Localization of Calretinin, Parvalbumin, and S100 Protein in Nothobranchius guentheri Retina: A Suitable Model for the Retina Aging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish and Tissue Treatment
2.2. Immunohistochemistry
2.3. Statistical Analysis
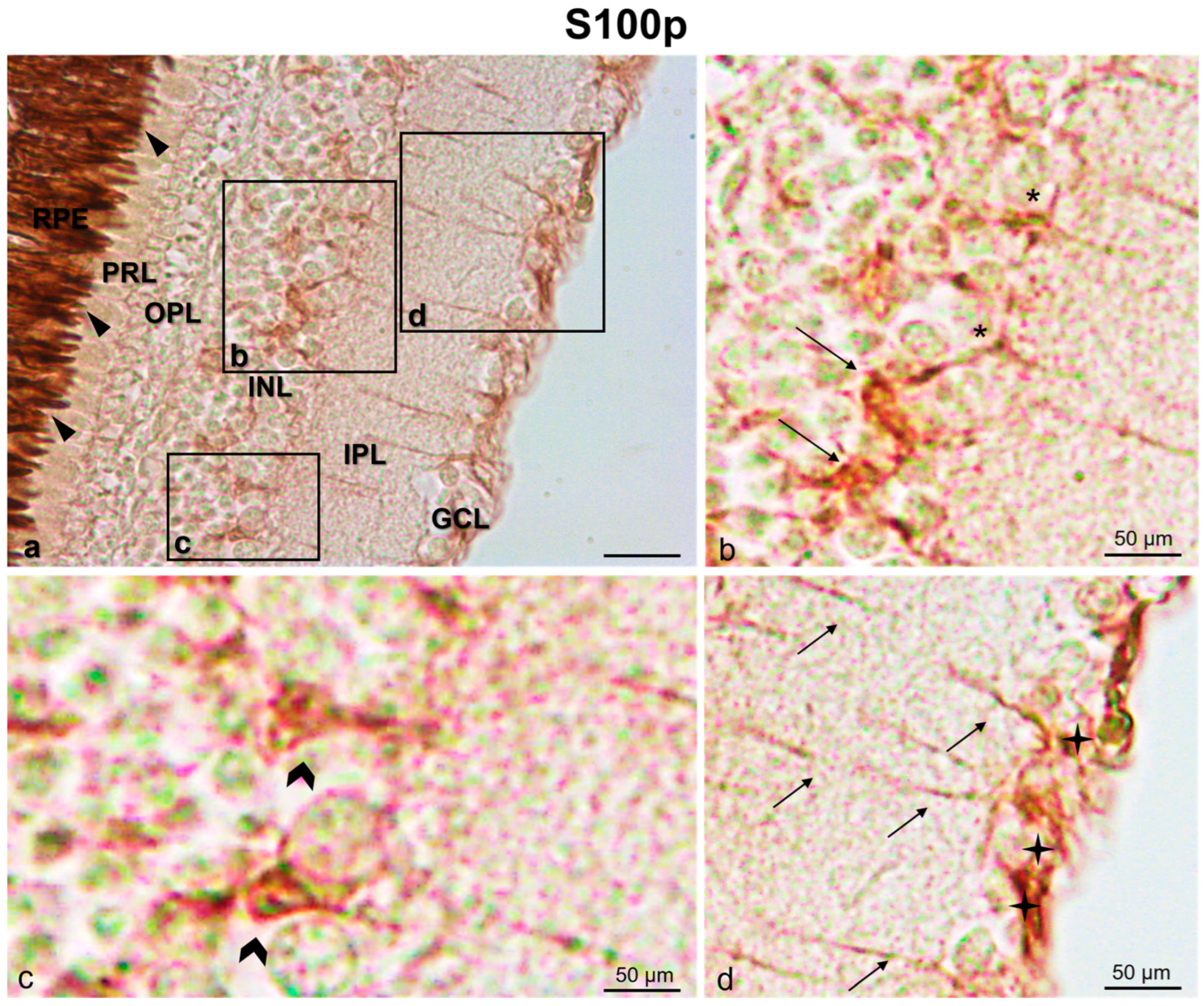
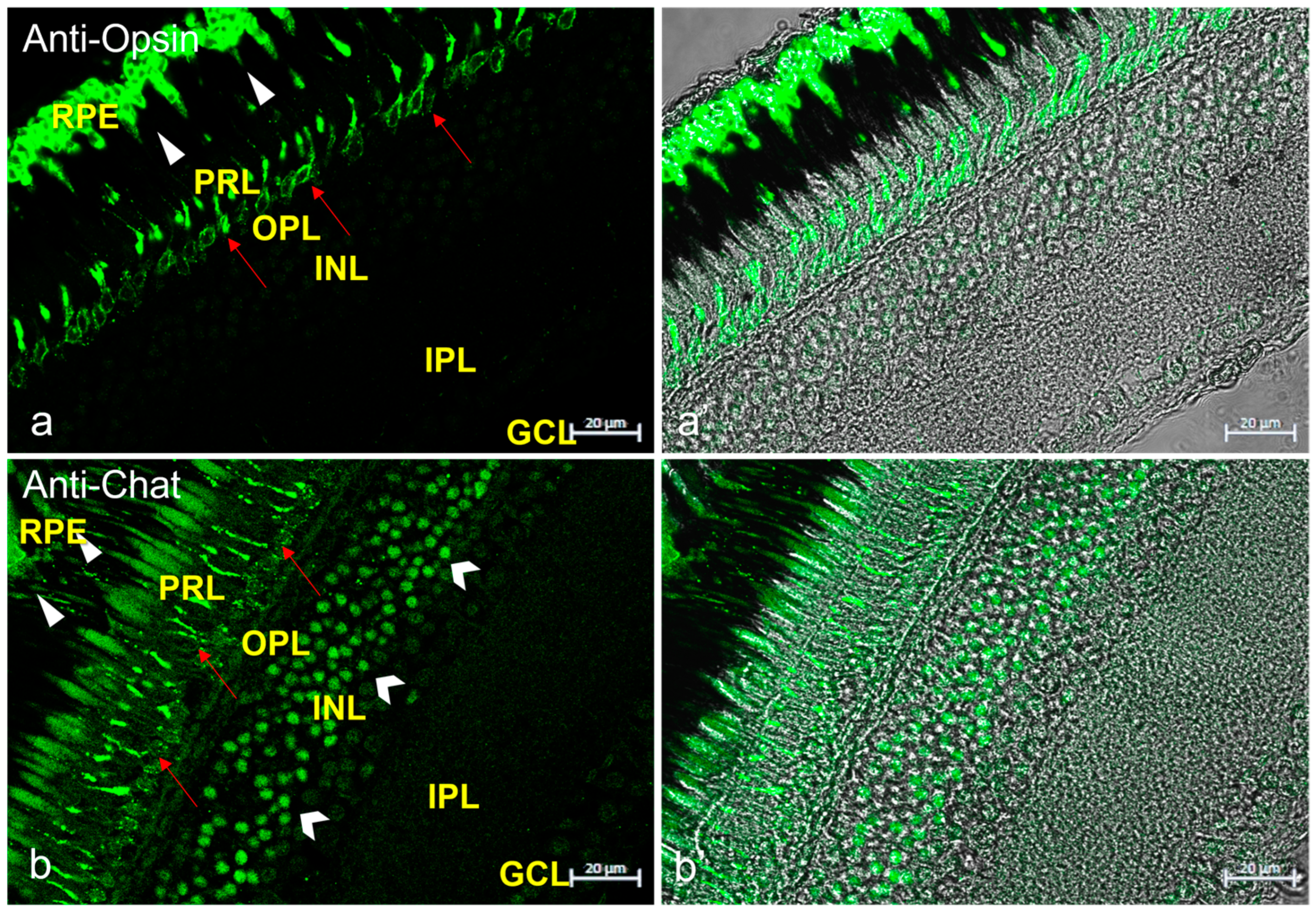
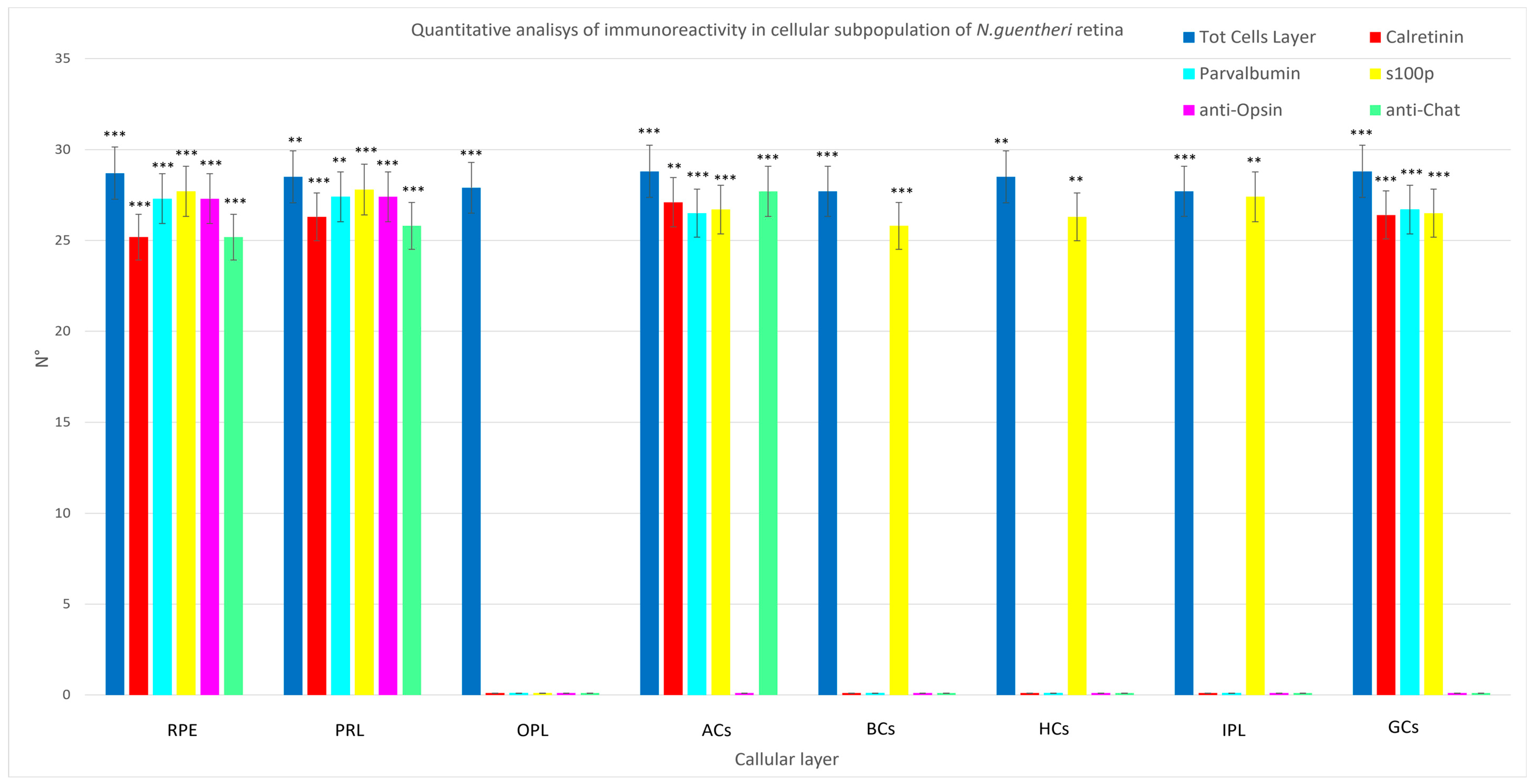
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Purves, D.; Augustine, G.; Fitzpatrick, D.; Hall, W.; LaMantia, A.; McNamara, E.; White, J.O. Neuroscience; Sinauer Associates: Sunderland, MA, USA, 2008; p. 857. [Google Scholar]
- Schoch, S.; Gundelfinger, E.D. Molecular organization of the presynaptic active zone. Cell Tissue Res. 2006, 326, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Südhof, T.C. The Synaptic Vesicle Cycle. Annu. Rev. Neurosci. 2004, 27, 509–547. [Google Scholar] [CrossRef]
- Zhai, R.G.; Bellen, H.J. The Architecture of the Active Zone in the Presynaptic Nerve Terminal. Physiology 2004, 19, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Dresbach, T.; Qualmann, B.; Kessels, M.M.; Garner, C.C.; Gundelfinger, E.D. The presynaptic cytomatrix of brain synapses. Cell. Mol. Life Sci. CMLS 2001, 58, 94–116. [Google Scholar] [CrossRef] [PubMed]
- Südhof, T.C. Neurotransmitter release: The last millisecond in the life of a synaptic vesicle. Neuron 2013, 80, 675–690. [Google Scholar] [CrossRef]
- Boczek, T.; Mackiewicz, J.; Sobolczyk, M.; Wawrzyniak, J.; Lisek, M.; Ferenc, B.; Guo, F.; Zylinska, L. The Role of G Protein-Coupled Receptors (GPCRs) and Calcium Signaling in Schizophrenia. Focus on GPCRs Activated by Neurotransmitters and Chemokines. Cells 2021, 10, 1228. [Google Scholar] [CrossRef] [PubMed]
- Dhyani, V.; Gare, S.; Gupta, R.K.; Swain, S.; Venkatesh, K.V.; Giri, L. GPCR mediated control of calcium dynamics: A systems perspective. Cell Signal. 2020, 74, 109717. [Google Scholar] [CrossRef]
- Roberts, W.M. Localization of calcium signals by a mobile calcium buffer in frog saccular hair cells. J. Neurosci. 1994, 14, 3246. [Google Scholar] [CrossRef]
- Neher, E. The influence of intracellular calcium concentration on degranulation of dialysed mast cells from rat peritoneum. J. Physiol. 1988, 395, 193–214. [Google Scholar] [CrossRef]
- Stern, M.D. Buffering of calcium in the vicinity of a channel pore. Cell Calcium 1992, 13, 183–192. [Google Scholar] [CrossRef]
- Castro, A.; Becerra, M.; Anadón, R.; Manso, M.J. Distribution of calretinin during development of the olfactory system in the brown trout, Salmo trutta fario: Comparison with other immunohistochemical markers. J. Chem. Neuroanat. 2008, 35, 306–316. [Google Scholar] [CrossRef]
- Castro, A.; Becerra, M.; Manso, M.J.; Anadón, R. Calretinin immunoreactivity in the brain of the zebrafish, Danio rerio: Distribution and comparison with some neuropeptides and neurotransmitter-synthesizing enzymes. I. Olfactory organ and forebrain. J. Comp. Neurol. 2006, 494, 435–459. [Google Scholar] [CrossRef]
- Germanà, A.; Paruta, S.; Germanà, G.P.; Ochoa-Erena, F.J.; Montalbano, G.; Cobo, J.; Vega, J.A. Differential distribution of S100 protein and calretinin in mechanosensory and chemosensory cells of adult zebrafish (Danio rerio). Brain Res. 2007, 1162, 48–55. [Google Scholar] [CrossRef]
- Levanti, M.B.; Montalbano, G.; Laurà, R.; Ciriaco, E.; Cobo, T.; García-Suarez, O.; Germanà, A.; Vega, J. Calretinin in the peripheral nervous system of the adult zebrafish. J. Anat. 2008, 212, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Münkle, M.C.; Waldvogel, H.J.; Faull, R.L. Calcium-binding protein immunoreactivity delineates the intralaminar nuclei of the thalamus in the human brain. Neuroscience 1999, 90, 485–491. [Google Scholar] [CrossRef]
- Yan, K.; Tang, Y.Z.; Carr, C.E. Calcium-binding protein immunoreactivity characterizes the auditory system of Gekko gecko. J. Comp. Neurol. 2010, 518, 3409–3426. [Google Scholar] [CrossRef]
- Fairless, R.; Williams, S.K.; Diem, R. Calcium-Binding Proteins as Determinants of Central Nervous System Neuronal Vulnerability to Disease. Int. J. Mol. Sci. 2019, 20, 2146. [Google Scholar] [CrossRef] [PubMed]
- Langeh, U.; Singh, S. Targeting S100B Protein as a Surrogate Biomarker and its Role in Various Neurological Disorders. Curr. Neuropharmacol. 2021, 19, 265–277. [Google Scholar] [CrossRef]
- Landfield, P.W. ‘Increased calcium-current’ hypothesis of brain aging. Neurobiol. Aging 1987, 8, 346–347. [Google Scholar] [CrossRef] [PubMed]
- Khachaturian, Z.S. Calcium Hypothesis of Alzheimer’s Disease and Brain Aginga. Ann. N. Y. Acad. Sci. 1994, 747, 1–11. [Google Scholar] [CrossRef]
- Fiuza, F.P.; Queiroz, J.P.G.; Aquino, A.C.Q.; Câmara, D.A.; Brandão, L.E.M.; Lima, R.H.; Cavalcanti, J.R.L.; Engelberth, R.C.G.; Cavalcante, J.S. Aging Alters Daily and Regional Calretinin Neuronal Expression in the Rat Non-image Forming Visual Thalamus. Front. Aging Neurosci. 2021, 13, 613305. [Google Scholar] [CrossRef] [PubMed]
- Lamerand, S.; Shahidehpour, R.; Ayala, I.; Gefen, T.; Mesulam, M.M.; Bigio, E.; Geula, C. Calbindin-D28K, parvalbumin, and calretinin in young and aged human locus coeruleus. Neurobiol. Aging 2020, 94, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chang, Q.; Wang, Y.; Su, F.; Zhang, S. Late-onset temperature reduction can retard the aging process in aged fish via a combined action of an anti-oxidant system and the insulin/insulin-like growth factor 1 signaling pathway. Rejuvenation Res. 2014, 17, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Du, X.; Zhou, Y.; Wang, S.; Su, F.; Zhang, S. Intermittent food restriction initiated late in life prolongs lifespan and retards the onset of age-related markers in the annual fish Nothobranchius guentheri. Biogerontology 2017, 18, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ren, Y.; Du, X.; Song, L.; Chen, F.; Su, F. Effects of late-onset dietary intake of salidroside on insulin/insulin-like growth factor-1 (IGF-1) signaling pathway of the annual fish Nothobranchius guentheri. Arch. Gerontol. Geriatr. 2020, 91, 104233. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Song, L.; Zhou, Y.; Yuan, J.; Zhang, S. Identification of Isthmin1 in the small annual fish, Nothobranchius guentheri, as a novel biomarker of aging and its potential rejuvenation activity. Biogerontology 2022, 23, 99–114. [Google Scholar] [CrossRef]
- Matsunaga, H.; Handa, J.T.; Aotaki-Keen, A.; Sherwood, S.W.; West, M.D.; Hjelmeland, L.M. Beta-galactosidase histochemistry and telomere loss in senescent retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 1999, 40, 197–202. [Google Scholar]
- Liu, C.; Wang, X.; Feng, W.; Li, G.; Su, F.; Zhang, S. Differential expression of aging biomarkers at different life stages of the annual fish Nothobranchius guentheri. Biogerontology 2012, 13, 501–510. [Google Scholar] [CrossRef]
- Yildirim, Z.; Ucgun, N.I.; Yildirim, F. The role of oxidative stress and antioxidants in the pathogenesis of age-related macular degeneration. J. Clin. 2011, 66, 743–746. [Google Scholar] [CrossRef]
- Aragona, M.; Porcino, C.; Guerrera, M.C.; Montalbano, G.; Levanti, M.; Abbate, F.; Laurà, R.; Germanà, A. Localization of Neurotrophin Specific Trk Receptors in Mechanosensory Systems of Killifish (Nothobranchius guentheri). Int. J. Mol. Sci. 2021, 22, 10411. [Google Scholar] [CrossRef]
- Abbate, F.; Guerrera, M.C.; Montalbano, G.; De Carlos, F.; Suárez, A.Á.; Ciriaco, E.; Germanà, A. Morphology of the european sea bass (Dicentrarchus labrax) tongue. Microsc. Res. Tech. 2012, 75, 643–649. [Google Scholar] [CrossRef]
- Lauriano, E.; Guerrera, M.; Laurà, R.; Capillo, G.; Pergolizzi, S.; Aragona, M.; Abbate, F.; Germanà, A. Effect of light on the calretinin and calbindin expression in skin club cells of adult zebrafish. Histochem. Cell Biol. 2020, 154, 495–505. [Google Scholar] [CrossRef]
- Lauriano, E.R.; Capillo, G.; Icardo, J.M.; Fernandes, J.M.O.; Kiron, V.; Kuciel, M.; Zuwala, K.; Guerrera, M.C.; Aragona, M.; Germana’, A.; et al. Neuroepithelial cells (NECs) and mucous cells express a variety of neurotransmitters and neurotransmitter receptors in the gill and respiratory air-sac of the catfish Heteropneustes fossilis (Siluriformes, Heteropneustidae): A possible role in local immune defence. Zoology 2021, 148, 125958. [Google Scholar] [CrossRef] [PubMed]
- Capillo, G.; Zaccone, G.; Cupello, C.; Fernandes, J.M.O.; Viswanath, K.; Kuciel, M.; Zuwala, K.; Guerrera, M.C.; Aragona, M.; Icardo, J.M.; et al. Expression of acetylcholine, its contribution to regulation of immune function and O2 sensing and phylogenetic interpretations of the African butterfly fish Pantodon buchholzi (Osteoglossiformes, Pantodontidae). Fish Shellfish Immunol. 2021, 111, 189–200. [Google Scholar] [CrossRef]
- Licata, P.; Tardugno, R.; Pergolizzi, S.; Capillo, G.; Aragona, M.; Colombo, A.; Gervasi, T.; Pellizzeri, V.; Cicero, N.; Calò, M. In vivo effects of PCB-126 and genistein on vitellogenin expression in zebrafish. Nat. Prod. Res. 2018, 33, 2507–2514. [Google Scholar] [CrossRef]
- United Nations. World Population Ageing 2019 Division; United Nations: New York, NY, USA, 2020; p. 64. [Google Scholar]
- Cao, W.; Li, T. COVID-19: Towards understanding of pathogenesis. Cell Res. 2020, 30, 367–369. [Google Scholar] [CrossRef]
- Vanhunsel, S.; Beckers, A.; Moons, L. Designing neuroreparative strategies using aged regenerating animal models. Ageing Res. Rev. 2020, 62, 101086. [Google Scholar] [CrossRef] [PubMed]
- Chader, G.J.; Taylor, A. Preface: The Aging Eye: Normal Changes, Age-Related Diseases, and Sight-Saving Approaches. Investig. Ophthalmol. Vis. Sci. 2013, 54, ORSF1–ORSF4. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Genade, T.; Benedetti, M.; Terzibasi, E.; Roncaglia, P.; Valenzano, D.R.; Cattaneo, A.; Cellerino, A. Annual fishes of the genus Nothobranchius as a model system for aging research. Aging Cell 2005, 4, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Terzibasi, E.; Valenzano, D.R.; Cellerino, A. The short-lived fish Nothobranchius furzeri as a new model system for aging studies. Exp. Gerontol. 2007, 42, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Valenzano, D.R.; Cellerino, A. Resveratrol and the Pharmacology of Aging: A New Vertebrate Model to Validate an Old Molecule. Cell Cycle 2006, 5, 1027–1032. [Google Scholar] [CrossRef]
- Valenzano, D.R.; Sharp, S.; Brunet, A. Transposon-mediated transgenesis in the short-lived African killifish Nothobranchius furzeri, a vertebrate model for aging. G3 Genes Genomes Genet. 2011, 1, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Tozzini, E.T.; Baumgart, M.; Battistoni, G.; Cellerino, A. Adult neurogenesis in the short-lived teleost Nothobranchius furzeri: Localization of neurogenic niches, molecular characterization and effects of aging. Aging Cell 2012, 11, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Tozzini, E.T.; Cellerino, A. Nothobranchius annual killifishes. EvoDevo 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, M.; Groth, M.; Priebe, S.; Savino, A.; Testa, G.; Dix, A.; Ripa, R.; Spallotta, F.; Gaetano, C.; Ori, M.; et al. RNA-seq of the aging brain in the short-lived fish N. furzeri–conserved pathways and novel genes associated with neurogenesis. Aging Cell 2014, 13, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, N.; Englert, C. A microinjection protocol for the generation of transgenic killifish (Species: Nothobranchius furzeri). Dev. Dyn. 2012, 241, 1133–1141. [Google Scholar] [CrossRef]
- Hartmann, N.; Reichwald, K.; Wittig, I.; Dröse, S.; Schmeisser, S.; Lück, C.; Hahn, C.; Graf, M.; Gausmann, U.; Terzibasi, E.; et al. Mitochondrial DNA copy number and function decrease with age in the short-lived fish Nothobranchius furzeri. Aging Cell 2011, 10, 824–831. [Google Scholar] [CrossRef]
- Nikiforov-Nikishin, D.L.; Irkha, V.A.; Kochetkov, N.I.; Kalita, T.L.; Nikiforov-Nikishin, A.L.; Blokhin, E.E.; Antipov, S.S.; Makarenkov, D.A.; Zhavnerov, A.N.; Glebova, I.A.; et al. Some Aspects of Development and Histological Structure of the Visual System of Nothobranchius Guentheri. Animals 2021, 11, 2755. [Google Scholar] [CrossRef]
- Tarboush, R.; Chapman, G.B.; Connaughton, V.P. Ultrastructure of the distal retina of the adult zebrafish, Danio rerio. Tissue Cell 2012, 44, 264–279. [Google Scholar] [CrossRef]
- Menke, A.L.; Spitsbergen, J.M.; Wolterbeek, A.P.; Woutersen, R.A. Normal anatomy and histology of the adult zebrafish. Toxicol. Pathol. 2011, 39, 759–775. [Google Scholar] [CrossRef] [PubMed]
- Kishi, S.; Slack, B.E.; Uchiyama, J.; Zhdanova, I.V. Zebrafish as a Genetic Model in Biological and Behavioral Gerontology: Where Development Meets Aging in Vertebrates—A Mini-Review. Gerontology 2009, 55, 430–441. [Google Scholar] [CrossRef]
- Hanus, J.; Anderson, C.; Wang, S. RPE necroptosis in response to oxidative stress and in AMD. Ageing Res. Rev. 2015, 24, 286–298. [Google Scholar] [CrossRef]
- Fabre, M.; Mateo, L.; Lamaa, D.; Baillif, S.; Pagès, G.; Demange, L.; Ronco, C.; Benhida, R. Recent Advances in Age-Related Macular Degeneration Therapies. Molecules 2022, 27, 5089. [Google Scholar] [CrossRef]
- Bollaerts, I.; Veys, L.; Geeraerts, E.; Andries, L.; De Groef, L.; Buyens, T.; Salinas-Navarro, M.; Moons, L.; Van Hove, I. Complementary research models and methods to study axonal regeneration in the vertebrate retinofugal system. Brain Struct. Funct. 2018, 223, 545–567. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, N.; Shi, H.; Oviatt, M.; Doustar, J.; Rentsendorj, A.; Fuchs, D.-T.; Sheyn, J.; Black, K.L.; Koronyo, Y.; Koronyo-Hamaoui, M. Alzheimer’s retinopathy: Seeing disease in the eyes. Front. Neurosci. 2020, 14, 921. [Google Scholar] [CrossRef]
- Guo, L.; Normando, E.M.; Shah, P.A.; De Groef, L.; Cordeiro, M.F. Oculo-visual abnormalities in Parkinson’s disease: Possible value as biomarkers. Mov. Disord. 2018, 33, 1390–1406. [Google Scholar] [CrossRef] [PubMed]
- Vandenabeele, M.; Veys, L.; Lemmens, S.; Hadoux, X.; Gelders, G.; Masin, L.; Serneels, L.; Theunis, J.; Saito, T.; Saido, T.C.; et al. The AppNL-G-F mouse retina is a site for preclinical Alzheimer’s disease diagnosis and research. Acta Neuropathol. Commun. 2021, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Veys, L.; Vandenabeele, M.; Ortuño-Lizarán, I.; Baekelandt, V.; Cuenca, N.; Moons, L.; De Groef, L. Retinal α-synuclein deposits in Parkinson’s disease patients and animal models. Acta Neuropathol. 2019, 137, 379–395. [Google Scholar] [CrossRef]
- Parnell, M.; Guo, L.; Abdi, M.; Cordeiro, M.F. Ocular Manifestations of Alzheimer’s Disease in Animal Models. Int. J. Alzheimer’s Dis. 2012, 2012, 786494. [Google Scholar] [CrossRef]
- Koronyo-Hamaoui, M.; Koronyo, Y.; Ljubimov, A.V.; Miller, C.A.; Ko, M.K.; Black, K.L.; Schwartz, M.; Farkas, D.L. Identification of amyloid plaques in retinas from Alzheimer’s patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. NeuroImage 2011, 54, S204–S217. [Google Scholar] [CrossRef] [PubMed]
- Cortes, L.; Malva, J.; Rego, A.C.; Pereira, C.F. Calcium Signaling in Aging and Neurodegenerative Diseases 2019. Int. J. Mol. Sci. 2020, 21, 1125. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.-G.; Bergert, H.; Funk, R. Neurodegenerative diseases of the retina and potential for protection and recovery. Curr. Neuropharmacol. 2008, 6, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Andressen, C.; Blümcke, I.; Celio, M.R. Calcium-binding proteins: Selective markers of nerve cells. Cell Tissue Res. 1993, 271, 181–208. [Google Scholar] [CrossRef]
- Schwaller, B. The use of transgenic mouse models to reveal the functions of Ca2+ buffer proteins in excitable cells. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2012, 1820, 1294–1303. [Google Scholar] [CrossRef]
- Chin, D.; Means, A.R. Calmodulin: A prototypical calcium sensor. Trends Cell Biol. 2000, 10, 322–328. [Google Scholar] [CrossRef]
- Germana, A.; Abbate, F.; González-Martínez, T.; Del Valle, M.; De Carlos, F.; Germanà, G.; Vega, J. S100 protein is a useful and specific marker for hair cells of the lateral line system in postembryonic zebrafish. Neurosci. Lett. 2004, 365, 186–189. [Google Scholar] [CrossRef]
- Germanà, A.; Marino, F.; Guerrera, M.C.; Campo, S.; de Girolamo, P.; Montalbano, G.; Germanà, G.P.; Ochoa-Erena, F.J.; Ciriaco, E.; Vega, J.A. Expression and distribution of S100 protein in the nervous system of the adult zebrafish (Danio rerio). Microsc. Res. Tech. 2008, 71, 248–255. [Google Scholar] [CrossRef]
- Parisi, V.; Guerrera, M.C.; Abbate, F.; Garcia-Suarez, O.; Viña, E.; Vega, J.A.; Germanà, A. Immunohistochemical characterization of the crypt neurons in the olfactory epithelium of adult zebrafish. Ann. Anat.-Anat. Anz. 2014, 196, 178–182. [Google Scholar] [CrossRef]
- Kántor, O.; Mezey, S.; Adeghate, J.; Naumann, A.; Nitschke, R.; Énzsöly, A.; Szabó, A.; Lukáts, Á.; Németh, J.; Somogyvári, Z.; et al. Calcium buffer proteins are specific markers of human retinal neurons. Cell Tissue Res. 2016, 365, 29–50. [Google Scholar] [CrossRef]
- Airaksinen, M.S.; Thoenen, H.; Meyer, M. Vulnerability of Midbrain Dopaminergic Neurons in Calbindin-D28k-deficient Mice: Lack of Evidence for a Neuroprotective Role of Endogenous Calbindin in MPTPtreated and Weaver Mice. Eur. J. Neurosci. 1997, 9, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Camp, A.J.; Wijesinghe, R. Calretinin: Modulator of neuronal excitability. Int. J. Biochem. Cell Biol. 2009, 41, 2118–2121. [Google Scholar] [CrossRef] [PubMed]
- Kovács-Öller, T.; Szarka, G.; Ganczer, A.; Tengölics, Á.; Balogh, B.; Völgyi, B. Expression of Ca2+-Binding Buffer Proteins in the Human and Mouse Retinal Neurons. Int. J. Mol. Sci. 2019, 20, 2229. [Google Scholar] [CrossRef] [PubMed]
- Verret, L.; Mann, E.O.; Hang, G.B.; Barth, A.M.I.; Cobos, I.; Ho, K.; Devidze, N.; Masliah, E.; Kreitzer, A.C.; Mody, I.; et al. Inhibitory Interneuron Deficit Links Altered Network Activity and Cognitive Dysfunction in Alzheimer Model. Cell 2012, 149, 708–721. [Google Scholar] [CrossRef]
- Germanà, A.; Guerrera, M.C.; Laurà, R.; Levanti, M.; Aragona, M.; Mhalhel, K.; Germanà, G.; Montalbano, G.; Abbate, F. Expression and Localization of BDNF/TrkB System in the Zebrafish Inner Ear. Int. J. Mol. Sci. 2020, 21, 5787. [Google Scholar] [CrossRef]
- Abbate, F.; Catania, S.; Germana, A.; González, T.; Diaz-Esnal, B.; Germana, G.; Vega, J. S-100 protein is a selective marker for sensory hair cells of the lateral line system in teleosts. Neurosci. Lett. 2002, 329, 133–136. [Google Scholar] [CrossRef]
- Abbate, F.; Guerrera, M.C.; Montalbano, G.; Ciriaco, E.; Germanà, A. Morphology of the tongue dorsal surface of gilthead seabream (Sparus aurata). Microsc. Res. Tech. 2012, 75, 1666–1671. [Google Scholar] [CrossRef]
- D’angelo, L. Brain Atlas of an Emerging Teleostean Model: Nothobranchius furzeri. Anat. Rec. 2013, 296, 681–691. [Google Scholar] [CrossRef]
- D’Angelo, L.; de Girolamo, P.; Cellerino, A.; Tozzini, E.T.; Castaldo, L.; Lucini, C. Neurotrophin Trk receptors in the brain of a teleost fish, Nothobranchius furzeri. Microsc. Res. Tech. 2012, 75, 81–88. [Google Scholar] [CrossRef]
- D’Angelo, L.; De Girolamo, P.; Lucini, C.; Terzibasi, E.T.; Baumgart, M.; Castaldo, L.; Cellerino, A. Brain-derived neurotrophic factor: mRNA expression and protein distribution in the brain of the teleost Nothobranchius furzeri. J. Comp. Neurol. 2014, 522, 1004–1030. [Google Scholar] [CrossRef]
- Leggieri, A.; Attanasio, C.; Palladino, A.; Cellerino, A.; Lucini, C.; Paolucci, M.; Terzibasi Tozzini, E.; de Girolamo, P.; D’Angelo, L. Identification and expression of neurotrophin-6 in the brain of Nothobranchius furzeri: One more piece in neurotrophin research. J. Clin. Med. 2019, 8, 595. [Google Scholar] [CrossRef] [PubMed]
- Aragona, M.; Porcino, C.; Guerrera, M.C.; Montalbano, G.; Laurà, R.; Cometa, M.; Levanti, M.; Abbate, F.; Cobo, T.; Capitelli, G.; et al. The BDNF/TrkB Neurotrophin System in the Sensory Organs of Zebrafish. Int. J. Mol. Sci. 2022, 23, 2621. [Google Scholar] [CrossRef] [PubMed]
- Aragona, M.; Porcino, C.; Guerrera, M.C.; Montalbano, G.; Laurà, R.; Levanti, M.; Abbate, F.; Cobo, T.; Capitelli, G.; Calapai, F.; et al. Localization of BDNF and Calretinin in Olfactory Epithelium and Taste Buds of Zebrafish (Danio rerio). Int. J. Mol. Sci. 2022, 23, 4696. [Google Scholar] [CrossRef]
- Copray, J.C.V.M.; Mantingh-Otter, I.J.; Brouwer, N. Expression of calcium-binding proteins in the neurotrophin-3-dependent subpopulation of rat embryonic dorsal root ganglion cells in culture. Dev. Brain Res. 1994, 81, 57–65. [Google Scholar] [CrossRef]
- Miguel, J.C.; Perez, S.E.; Malek-Ahmadi, M.; Mufson, E.J. Cerebellar Calcium-Binding Protein and Neurotrophin Receptor Defects in Down Syndrome and Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 79. [Google Scholar] [CrossRef] [PubMed]
- Germana, A.; Montalbano, G.; Laura, R.; Ciriaco, E.; Del Valle, M.; Vega, J.A. S100 protein-like immunoreactivity in the crypt olfactory neurons of the adult zebrafish. Neurosci. Lett. 2004, 371, 196–198. [Google Scholar] [CrossRef]
- Porcino, C.; Briglia, M.; Aragona, M.; Mhalhel, K.; Laurà, R.; Levanti, M.; Abbate, F.; Montalbano, G.; Germanà, G.; Lauriano, E.R.; et al. Potential Neuroprotective Role of Calretinin-N18 and Calbindin-D28k in the Retina of Adult Zebrafish Exposed to Different Wavelength Lights. Int. J. Mol. Sci. 2023, 24, 1087. [Google Scholar] [CrossRef]
- Hwang, I.K.; Yoo, K.Y.; Kim, D.S.; Jung, J.Y.; Shin, M.C.; Seo, K.; Kim, K.S.; Kang, T.C.; Won, M.H. Comparative Study on Calretinin Immunoreactivity in Gerbil and Rat Retina. Anat. Histol. Embryol. 2005, 34, 129–131. [Google Scholar] [CrossRef]
- Nag, T.C.; Wadhwa, S. Calbindin and parvalbumin immunoreactivity in the developing and adult human retina. Dev. Brain Res. 1996, 93, 23–32. [Google Scholar] [CrossRef]
- Nag, T.C.; Wadhwa, S. Developmental expression of calretinin immunoreactivity in the human retina and a comparison with two other EF-hand calcium-binding proteins. Neuroscience 1999, 91, 41–50. [Google Scholar] [CrossRef]
- Hamano, K.; Kiyama, H.; Emson, P.C.; Manabe, R.; Nakauchi, M.; Tohyama, M. Localization of two calcium binding proteins, calbindin (28 kD) and parvalbumin (12 kD), in the vertebrate retina. J. Comp. Neurol. 1990, 302, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.S.; Weltzien, F.; Madigan, M.C.; Martin, P.R.; Grünert, U. Identification of AII amacrine, displaced amacrine, and bistratified ganglion cell types in human retina with antibodies against calretinin. J. Comp. Neurol. 2016, 524, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Takahashi-Iwanaga, H.; Shimoda, H. The three-dimensional microanatomy of Meissner corpuscles in monkey palmar skin. J. Neurocytol. 2003, 32, 363–371. [Google Scholar] [CrossRef]
- Fu, Z.; Nian, S.; Li, S.-Y.; Wong, D.; Chung, S.K.; Lo, A.C.Y. Deficiency of aldose reductase attenuates inner retinal neuronal changes in a mouse model of retinopathy of prematurity. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 1503–1513. [Google Scholar] [CrossRef]
- Gábriel, R.; Erdélyi, F.; Szabó, G.; Lawrence, J.J.; Wilhelm, M. Ectopic transgene expression in the retina of four transgenic mouse lines. Brain Struct. Funct. 2016, 221, 3729–3741. [Google Scholar] [CrossRef]
- Haverkamp, S.; Wässle, H. Immunocytochemical analysis of the mouse retina. J. Comp. Neurol. 2000, 424, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Celio, M.R. Calbindin D-28k and parvalbumin in the rat nervous system. Neuroscience 1990, 35, 375–475. [Google Scholar] [CrossRef]
- Kovács-Öller, T.; Debertin, G.; Balogh, M.; Ganczer, A.; Orbán, J.; Nyitrai, M.; Balogh, L.; Kántor, O.; Völgyi, B. Connexin36 expression in the mammalian retina: A multiple-species comparison. Front. Cell. Neurosci. 2017, 11, 65. [Google Scholar] [CrossRef]
- Kovács-Öller, T.; Raics, K.; Orbán, J.; Nyitrai, M.; Völgyi, B. Developmental changes in the expression level of connexin36 in the rat retina. Cell Tissue Res. 2014, 358, 289–302. [Google Scholar] [CrossRef]
- Kovács-Valasek, A.; Pöstyéni, E.; Dénes, V.; Mester, A.; Sétáló Jr, G.; Gábriel, R. Age-Related Alterations of Proteins in Albino Wistar Rat Retina. Cells Tissues Organs 2021, 210, 135–150. [Google Scholar] [CrossRef]
- Trost, A.; Schroedl, F.; Marschallinger, J.; Rivera, F.J.; Bogner, B.; Runge, C.; Couillard-Despres, S.; Aigner, L.; Reitsamer, H.A. Characterization of dsRed2-positive cells in the doublecortin-dsRed2 transgenic adult rat retina. Histochem. Cell Biol. 2014, 142, 601–617. [Google Scholar] [CrossRef]
- Massey, S.C.; Mills, S.L. A calbindin-immunoreactive cone bipolar cell type in the rabbit retina. J. Comp. Neurol. 1996, 366, 15–33. [Google Scholar] [CrossRef]
- Kwon, O.-J.; Kim, J.-Y.; Kim, S.-Y.; Jeon, C.-J. Alterations in the localization of calbindin D28K-, calretinin-, and parvalbumin-immunoreactive neurons of rabbit retinal ganglion cell layer from ischemia and reperfusion. Mol. Cells 2005, 19, 382–390. [Google Scholar]
- Jeon, M.-H.; Jeon, C.-J. Immunocytochemical localization of calretinin containing neurons in retina from rabbit, cat, and dog. Neurosci. Res. 1998, 32, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Chang-Jin, J.; Enrica, S.; Richard, H.M. The Major Cell Populations of the Mouse Retina. J. Neurosci. 1998, 18, 8936. [Google Scholar] [CrossRef]
- Casini, G.; Rickman, D.W.; Brecha, N.C. AII amacrine cell population in the rabbit retina: Identification by parvalbumin immunoreactivity. J. Comp. Neurol. 1995, 356, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Wässle, H.; Peichl, L.; Airaksinen, M.S.; Meyer, M. Calcium-binding proteins in the retina of a calbindin-null mutant mouse. Cell Tissue Res. 1998, 292, 211–218. [Google Scholar] [CrossRef]
- Chun, M.-H.; Kim, I.-B.; Ju, W.-K.; Kim, K.-Y.; Lee, M.-Y.; Joo, C.-K.; Chung, J.-W. Horizontal cells of the rat retina are resistant to degenerative processes induced by ischemia-reperfusion. Neurosci. Lett. 1999, 260, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Bordt, A.S.; Hoshi, H.; Yamada, E.S.; Perryman-Stout, W.C.; Marshak, D.W. Synaptic input to OFF parasol ganglion cells in macaque retina. J. Comp. Neurol. 2006, 498, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Chiquet, C.; Dkhissi-Benyahya, O.; Cooper, H.M. Calcium-binding protein distribution in the retina of strepsirhine and haplorhine primates. Brain Res. Bull. 2005, 68, 185–194. [Google Scholar] [CrossRef]
- Röhrenbeck, J.; Wässle, H.; Boycott, B.B. Horizontal Cells in the Monkey Retina: Immunocytochemical staining with antibodies against calcium binding proteins. Eur. J. Neurosci. 1989, 1, 407–420. [Google Scholar] [CrossRef]
- Röhrenbeck, J.; Wässle, H.; Heizmann, C.W. Immunocytochemical labelling of horizontal cells in mammalian retina using antibodies against calcium-binding proteins. Neurosci. Lett. 1987, 77, 255–260. [Google Scholar] [CrossRef]
- Martin, P.R.; Grünert, U. Spatial density and immunoreactivity of bipolar cells in the macaque monkey retina. J. Comp. Neurol. 1992, 323, 269–287. [Google Scholar] [CrossRef] [PubMed]
- Roski, C.; Langrock, C.; Körber, N.; Habermann, G.; Buse, E.; Reichenbach, A.; Pannicke, T.; Francke, M. Comparison of cellular localisation of the Ca2+-binding proteins calbindin, calretinin and parvalbumin in the retina of four different Macaca species. Anat. Histol. Embryol. 2018, 47, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Youn, H.-Y.; Chou, B.R.; Cullen, A.P.; Sivak, J.G. Effects of 400nm, 420nm, and 435.8nm radiations on cultured human retinal pigment epithelial cells. J. Photochem. Photobiol. B Biol. 2009, 95, 64–70. [Google Scholar] [CrossRef]
- Pochet, R.; Pasteels, B.; Seto-ohshima, A.; Bastianelli, E.; Kitajima, S.; Van Eldik, L.J. Calmodulin and calbindin localization in retina from six vertebrate species. J. Comp. Neurol. 1991, 314, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Haley, T.L.; Pochet, R.; Baizer, L.; Burton, M.D.; Crabb, J.W.; Parmentier, M.; Polans, A.S. Calbindin D-28K immunoreactivity of human cone cells varies with retinal position. Vis. Neurosci. 1995, 12, 301–307. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Zhou, A.; Ye, Q.; Feng, Y.; Wang, Z.; Wang, S.; Xu, G.; Zou, J. Species-specific effect of microplastics on fish embryos and observation of toxicity kinetics in larvae. J. Hazard. Mater. 2021, 403, 123948. [Google Scholar] [CrossRef]
- Pöstyéni, E.; Szabadfi, K.; Sétáló, G.; Gabriel, R. A Promising Combination: PACAP and PARP Inhibitor Have Therapeutic Potential in Models of Diabetic and Hypertensive Retinopathies. Cells 2021, 10, 3470. [Google Scholar] [CrossRef]
- Kim, S.A.; Jung, C.K.; Kang, T.-H.; Jeon, J.H.; Cha, J.; Kim, I.-B.; Chun, M.-H. Synaptic connections of calbindin-immunoreactive cone bipolar cells in the inner plexiform layer of rabbit retina. Cell Tissue Res. 2010, 339, 311–320. [Google Scholar] [CrossRef]
- Sharma, R.K.; O’Leary, T.E.; Fields, C.M.; Johnson, D.A. Development of the outer retina in the mouse. Dev. Brain Res. 2003, 145, 93–105. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, X.; Hu, F.; Wu, J. Fine structure of the human retina defined by confocal microscopic immunohistochemistry. Br. J. Biomed. Sci. 2021, 78, 28–34. [Google Scholar] [CrossRef]
- Endo, T.; Kobayashi, M.; Kobayashi, S.; Onaya, T. Immunocytochemical and biochemical localization of parvalbumin in the retina. Cell Tissue Res. 1986, 243, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Nivison-Smith, L.; Khoo, P.; Acosta, M.L.; Kalloniatis, M. Pre-treatment with vinpocetine protects against retinal ischemia. Exp. Eye Res. 2017, 154, 126–138. [Google Scholar] [CrossRef]
- Kim, D.; Kim, M.J.; Lee, J.H.; Im, J.O.; Won, Y.J.; Yoon, S.-Y.; Hong, H.N. Concomitant distribution shift of glial GABA transporter and S100 calcium-binding proteins in the rat retina after kainate-induced excitotoxic injury. Neurosci. Lett. 2003, 353, 17–20. [Google Scholar] [CrossRef]
- Iwanaga, T.; Takahashi, Y.; Fujita, T. Immunohistochemical localization of S-100 protein in the retina, ciliary body and iris of human fetuses. Cell Tissue Res. 1985, 239, 505–510. [Google Scholar] [CrossRef]
- Uesugi, R.; Yamada, M.; Mizuguchi, M.; Baimbridge, K.G.; Kim, S.U. Calbindin D-28k and parvalbumin immunohistochemistry in developing rat retina. Exp. Eye Res. 1992, 54, 491–499. [Google Scholar] [CrossRef]
- Wäussle, H.; Grüunert, U.; Röhrenbeck, J. Immunocytochemical staining of AII-amacrine cells in the rat retina with antibodies against parvalbumin. J. Comp. Neurol. 1993, 332, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Oguni, M.; Setogawa, T.; Shinohara, H.; Kato, K.; Semba, R. Distribution of γ-Aminobutyric Acid (GABA) and the Calcium-binding Protein Parvalbumin in Rat Retina during Development. Acta Histochem. Cytochem. 1997, 30, 237–242. [Google Scholar] [CrossRef]
- Ho, T.; Vessey, K.A.; Fletcher, E.L. Immunolocalization of the P2X4 receptor on neurons and glia in the mammalian retina. Neuroscience 2014, 277, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Pasteels, B.; Rogers, J.; Blachier, F.; Pochet, R. Calbindin and calretinin localization in retina from different species. Vis. Neurosci. 1990, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, C.; Guo, M. Use of ecofriendly alternatives for the control of bacterial infection in aquaculture of sea cucumber Apostichopus japonicus. Aquaculture 2021, 545, 737185. [Google Scholar] [CrossRef]
- Sanna, P.P.; Keyser, K.T.; Battenberg, E.; Bloom, F.E. Parvalbumin immunoreactivity in the rat retina. Neurosci. Lett. 1990, 118, 136–139. [Google Scholar] [CrossRef]
- Miller, R.J. Regulation of calcium homoeostasis in neurons: The role of calcium-binding proteins. Biochem. Soc. Trans. 1995, 23, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, C.; Friauf, E. Distribution of the calcium-binding proteins parvalbumin and calretinin in the auditory brainstem of adult and developing rats. J. Comp. Neurol. 1996, 367, 90–109. [Google Scholar] [CrossRef]
- Kim, I.-J.; Zhang, Y.; Meister, M.; Sanes, J.R. Laminar Restriction of Retinal Ganglion Cell Dendrites and Axons: Subtype-Specific Developmental Patterns Revealed with Transgenic Markers. J. Neurosci. 2010, 30, 1452. [Google Scholar] [CrossRef]
- Park, H.-S.; Park, S.-J.; Park, S.-H.; Chun, M.-H.; Oh, S.-J. Shifting of parvalbumin expression in the rat retina in experimentally induced diabetes. Acta Neuropathol. 2008, 115, 241–248. [Google Scholar] [CrossRef]
- Hernandez, M.; Rodriguez, F.D.; Sharma, S.C.; Vecino, E. Immunohistochemical changes in rat retinas at various time periods of elevated intraocular pressure. Mol. Vis. 2009, 15, 2696–2709. [Google Scholar]
- Gunn, D.J.; Gole, G.A.; Barnett, N.L. Specific amacrine cell changes in an induced mouse model of glaucoma. Clin. Exp. Ophthalmol. 2011, 39, 555–563. [Google Scholar] [CrossRef]
- Huang, J.-F.; Shang, L.; Zhang, M.-Q.; Wang, H.; Chen, D.; Tong, J.-B.; Huang, H.; Yan, X.-X.; Zeng, L.-P.; Xiong, K. Differential neuronal expression of receptor interacting protein 3 in rat retina: Involvement in ischemic stress response. BMC Neurosci. 2013, 14, 16. [Google Scholar] [CrossRef]



| Primary Antibodies | Supplier | Catalog Number | Source | Dilution | Antibody ID |
| Calretinin-N18 | Santa Cruz Biotechnology, Inc., Dallas, TX, USA | sc-11644 | goat | 1:100 | AB_634545 |
| Parvalbumin clone PA235 | Sigma-Aldrich, Inc., St. Louis, MO, USA | P-3171 | mouse | 1:1000 | AB_2313693 |
| S100 | Dako Agilent, Santa Clara, CA, USA | Z0311 | rabbit | 1:100 | AB_10013383 |
| Anti-Opsina Clone RET-P1 | Sigma-Aldrich, Inc., St. Louis, MO, USA | O4886 | mouse | 1:100 | AB_260838 |
| Anti-Chat | Sigma-Aldrich, Inc., St. Louis, MO, USA | AMAB91130 | mouse | 1:100 | AB_2665812 |
| Secondary Antibodies | Supplier | Catalog Number | Source | Dilution | Antibody ID |
| Antigoat IgG (H + L) Alexa Fluor 594 | Molecular Probes, Invitrogen, Waltham, MA, USA | A-11058 | donkey | 1:300 | AB_2534105 |
| Antimouse IgG (H + L) Alexa Fluor 488 | Molecular Probes, Invitrogen, Waltham, MA, USA | A-11001 | goat | 1:300 | AB_2534069 |
| Antirabbit IgG peroxidase conjugate | Amersham Bioscences, Amersham, United Kingdom | NA934 | donkey | 1:100 | AB_772206 |
| Total Cells of a Layer | Calretinin N-18 | Parvalbumin | S100 | Anti-Opsin | Anti-Chat | |
|---|---|---|---|---|---|---|
| Mean ± ∆σ in RPE | 28.7 ± 4.9 *** | 25.2 ± 4.5 *** | 27.3 ± 3.57 *** | 27.7 ± 5.36 *** | 27.3 ± 3.57 *** | 25.2 ± 4.5 *** |
| Mean ± ∆σ in PRL | 28.5 ± 4.7 ** | 26.3 ± 3.1 *** | 27.4 ± 4.8 ** | 27.8 ± 5.97 *** | 27.4 ± 4.8 ** | 28.5 ± 4.7 ** |
| Mean ± ∆σ in OPL | 27.9 ± 5.6 *** | n/a | n/a | n/a | n/a | n/a |
| Mean ± ∆σ in ACs | 28.8 ± 5.11 *** | 27.1 ± 4.08 ** | 26.5 ± 5.48 *** | 26.7 ± 4.64 *** | n/a | 27.7 ± 5.36 *** |
| Mean ± ∆σ in BCs | 27.7 ± 5.36 *** | n/a | n/a | 25.8 ± 4.6 *** | n/a | n/a |
| Mean ± ∆σ in HCs | 28.5 ± 4.7 ** | n/a | n/a | 26.3 ± 4 ** | n/a | n/a |
| Mean ± ∆σ in IPL | 27.7 ± 5.36 *** | n/a | n/a | 27.4 ± 4.84 *** | n/a | n/a |
| Mean ± ∆σ in GCs | 28.8 ± 5.11 *** | 26.4 ± 5.40 *** | 26.7 ± 4.42 *** | 26.5 ± 5.12 *** | n/a | n/a |
| Species | Nothobranchius guentheri * | Zebrafish | Ref | Rat | Ref | Mouse | Ref | Human | Ref | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibodies | Calretinin N-18 | Parvalbumin | S100p | Calretini N-18 | Parvalbumin | S100p | Calretinin N-18 | Parvalbumin | S100p | Calretini N-18 | Parvalbumin | S100p | Calretini N-18 | Parvalbumin | S100p | ||||
| RPE | + | + | + | + | n/a | n/a | [91] | + | n/a | n/a | [92] | n/a | n/a | n/a | n/a | n/a | + | [93,94] | |
| PRL | + | + | − | + | + | n/a | [119] | + | n/a | n/a | [92,95] | n/a | n/a | n/a | [120] | − | n/a | n/a | [76,95,101,121,122] |
| OPL | − | − | − | − | n/a | n/a | [119] | + | n/a | + | [95,123,124] | n/a | + | n/a | [100,125] | − | n/a | + | [93,94,95,126] |
| INL | + | + | + | n/a | + | n/a | [119] | + | n/a | + | [92,95,103,110,123,127,128,129] | + | n/a | [98,99] | + | n/a | + | [73,96,130] | |
| Bipolar Cells (INL) | − | − | + | − | n/a | n/a | [119] | − | n/a | n/a | [92,131] | − | n/a | [76] | + | + | + | [76,94,95,96] | |
| Amacrine Cells (INL) | + | + | + | − | n/a | n/a | + | + | n/a | [95,101,103,104,105,132,133] | + | + | [76,100,134] | + | + | n/a | [76,95,96,101] | ||
| IPL | − | − | + | − | + | n/a | [119] | + | + | n/a | [92,95,99,103,123,128] | + | + | n/a | [98,99,100,134,135] | + | n/a | n/a | [93,96,136] |
| GLC | + | + | + | − | + | n/a | [119] | + | + | + | [92,95,101,104,105,110,123,127,128,137] | + | + | n/a | [76,99,100,134] | + | + | + | [76,94,95,96,101,122,130] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aragona, M.; Briglia, M.; Porcino, C.; Mhalhel, K.; Cometa, M.; Germanà, P.G.; Montalbano, G.; Levanti, M.; Laurà, R.; Abbate, F.; et al. Localization of Calretinin, Parvalbumin, and S100 Protein in Nothobranchius guentheri Retina: A Suitable Model for the Retina Aging. Life 2023, 13, 2050. https://doi.org/10.3390/life13102050
Aragona M, Briglia M, Porcino C, Mhalhel K, Cometa M, Germanà PG, Montalbano G, Levanti M, Laurà R, Abbate F, et al. Localization of Calretinin, Parvalbumin, and S100 Protein in Nothobranchius guentheri Retina: A Suitable Model for the Retina Aging. Life. 2023; 13(10):2050. https://doi.org/10.3390/life13102050
Chicago/Turabian StyleAragona, Marialuisa, Marilena Briglia, Caterina Porcino, Kamel Mhalhel, Marzio Cometa, Patrizia Germana Germanà, Giuseppe Montalbano, Maria Levanti, Rosaria Laurà, Francesco Abbate, and et al. 2023. "Localization of Calretinin, Parvalbumin, and S100 Protein in Nothobranchius guentheri Retina: A Suitable Model for the Retina Aging" Life 13, no. 10: 2050. https://doi.org/10.3390/life13102050
APA StyleAragona, M., Briglia, M., Porcino, C., Mhalhel, K., Cometa, M., Germanà, P. G., Montalbano, G., Levanti, M., Laurà, R., Abbate, F., Germanà, A., & Guerrera, M. C. (2023). Localization of Calretinin, Parvalbumin, and S100 Protein in Nothobranchius guentheri Retina: A Suitable Model for the Retina Aging. Life, 13(10), 2050. https://doi.org/10.3390/life13102050









