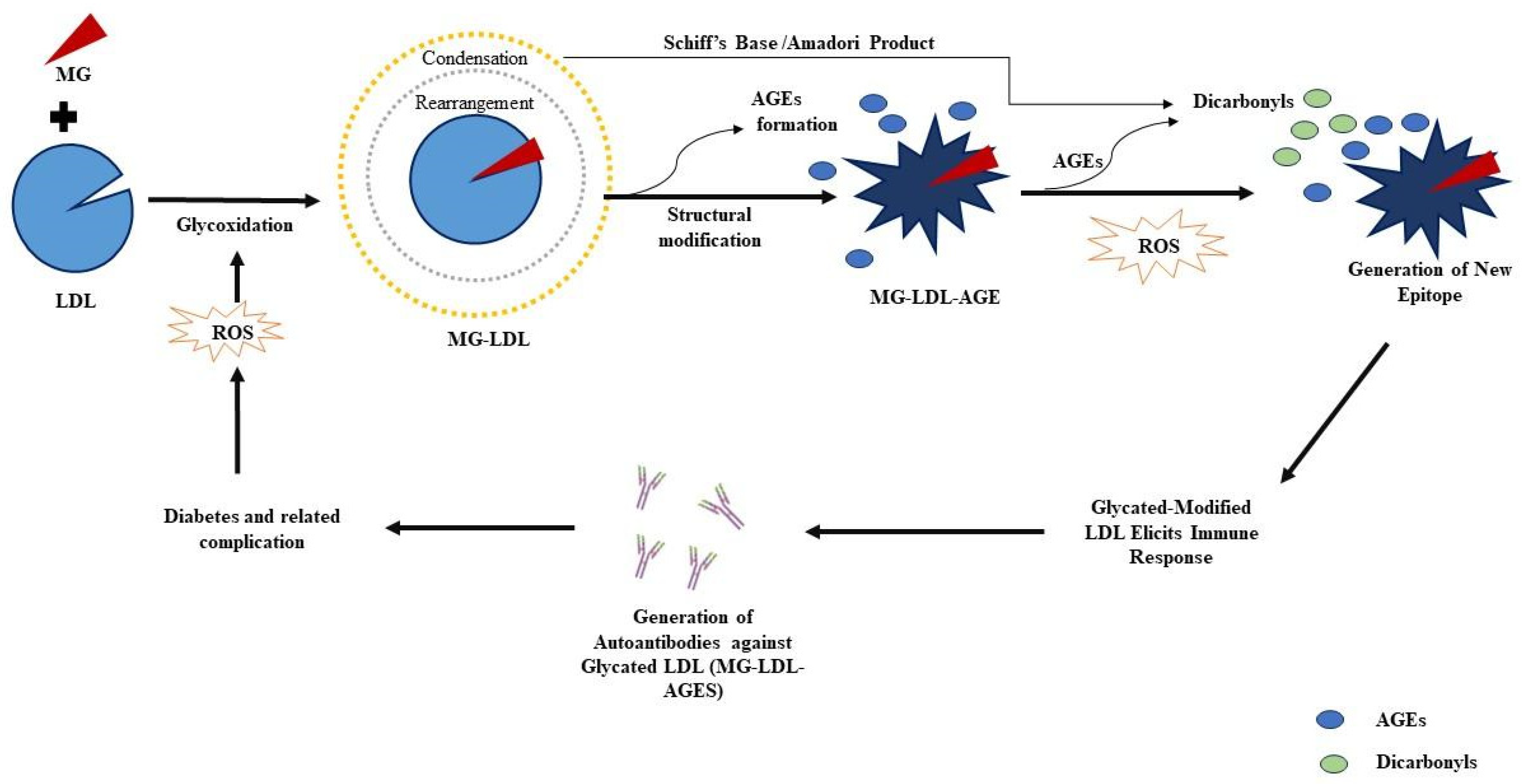
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
4.2. Precipitation of LDL from Human Plasma
4.3. Glycation of LDL
4.4. Early Glycation Detection-Nitroblue Tetrazolium (NBT) Assay
4.5. Hydroxymethylfurfural (HMF) Estimation
4.6. Glycation Intermediates-Protein Carbonyl Estimation
4.7. Evaluation of Nonfluorogenic CML-AGE
4.8. Preparation of Anti-MG-LDL Antibodies (MG-LDL-IgG)
4.9. Direct Binding ELISA
4.10. Inhibition ELISA-Glycative Lesion Detection
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yazihan, N.; Kacar, M. The Roles of HIFs in the Complications of Diabetes. In Biochemistry of Cardiovascular Dysfunction in Obesity; Springer: Cham, Switzerland, 2020; pp. 145–160. [Google Scholar]
- Negre-Salvayre, A.; Salvayre, R.; Auge, N.; Pamplona, R.; Portero-Otin, M. Hyperglycemia and glycation in diabetic complications.Antioxid. Redox Signal 2009, 11, 3071–3109. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Xue, M.; Thornalley, P.J. Dicarbonyl stress, protein glycation and the unfolded protein response. Glycoconj. J. 2021, 38, 331–340. [Google Scholar] [CrossRef]
- Schalkwijk, C.G.; Stehouwer, C.D.A. Methylglyoxal, a highly reactive dicarbonyl compound, in diabetes, its vascular complications, and other age-related diseases. Physiol. Rev. 2020, 100, 407–461. [Google Scholar] [CrossRef]
- Dhar, A.; Desai, K.; Kazachmov, M.; Yu, P.; Wu, L. Methylglyoxal production in vascular smooth muscle cells from different metabolic precursors. Metabolism 2008, 57, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Sun, H.; Sun, Z. Advanced glycation end products (AGEs) increase renal lipid accumulation: A pathogenic factor of diabetic nephropathy (DN). Lipids Health Dis. 2017, 16, 126. [Google Scholar] [CrossRef] [PubMed]
- Van Eupen, M.G.A.; Schram, M.T.; Colhoun, H.M.; Hanssen, N.M.J.; Niessen, H.W.M.; Tarnow, L.; Schalkwijk, C.G. The methylglyoxal-derived AGE tetrahydropyrimidine is increased in plasma of individuals with type 1 diabetes mellitus and in atherosclerotic lesions and is associated with sVCAM-1. Diabetologia 2013, 56, 1845–1855. [Google Scholar] [CrossRef]
- Chehade, J.M.; Gladysz, M.; Mooradian, A.D. Dyslipidemia in type 2 diabetes: Prevalence, pathophysiology, and management. Drugs 2013, 73, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Mooradian, A.D. Dyslipidemia in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2009, 5, 150–159. [Google Scholar] [CrossRef]
- Banach, M.; Surma, S.; Reiner, Z.; Katsiki, N.; Penson, P.E.; Fras, Z.; Sahebkar, A.; Paneni, F.; Rizzo, M.; Kastelein, J. Personalized management of dyslipidemias in patients with diabetes—It is time for a new approach. Cardiovasc. Diabetol. 2022, 21, 263. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.V.; Sukhorukov, V.N.; Surkova, R.; Orekhov, N.A.; Orekhov, A.N. Glycation of LDL: AGEs, impact on lipoprotein function, and involvement in atherosclerosis. Front. Cardiovasc. Med. 2023, 10, 1094188. [Google Scholar] [CrossRef] [PubMed]
- Waqas, K.; Muller, M.; Koedam, M.; El Kadi, Y.; Zillikens, M.C.; van der Eerden, B.C.J. Methylglyoxal–an advanced glycation end products (AGEs) precursor–Inhibits differentiation of human MSC-derived osteoblasts in vitro independently of receptor for AGEs (RAGE). Bone 2022, 164, 116526. [Google Scholar] [CrossRef]
- Karve, T.M.; Cheema, A.K. Small changes huge impact: The role of protein posttranslational modifications in cellular homeostasis and disease. J. Amino Acids. 2011, 2011, 207691. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Quesada, J.L.; Pérez, A. Modified lipoproteins as biomarkers of cardiovascular risk in diabetes mellitus. Endocrinol. Nutr. 2013, 60, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, F.; Habibi-Rezaei, M.; Amani, M.; Saboury, A.A.; Moosavi-Movahedi, A.A. The status of glycation in protein aggregation. Int. J. Biol. Macromol. 2017, 100, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Y.; Alouffi, S.; Ahmad, S. Immunochemical studies on native and glycated LDL–An approach to uncover the structural perturbations. Int. J. Biol. Macromol. 2018, 115, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Y.; Alouffi, S.; Khan, M.S.; Husain, F.M.; Akhter, F.; Ahmad, S. The neoepitopes on methylglyoxal (MG) glycated LDL create autoimmune response; autoimmunity detection in T2DM patients with varying disease duration. Cell. Immunol. 2020, 351, 104062. [Google Scholar] [CrossRef] [PubMed]
- Rizwee, M.M.; Abidi, M.; Habib, S.; Mir, A.R.; Ali, A.; Uddin, M. Characterization of Glyoxal Modified LDL: Role in the Generation of Circulating Autoantibodies in Type 2 Diabetes Mellitus and Coronary Artery Disease. Curr. Drug Targets 2021, 18, 1027–1040. [Google Scholar]
- Siddiqui, Z.; Faisal, M.; Alatar, A.R.; Ahmad, S. Prevalence of auto-antibodies against D-ribose-glycated-hemoglobin in diabetes mellitus. Glycobiology 2019, 29, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Imanaga, Y.; Sakata, N.; Takebayashi, S.; Matsunaga, A.; Sasaki, J.; Arakawa, K.; Nagai, R.; Horiuchi, S.; Itabe, H.; Takano, T. In vivo and in vitro evidence for the glycoxidation of low density lipoprotein in human atherosclerotic plaques. Atherosclerosis 2000, 150, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Shahab, U.; Ahmad, S.; Dixit, K.; Abidi, S.A.; Alam, K.; Ali, A. Acquired immunogenicity of human DNA damaged by N-hydroxy-N-acetyl-4-aminobiphenyl. IUBMB Life 2012, 64, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Virella, G.; Thorpe, S.R.; Alderson, N.L.; Stephan, E.M.; Atchley, D.; Wagner, F.; Lopes-Virella, M.F. Autoimmune response to advanced glycosylation end-products of human LDL. J. Lipid Res. 2003, 44, 487–493. [Google Scholar] [CrossRef]
- Vlassara, H. Advanced glycation end-products and atherosclerosis. Ann. Med. 1996, 28, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Shuck, S.C.; Achenbach, P.; Roep, B.O.; Termini, J.S.; Hernandez-Castillo, C.; Winkler, C.; Weiss, A.; Ziegler, A.G. Methylglyoxal products in pre-symptomatic type 1 diabetes. Front. Endocrinol. 2023, 14, 1108910. [Google Scholar] [CrossRef]
- Wieland, H.; Seidel, D.A. simple specific method for precipitation of low density lipoproteins. J. Lipid Res. 1983, 24, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Sugiuchi, H.; Irie, T.; Uji, Y.; Ueno, T.; Chaen, T.; Uekama, K.; Okabe, H. Homogeneous assay for measuring low-density lipoprotein cholesterol in serum with triblock copolymer and α-cyclodextrin sulfate. Clin. Chem. 1998, 44, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Alouffi, S.; Khan, S.; Khan, M.; Akasha, R.; Ashraf, J.M.; Farhan, M.; Shahab, U.; Khan, M.Y. Physicochemical Characterization of In Vitro LDL Glycation and Its Inhibition by Ellagic Acid (EA): An In Vivo Approach to Inhibit Diabetes in Experimental Animals. Biomed. Res. Int. 2022, 2022, 5583298. [Google Scholar] [CrossRef]
- Alenazi, F.; Saleem, M.; Khaja, A.S.; Zafar, M.; Alharbi, M.S.; Al Hagbani, T.; Khan, M.Y.; Ahmad, S. Glycation of Immunoglobulin-G from Pentose Sugar: A Cause for Structural Perturbations. Curr. Protein Pept. Sci. 2022, 23, 773–781. [Google Scholar] [CrossRef]
- Rufián-Henares, J.A.; De la Cueva, S.P. Assessment of hydroxymethylfurfural intake in the Spanish diet. Food Addit. Contam. 2008, 25, 1306–1312. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef]
- Rehman, S.; Alouffi, S.; Faisal, M.; Qahtan, A.A.; Alatar, A.A.; Ahmad, S. Methylglyoxal mediated glycation leads to neo-epitopes generation in fibrinogen: Role in the induction of adaptive immune response. Int. J. Biol. Macromol. 2021, 175, 535–543. [Google Scholar] [CrossRef]
- Shahab, U.; Ahmad, S.; Dixit, K.; Habib, S.; Alam, K.; Ali, A. Genotoxic effect of N-hydroxy-4-acetylaminobiphenyl on human DNA: Implications in bladder cancer. PLoS ONE 2013, 8, e53205. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alyahyawi, A.R.; Khan, M.Y.; Alouffi, S.; Maarfi, F.; Akasha, R.; Khan, S.; Rafi, Z.; Alharazi, T.; Shahab, U.; Ahmad, S. Identification of Glycoxidative Lesion in Isolated Low-Density Lipoproteins from Diabetes Mellitus Subjects. Life 2023, 13, 1986. https://doi.org/10.3390/life13101986
Alyahyawi AR, Khan MY, Alouffi S, Maarfi F, Akasha R, Khan S, Rafi Z, Alharazi T, Shahab U, Ahmad S. Identification of Glycoxidative Lesion in Isolated Low-Density Lipoproteins from Diabetes Mellitus Subjects. Life. 2023; 13(10):1986. https://doi.org/10.3390/life13101986
Chicago/Turabian StyleAlyahyawi, Amjad R., Mohd Yasir Khan, Sultan Alouffi, Farah Maarfi, Rihab Akasha, Saif Khan, Zeeshan Rafi, Talal Alharazi, Uzma Shahab, and Saheem Ahmad. 2023. "Identification of Glycoxidative Lesion in Isolated Low-Density Lipoproteins from Diabetes Mellitus Subjects" Life 13, no. 10: 1986. https://doi.org/10.3390/life13101986
APA StyleAlyahyawi, A. R., Khan, M. Y., Alouffi, S., Maarfi, F., Akasha, R., Khan, S., Rafi, Z., Alharazi, T., Shahab, U., & Ahmad, S. (2023). Identification of Glycoxidative Lesion in Isolated Low-Density Lipoproteins from Diabetes Mellitus Subjects. Life, 13(10), 1986. https://doi.org/10.3390/life13101986







