Outcomes after Microvascular Decompression for Hemifacial Spasm without Definite Radiological Neurovascular Compression at the Root Exit Zone
Abstract
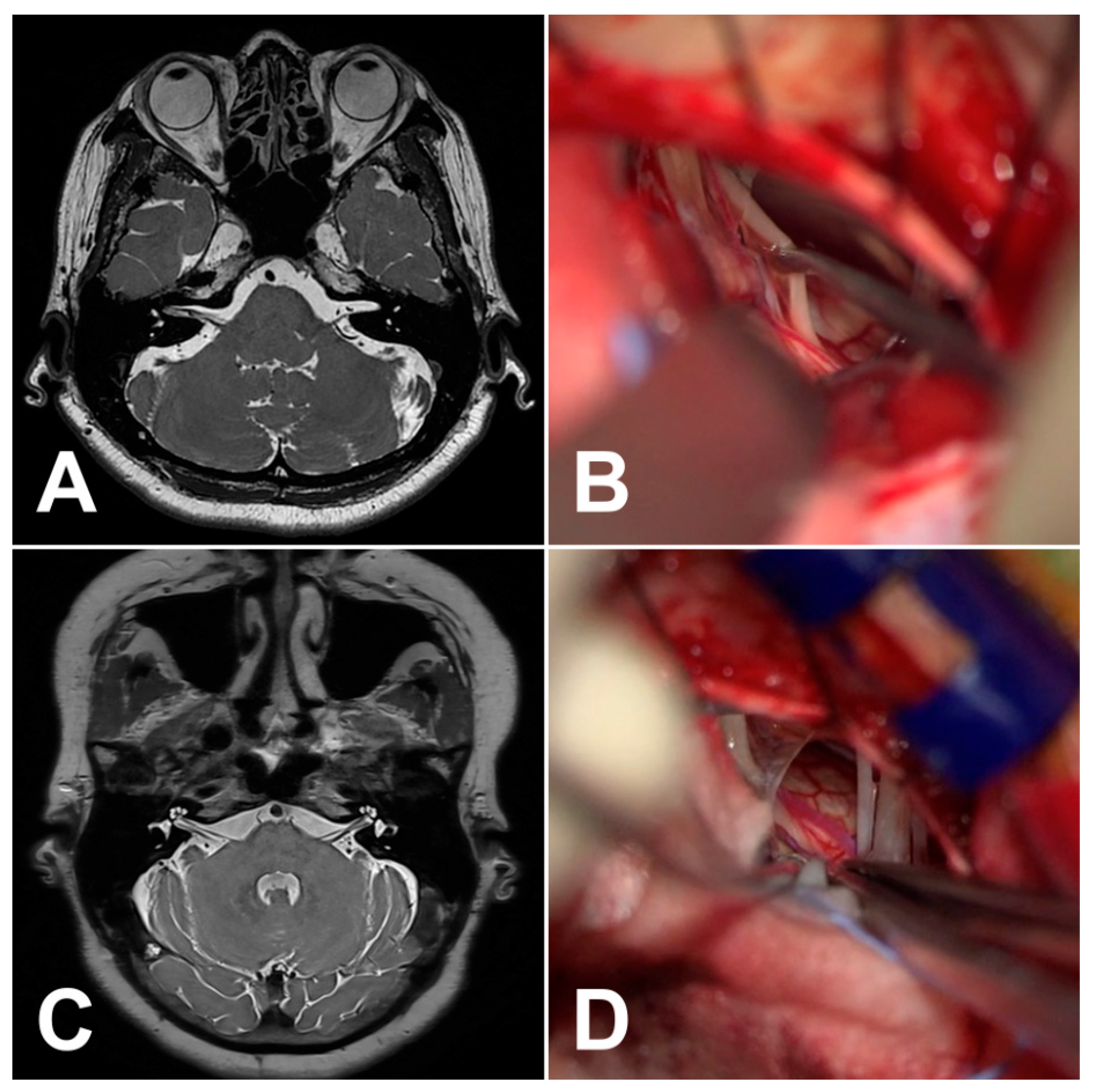
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Traylor, K.S.; Sekula, R.F.; Eubanks, K.; Muthiah, N.; Chang, Y.F.; Hughes, M.A. Prevalence and severity of neurovascular compression in hemifacial spasm patients. Brain 2021, 144, 1482–1487. [Google Scholar] [CrossRef]
- Wang, A.; Jankovic, J. Hemifacial spasm: Clinical findings and treatment. Muscle Nerve 1998, 21, 1740–1747. [Google Scholar] [CrossRef]
- Hermier, M. Imaging of hemifacial spasm. Neurochirurgie 2018, 64, 117–123. [Google Scholar] [CrossRef]
- Campos-Benitez, M.; Kaufmann, A.M. Neurovascular compression findings in hemifacial spasm. J. Neurosurg. 2008, 109, 416–420. [Google Scholar] [CrossRef]
- Hyun, S.J.; Kong, D.S.; Park, K. Microvascular decompression for treating hemifacial spasm: Lessons learned from a prospective study of 1,174 operations. Neurosurg. Rev. 2010, 33, 325–334, discussion 334. [Google Scholar] [CrossRef]
- Menna, G.; Battistelli, M.; Rapisarda, A.; Izzo, A.; D'Ercole, M.; Olivi, A.; Montano, N. Factors Related to Hemifacial Spasm Recurrence in Patients Undergoing Microvascular Decompression—A Systematic Review and Meta-Analysis. Brain Sci. 2022, 12, 583. [Google Scholar] [CrossRef]
- Chen, S.R. Neurological Imaging for Hemifacial Spasm. Int. Ophthalmol. Clin. 2018, 58, 97–109. [Google Scholar] [CrossRef]
- El Refaee, E.; Marx, S.; Rosenstengel, C.; Baldauf, J.; Schroeder, H.W.S. Arachnoid bands and venous compression as rare causes of hemifacial spasm: Analysis of etiology in 353 patients. Acta Neurochir. (Wien) 2020, 162, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Toda, H.; Iwasaki, K.; Yoshimoto, N.; Miki, Y.; Hashikata, H.; Goto, M.; Nishida, N. Bridging veins and veins of the brainstem in microvascular decompression surgery for trigeminal neuralgia and hemifacial spasm. Neurosurg. Focus 2018, 45, E2. [Google Scholar] [CrossRef]
- Jannetta, P.J. Hemifacial spasm caused by a venule: Case report. Neurosurgery 1984, 14, 89–92. [Google Scholar] [CrossRef]
- Lefaucheur, J.P.; Ben Daamer, N.; Sangla, S.; Le Guerinel, C. Diagnosis of primary hemifacial spasm. Neurochirurgie 2018, 64, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.S.; Kim, H.Y.; Chung, S.S.; Chang, J.W. Microneurovascular decompression in patients with hemifacial spasm caused by vascular compression of facial nerve at cisternal portion. Acta Neurochir. (Wien) 2010, 152, 2105–2111. [Google Scholar] [CrossRef] [PubMed]
- Gardner, W.J. Concerning the mechanism of trigeminal neuralgia and hemifacial spasm. J. Neurosurg. 1962, 19, 947–958. [Google Scholar] [CrossRef]
- Jannetta, P.J.; Hackett, E.; Ruby, J.R. Electromyographic and electron microscopic correlates in hemifacial spasm treated by microsurgical relief of neurovascular compression. Surg. Forum 1970, 21, 449–451. [Google Scholar]
- Jannetta, P.J. The cause of hemifacial spasm: Definitive microsurgical treatment at the brainstem in 31 patients. Trans. Sect. Ophthalmol. Am. Acad. Ophthalmol. Otolaryngol. 1975, 80, 319–322. [Google Scholar]
- Jannetta, P.J. Trigeminal neuralgia and hemifacial spasm--etiology and definitive treatment. Trans. Am. Neurol. Assoc. 1975, 100, 89–91. [Google Scholar]
- Jannetta, P.J.; Abbasy, M.; Maroon, J.C.; Ramos, F.M.; Albin, M.S. Etiology and definitive microsurgical treatment of hemifacial spasm. Operative techniques and results in 47 patients. J. Neurosurg. 1977, 47, 321–328. [Google Scholar] [CrossRef]
- Chung, S.S.; Chang, J.H.; Choi, J.Y.; Chang, J.W.; Park, Y.G. Microvascular decompression for hemifacial spasm: A long-term follow-up of 1,169 consecutive cases. Stereotact. Funct. Neurosurg. 2001, 77, 190–193. [Google Scholar] [CrossRef]
- Barker, F.G., 2nd; Jannetta, P.J.; Bissonette, D.J.; Shields, P.T.; Larkins, M.V.; Jho, H.D. Microvascular decompression for hemifacial spasm. J. Neurosurg. 1995, 82, 201–210. [Google Scholar] [CrossRef]
- Park, J.S.; Kong, D.S.; Lee, J.A.; Park, K. Hemifacial spasm: Neurovascular compressive patterns and surgical significance. Acta Neurochir. (Wien) 2008, 150, 235–241, discussion 241. [Google Scholar] [CrossRef]
- Kim, H.R.; Rhee, D.J.; Kong, D.S.; Park, K. Prognostic factors of hemifacial spasm after microvascular decompression. J. Korean Neurosurg. Soc. 2009, 45, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Jo, K.W.; Kong, D.S.; Park, K. Microvascular decompression for hemifacial spasm: Long-term outcome and prognostic factors, with emphasis on delayed cure. Neurosurg. Rev. 2013, 36, 297–301, discussion 301–292. [Google Scholar] [CrossRef] [PubMed]
- Haller, S.; Etienne, L.; Kovari, E.; Varoquaux, A.D.; Urbach, H.; Becker, M. Imaging of Neurovascular Compression Syndromes: Trigeminal Neuralgia, Hemifacial Spasm, Vestibular Paroxysmia, and Glossopharyngeal Neuralgia. Am. J. Neuroradiol. 2016, 37, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- House, J.W.; Brackmann, D.E. Facial nerve grading system. Otolaryngol. Head Neck Surg. 1985, 93, 146–147. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Park, S.K.; Lee, S.; Lee, J.A.; Park, K. Lateral spread response of different facial muscles during microvascular decompression in hemifacial spasm. Clin. Neurophysiol. 2021, 132, 2503–2509. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Joo, K.M.; Park, K. Challenging Microvascular Decompression Surgery for Hemifacial Spasm. World Neurosurg. 2021, 151, e94–e99. [Google Scholar] [CrossRef]
- Lee, J.A.; Park, K. Short-term versus long-term outcomes of microvascular decompression for hemifacial spasm. Acta Neurochir. (Wien) 2019, 161, 2027–2033. [Google Scholar] [CrossRef]
- Lee, S.; Park, S.K.; Joo, B.E.; Lee, J.A.; Park, K. Vascular Complications in Microvascular Decompression: A Survey of 4000 Operations. World Neurosurg. 2019, 130, e577–e582. [Google Scholar] [CrossRef]
- Lee, S.; Park, S.K.; Lee, J.A.; Joo, B.E.; Park, K. Missed Culprits in Failed Microvascular Decompression Surgery for Hemifacial Spasm and Clinical Outcomes of Redo Surgery. World Neurosurg. 2019, 129, e627–e633. [Google Scholar] [CrossRef]
- Polo, G.; Fischer, C.; Sindou, M.P.; Marneffe, V. Brainstem auditory evoked potential monitoring during microvascular decompression for hemifacial spasm: Intraoperative brainstem auditory evoked potential changes and warning values to prevent hearing loss--prospective study in a consecutive series of 84 patients. Neurosurgery 2004, 54, 97–104, discussion 104–106. [Google Scholar] [CrossRef]
- McLaughlin, M.R.; Jannetta, P.J.; Clyde, B.L.; Subach, B.R.; Comey, C.H.; Resnick, D.K. Microvascular decompression of cranial nerves: Lessons learned after 4400 operations. J. Neurosurg. 1999, 90, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Joo, B.E.; Kim, J.S.; Deletis, V.; Park, K.S. Advances in Intraoperative Neurophysiology During Microvascular Decompression Surgery for Hemifacial Spasm. J. Clin. Neurol. 2022, 18, 410–420. [Google Scholar] [CrossRef]
- Park, S.K.; Joo, B.E.; Kwon, J.; Kim, M.; Lee, S.; Lee, J.A.; Park, K. A prewarning sign for hearing loss by brainstem auditory evoked potentials during microvascular decompression surgery for hemifacial spasm. Clin. Neurophysiol. 2021, 132, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Joo, B.E.; Park, S.K.; Lee, M.H.; Lee, S.; Lee, J.A.; Park, K. Significance of wave I loss of brainstem auditory evoked potentials during microvascular decompression surgery for hemifacial spasm. Clin. Neurophysiol. 2020, 131, 809–815. [Google Scholar] [CrossRef]
- Park, S.K.; Joo, B.E.; Park, K. Intraoperative Neurophysiological Monitoring during Microvascular Decompression Surgery for Hemifacial Spasm. J. Korean Neurosurg. Soc. 2019, 62, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Dolati, P.; Golby, A.; Eichberg, D.; Abolfotoh, M.; Dunn, I.F.; Mukundan, S.; Hulou, M.M.; Al-Mefty, O. Pre-operative image-based segmentation of the cranial nerves and blood vessels in microvascular decompression: Can we prevent unnecessary explorations? Clin. Neurol. Neurosurg. 2015, 139, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Busse, S.; Taylor, J.; Field, M. Correlation of Preoperative High-Resolution Neurovascular Imaging and Surgical Success in Neurovascular Compression Syndromes. World Neurosurg. 2023, 172, e593–e598. [Google Scholar] [CrossRef]
- Lee, M.H.; Lee, H.S.; Jee, T.K.; Jo, K.I.; Kong, D.S.; Lee, J.A.; Park, K. Cerebellar retraction and hearing loss after microvascular decompression for hemifacial spasm. Acta Neurochir. (Wien) 2015, 157, 337–343. [Google Scholar] [CrossRef]
- Jin, Z.; Li, Z. Clinical Application of Diffusion Tensor Imaging in Diagnosis and Prognosis of Hemifacial Spasm. World Neurosurg. 2021, 145, e14–e20. [Google Scholar] [CrossRef]
- Campbell, E.; Keedy, C. Hemifacial spasm; a note on the etiology in two cases. J. Neurosurg. 1947, 4, 342–347. [Google Scholar] [CrossRef]
- Ko, H.C.; Lee, S.H.; Shin, H.S. Facial Nerve Indentation in Hemifacial Spasm: An Analysis of Factors Contributing to the Formation of and Consequent Effects Associated with Indentation. World Neurosurg. 2021, 146, e1083–e1091. [Google Scholar] [CrossRef] [PubMed]
- Leal, P.R.; Hermier, M.; Souza, M.A.; Cristino-Filho, G.; Froment, J.C.; Sindou, M. Visualization of vascular compression of the trigeminal nerve with high-resolution 3T MRI: A prospective study comparing preoperative imaging analysis to surgical findings in 40 consecutive patients who underwent microvascular decompression for trigeminal neuralgia. Neurosurgery 2011, 69, 15–25, discussion 26. [Google Scholar] [CrossRef] [PubMed]
- Tanrikulu, L.; Scholz, T.; Nikoubashman, O.; Wiesmann, M.; Clusmann, H. Preoperative MRI in neurovascular compression syndromes and its role for microsurgical considerations. Clin. Neurol. Neurosurg. 2015, 129, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Muller, S.; Khadhraoui, E.; Khanafer, A.; Psychogios, M.; Rohde, V.; Tanrikulu, L. Differentiation of arterial and venous neurovascular conflicts estimates the clinical outcome after microvascular decompression in trigeminal neuralgia. BMC Neurol. 2020, 20, 279. [Google Scholar] [CrossRef]
- Sekula, R.F., Jr.; Frederickson, A.M.; Branstetter, B.F.t.; Oskin, J.E.; Stevens, D.R.; Zwagerman, N.T.; Grandhi, R.; Hughes, M.A. Thin-slice T2 MRI imaging predicts vascular pathology in hemifacial spasm: A case-control study. Mov. Disord. 2014, 29, 1299–1303. [Google Scholar] [CrossRef]
- Miller, L.E.; Miller, V.M. Safety and effectiveness of microvascular decompression for treatment of hemifacial spasm: A systematic review. Br. J. Neurosurg. 2012, 26, 438–444. [Google Scholar] [CrossRef]
- Huang, C.I.; Chen, I.H.; Lee, L.S. Microvascular decompression for hemifacial spasm: Analyses of operative findings and results in 310 patients. Neurosurgery 1992, 30, 53–56, discussion 56–57. [Google Scholar] [CrossRef]
- Zhong, J.; Xia, L.; Dou, N.N.; Ying, T.T.; Zhu, J.; Liu, M.X.; Li, S.T. Delayed relief of hemifacial spasm after microvascular decompression: Can it be avoided? Acta Neurochir. (Wien) 2015, 157, 93–98, discussion 98–99. [Google Scholar] [CrossRef]
- Naraghi, R.; Tanrikulu, L.; Troescher-Weber, R.; Bischoff, B.; Hecht, M.; Buchfelder, M.; Hastreiter, P. Classification of neurovascular compression in typical hemifacial spasm: Three-dimensional visualization of the facial and the vestibulocochlear nerves. J. Neurosurg. 2007, 107, 1154–1163. [Google Scholar] [CrossRef]
- Han, I.B.; Chang, J.H.; Chang, J.W.; Huh, R.; Chung, S.S. Unusual causes and presentations of hemifacial spasm. Neurosurgery 2009, 65, 130–137, discussion 137. [Google Scholar] [CrossRef]
- Samii, M.; Gunther, T.; Iaconetta, G.; Muehling, M.; Vorkapic, P.; Samii, A. Microvascular decompression to treat hemifacial spasm: Long-term results for a consecutive series of 143 patients. Neurosurgery 2002, 50, 712–718, discussion 718–719. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Zhu, J.; Sun, H.; Dou, N.N.; Wang, Y.N.; Ying, T.T.; Xia, L.; Liu, M.X.; Tao, B.B.; Li, S.T. Microvascular decompression surgery: Surgical principles and technical nuances based on 4000 cases. Neurol. Res. 2014, 36, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, T.; Rhoton, A.L., Jr.; de Oliveira, E.; Peace, D. Microsurgical anatomy of the veins of the posterior fossa. J. Neurosurg. 1983, 59, 63–105. [Google Scholar] [CrossRef] [PubMed]
- Rhoton, A.L., Jr. The cerebellopontine angle and posterior fossa cranial nerves by the retrosigmoid approach. Neurosurgery 2000, 47, S93–S129. [Google Scholar] [CrossRef]
- Rhoton, A.L., Jr. The posterior fossa veins. Neurosurgery 2000, 47, S69–S92. [Google Scholar] [CrossRef]
- Mercier, P.; Sindou, M. The conflicting vessels in hemifacial spasm: Literature review and anatomical-surgical implications. Neurochirurgie 2018, 64, 94–100. [Google Scholar] [CrossRef]
- Sindou, M.; Mercier, P. Microvascular decompression for hemifacial spasm: Surgical techniques and intraoperative monitoring. Neurochirurgie 2018, 64, 133–143. [Google Scholar] [CrossRef]
- Nagahiro, S.; Takada, A.; Matsukado, Y.; Ushio, Y. Microvascular decompression for hemifacial spasm. Patterns of vascular compression in unsuccessfully operated patients. J. Neurosurg. 1991, 75, 388–392. [Google Scholar] [CrossRef]
- Yeh, H.S.; Tew, J.M., Jr.; Ramirez, R.M. Microsurgical treatment of intractable hemifacial spasm. Neurosurgery 1981, 9, 383–386. [Google Scholar] [CrossRef]
- Kawashima, M.; Yamada, M.; Sato, S.; Oka, H.; Fujii, K.; Matsushima, T. Hemifacial spasm caused by vascular compression of the distal portion of the facial nerve associated with configuration variation of the facial and vestibulocochlear nerve complex. Turk. Neurosurg. 2009, 19, 269–275. [Google Scholar]
- Ryu, H.; Yamamoto, S.; Sugiyama, K.; Uemura, K.; Miyamoto, T. Hemifacial spasm caused by vascular compression of the distal portion of the facial nerve. Report of seven cases. J. Neurosurg. 1998, 88, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Son, B.C.; Ko, H.C.; Choi, J.G. Hemifacial Spasm Caused by Vascular Compression in the Cisternal Portion of the Facial Nerve: Report of Two Cases with Review of the Literature. Case Rep. Neurol. Med. 2019, 2019, 8526157. [Google Scholar] [CrossRef] [PubMed]
| Case No. | Age (Years, Sex) | Side | Symptom Duration | Offending Vessel | Compression Site | Compression Type | Outcome | Recurrence | Complication | FU |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 44, F | Left | 24 | Arteriole | Cisternal (distal) | Arachnoid | Partial | No | No | 60 |
| 2 | 69, F | Left | 240 | AICA | Cisternal | Arachnoid | Complete | No | Transient facial palsy (HB Gr3) | 12 |
| 3 | 71, F | Right | 36 | AICA | Cisternal | Arachnoid | Complete | Yes | No | 56 |
| 4 | 48, F | Left | 38 | Vein | Cisternal | NA | Complete | No | Transient facial palsy (HB Gr3) | 40 |
| 5 | 56, F | Left | 40 | Arteriole | Cisternal | Arachnoid | Complete | No | No | 12 |
| 6 | 58, M | Left | 32 | Arteriole | Cisternal | Arachnoid | Complete | No | No | 12 |
| 7 | 58, M | Left | 6 | Vein | REZ | NA | Complete | No | Transient facial palsy (HB Gr3) | 12 |
| 8 | 70, F | Right | 12 | AICA | REZ (medial) | Arachnoid | Complete | No | No | 12 |
| 9 | 55, F | Left | 4 | AICA | Cisternal (medial) | Perforator | Complete | No | No | 12 |
| 10 | 64, F | Left | 84 | Arteriole | Cisternal (medial) | Loop | Partial | No | No | 6 |
| 11 | 53, F | Left | 36 | AICA | Cisternal | Perforator | Complete | No | No | 12 |
| 12 | 62, F | Left | 60 | AICA | REZ (medial) | Perforator | Complete | No | No | 24 |
| 13 | 68, F | Left | 24 | AICA | Cisternal (between CN VII and CN VIII) | Perforator | Complete | No | Transient facial palsy (HB Gr4) | 24 |
| 14 | 63, F | Left | 84 | PICA (small branch) + vein | REZ + cisternal | Sandwich | Complete | No | No | 24 |
| 15 | 62, F | Right | 240 | Arteriole + vein | Cisternal | Sandwich | Complete | No | No | 12 |
| 16 | 57, F | Right | 72 | AICA | Cisternal (distal) | Arachnoid | Complete | No | No | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, C.; Kim, M.; Lee, H.-S.; Kong, D.-S.; Park, K. Outcomes after Microvascular Decompression for Hemifacial Spasm without Definite Radiological Neurovascular Compression at the Root Exit Zone. Life 2023, 13, 2064. https://doi.org/10.3390/life13102064
Jeon C, Kim M, Lee H-S, Kong D-S, Park K. Outcomes after Microvascular Decompression for Hemifacial Spasm without Definite Radiological Neurovascular Compression at the Root Exit Zone. Life. 2023; 13(10):2064. https://doi.org/10.3390/life13102064
Chicago/Turabian StyleJeon, Chiman, Minsoo Kim, Hyun-Seok Lee, Doo-Sik Kong, and Kwan Park. 2023. "Outcomes after Microvascular Decompression for Hemifacial Spasm without Definite Radiological Neurovascular Compression at the Root Exit Zone" Life 13, no. 10: 2064. https://doi.org/10.3390/life13102064





