Navigating the Post-COVID-19 Immunological Era: Understanding Long COVID-19 and Immune Response
Abstract
:1. Introduction
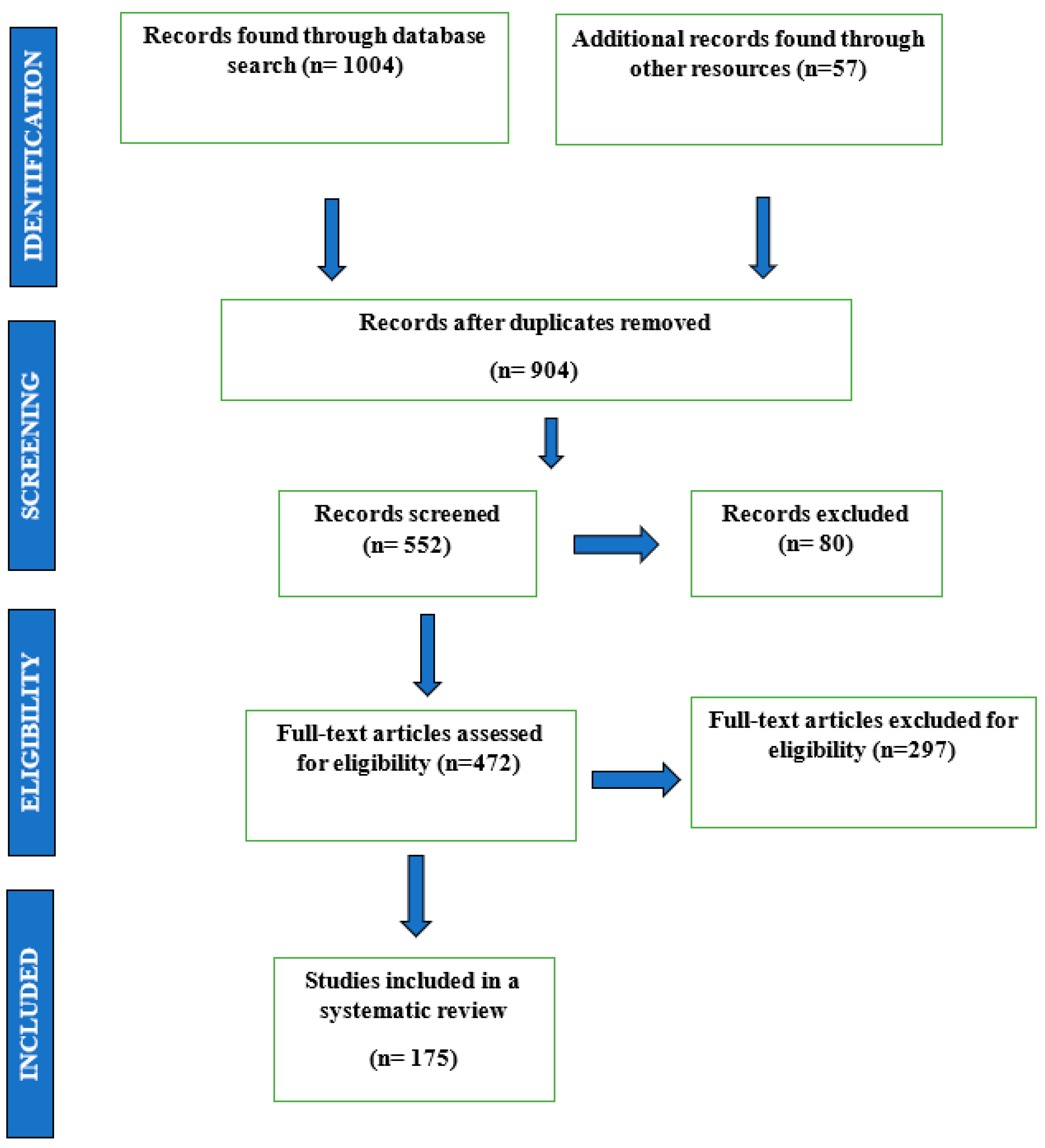
2. Materials and Methods
3. Results
4. Long COVID-19 and Immune Pathology
4.1. Definition and Prevalence of Long COVID-19
4.2. Overview of the Immune Response to COVID-19 and How It Relates to Long COVID-19
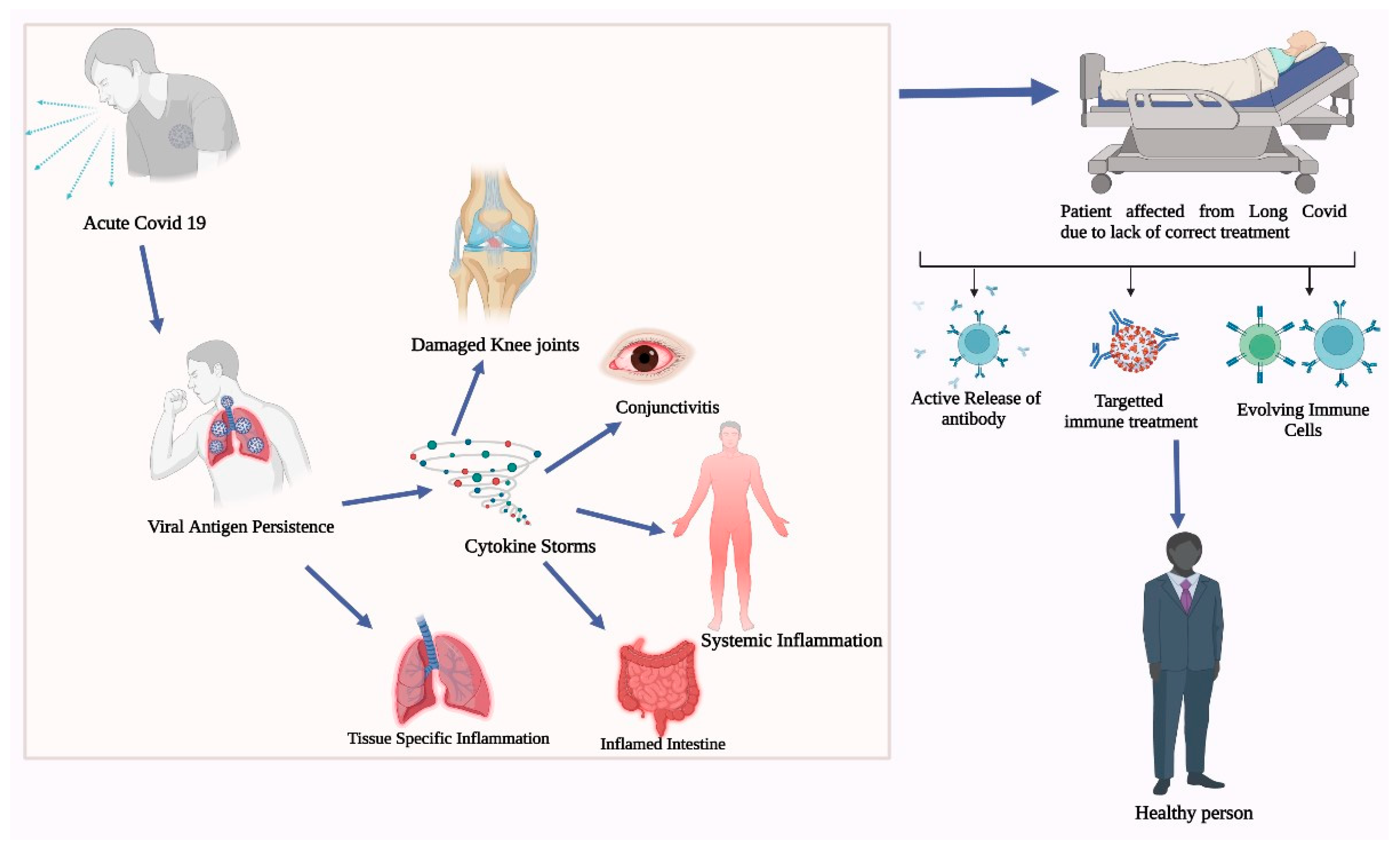
4.3. Mechanism behind Post-COVID-19 Immune Pathology
4.4. Impact of Post-COVID-19 Immune Pathology on Patient Outcomes
| Immune Cell | Treatment Options | Limitations | Advantages | References |
|---|---|---|---|---|
| T cells | Adoptive T-cell therapy | Limited availability of matched donors | Enhanced immune response | [64] |
| Potential for graft-versus-host disease, potential for cytokine release syndrome | Targeted elimination of infected cells | [65] | ||
| B cells | Monoclonal antibody therapy | Infusion-related reactions | Neutralization of viral particles | [66] |
| Potential for allergic reactions | Reduction in disease severity, enhanced clearance of infected cells | [67,68] | ||
| Natural killer (NK) cells | Adoptive NK-cell therapy | Limited availability of matched donors | Efficient elimination of infected cells | [69] |
| Potential for graft-versus-host disease, potential for cytokine release syndrome | Potential for combination therapy | [70] | ||
| Dendritic cells | Dendritic cell-based vaccines | Need for specialized equipment and facilities | Induction of specific immune responses | [71] |
| Variable efficacy based on individual response, ethical considerations | Potential for personalized vaccines | [72] | ||
| Macrophages | Immunomodulatory therapies | Risk of immune suppression | Regulation of immune responses | [73] |
| Secondary infections | Potential for reducing inflammation, modulation of disease progression | [74] |
5. Overview of Autoimmunity in COVID-19 Patients
6. Overview of Autoimmunity in Post-COVID-19 Patients
Mechanisms behind the Development of Autoimmunity in Post-COVID-19 Patients
7. Current Treatments for Long COVID-19
8. Promising New Treatments, Immune Therapies, and Their Limitations
| Treatment/Therapy | Limitations | Advantages | References |
|---|---|---|---|
| Monoclonal antibody | Infusion-related reactions | Reduction in viral load | [156] |
| Allergic reactions | Improved clinical outcomes | [157] | |
| Limited availability | [134,158] | ||
| Antiviral therapies | Potential side effects (e.g., immune reactions, | Reduction in disease severity | [139] |
| gastrointestinal symptoms, | Improved clinical recovery | [67] | |
| hypersensitivity reactions) | [159] | ||
| Paxlovid treatment | Limited availability, side effects, effectiveness varies against specific strains of the virus | Reduction in viral load, authorized for emergency use, complement to vaccination, alleviate symptoms | [139,140] |
| Immune modulators | Risk of immune suppression | Reduction in inflammation | [67] |
| Increased risk of secondary infections | Improved outcomes in severe cases | [159,160,161] | |
| Convalescent plasma therapy | Allergic reactions | Potential reduction in disease severity | [162] |
| Transfusion-related lung injury | Improved outcomes when administered early | [142] | |
| Novel immune therapies | Potential risks (e.g., immune reactions) | Novel therapeutic approaches | [143] |
| Thrombosis | Potential for reducing inflammation | [147] | |
| mRNA vaccines | Cold storage requirements | High efficacy in preventing COVID-19 | [163] |
| Limited global supply | Induction of robust immune response | [164] | |
| Possible adverse events (e.g., allergic reactions) | Potential for reducing inflammation | [165] | |
| Nanoparticle vaccines | Development and manufacturing challenges | Enhanced stability and shelf life | [166] |
| Need for further clinical validations | Potential for targeted delivery | [167] | |
| Cell-based therapies | Limited availability | Potential for personalized treatment | [168] |
| Immune rejection | Multimodal immunomodulatory effects | [169] | |
| Long-term safety concerns | Potential for targeted delivery | [170,171] | |
| Gene editing technologies | Off-target effects | Precise targeting of viral genetic material | [172] |
| Ethical considerations | Potential for preventing viral replication | [173] | |
| Limited clinical applications | Enhanced stability and shelf life | [174,175] |
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Vabret, N.; Britton, G.J.; Gruber, C.; Hegde, S.; Kim, J.; Kuksin, M.; Levantovsky, R.; Malle, L.; Moreira, A.; Park, M.D.; et al. Immunology of COVID-19: Current state of the science. Immunity 2020, 52, 910–941. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical characteristics of Coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel Coronavirus from patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Abu-Rumeileh, S.; Abdelhak, A.; Foschi, M.; Tumani, H.; Otto, M. Guillain-Barré syndrome spectrum associated with COVID-19: An up-to-date systematic review of 73 cases. J. Neurol. 2021, 268, 1133–1170. [Google Scholar] [CrossRef]
- Feldstein, L.R.; Rose, E.B.; Horwitz, S.M.; Collins, J.P.; Newhams, M.M.; Son, M.B.F.; Newburger, J.W.; Kleinman, L.C.; Heidemann, S.M.; Martin, A.A.; et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N. Engl. J. Med. 2020, 383, 334–346. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Tenforde, M.W.; Kim, S.S.; Lindsell, C.J.; Billig Rose, E.; Shapiro, N.I.; Files, D.C.; Gibbs, K.W.; Erickson, H.L.; Steingrub, J.S.; Smithline, H.A.; et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network—United States, March-June 2020. MMWR Morb. Mortal Wkly. Rep. 2020, 69, 993–998. [Google Scholar] [CrossRef]
- Sansone, D.; Tassinari, A.; Valentinotti, R.; Kontogiannis, D.; Ronchese, F.; Centonze, S.; Maggiore, A.; Cegolon, L.; Filon, F.L. Persistence of Symptoms 15 Months since COVID-19 Diagnosis: Prevalence, Risk Factors and Residual Work Ability. Life 2022, 13, 97. [Google Scholar] [CrossRef]
- Yong, S.J. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect Dis. 2021, 53, 737–754. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Cortinovis, M.; Perico, N.; Remuzzi, G. Long-term follow-up of recovered patients with COVID-19. Lancet 2021, 397, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Logue, J.K.; Franko, N.M.; McCulloch, D.J.; McDonald, D.; Magedson, A.; Wolf, C.R.; Chu, H.Y. Sequelae in adults at 6 months after COVID-19 Infection. JAMA Netw. Open 2021, 4, e210830. [Google Scholar] [CrossRef]
- Raveendran, A.V.; Jayadevan, R.; Sashidharan, S. Long COVID: An overview. Diabetes Metab. Syndr. 2021, 15, 869–875. [Google Scholar] [CrossRef]
- Callard, F.; Perego, E. How and why patients made Long Covid. Soc. Sci. Med. 2021, 268, 113426. [Google Scholar] [CrossRef]
- Garg, M.; Maralakunte, M.; Garg, S.; Dhooria, S.; Sehgal, I.; Bhalla, A.S.; Vijayvergiya, R.; Grover, S.; Bhatia, V.; Jagia, P.; et al. The conundrum of ‘Long-COVID-19’: A narrative review. Int. J. Gen. Med. 2021, 14, 2491–2506. [Google Scholar] [CrossRef]
- Paces, J.; Strizova, Z.; Smrz, D.; Cerny, J. COVID-19 and the immune system. Physiol. Res. 2020, 69, 379–388. [Google Scholar] [CrossRef]
- Low, R.N.; Low, R.J.; Akrami, A. A review of cytokine-based pathophysiology of Long COVID symptoms. Front. Med. 2023, 10, 1011936. [Google Scholar] [CrossRef]
- Cleary, S.J.; Pitchford, S.C.; Amison, R.T.; Carrington, R.; Robaina Cabrera, C.L.; Magnen, M.; Looney, M.R.; Gray, E.; Page, C.P. Animal models of mechanisms of SARS-CoV-2 infection and COVID-19 pathology. Br. J. Pharmacol. 2020, 177, 4851–4865. [Google Scholar] [CrossRef]
- Mohandas, S.; Jagannathan, P.; Henrich, T.J.; Sherif, Z.A.; Bime, C.; Quinlan, E.; Portman, M.A.; Gennaro, M.; Rehman, J.; RECOVER Mechanistic Pathways Task Force. Immune mechanisms underlying COVID-19 pathology and post-acute sequelae of SARS-CoV-2 infection (PASC). Elife 2023, 12, e86014. [Google Scholar] [CrossRef]
- Eskandarian Boroujeni, M.; Sekrecka, A.; Antonczyk, A.; Hassani, S.; Sekrecki, M.; Nowicka, H.; Lopacinska, N.; Olya, A.; Kluzek, K.; Wesoly, J.; et al. Dysregulated interferon response and immune hyperactivation in severe COVID-19: Targeting STATs as a novel therapeutic strategy. Front. Immunol. 2022, 13, 888897. [Google Scholar] [CrossRef] [PubMed]
- Altmann, D.M.; Boyton, R.J. SARS-CoV-2 T cell immunity: Specificity, function, durability, and role in protection. Sci. Immunol. 2020, 5, eabd6160. [Google Scholar] [CrossRef] [PubMed]
- Gnanaraj, J.; Parnes, A.; Francis, C.W.; Go, R.S.; Takemoto, C.M.; Hashmi, S.K. Approach to pancytopenia: Diagnostic algorithm for clinical hematologists. Blood Rev. 2018, 32, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, O.B.; Nissen, J.; Cantwell, L.; Schwinn, M.; Tulstrup, M.; Westergaard, D.; Ullum, H.; Brunak, S.; Tommerup, N.; et al. Acute and persistent symptoms in non-hospitalized PCR-confirmed COVID-19 patients. Sci. Rep. 2021, 11, 13153. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, M.G.; De Lorenzo, R. Long-term consequences of COVID-19 on cognitive functioning up to 6 months after discharge: Role of depression and impact on quality of life. Eur. Arch. Psychiatry Clin. Neurosci. 2022, 272, 773–782. [Google Scholar] [CrossRef]
- Dennis, A.; Wamil, M.; Alberts, J.; Oben, J.; Cuthbertson, D.J.; Wootton, D.; Crooks, M.; Gabbay, M.; Brady, M.; Hishmeh, L.; et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: A prospective, community-based study. BMJ Open 2021, 11, e048391. [Google Scholar] [CrossRef]
- Huang, L.; Yao, Q.; Gu, X.; Wang, Q.; Ren, L.; Wang, Y.; Hu, P.; Guo, L.; Liu, M.; Xu, J.; et al. 1-year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet 2021, 398, 747–758. [Google Scholar] [CrossRef]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef]
- Buonsenso, D.; Munblit, D.; De Rose, C.; Sinatti, D.; Ricchiuto, A.; Carfi, A.; Valentini, P. Preliminary evidence on long COVID in children. Acta Paediatr. 2021, 110, 2208–2211. [Google Scholar] [CrossRef]
- The Lancet. Facing up to long COVID. Lancet 2020, 396, 1861. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Guo, R.; Lei, L.; Liu, H.; Wang, Y.; Wang, Y.; Qian, H.; Dai, T.; Zhang, T.; Lai, Y.; et al. Frontline science: COVID-19 infection induces readily detectable morphologic and inflammation-related phenotypic changes in peripheral blood monocytes. J. Leukoc. Biol. 2021, 109, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Alwan, N.A. The road to addressing Long Covid. Science 2021, 373, 491–493. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T cell responses to SARS-CoV-2 Coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 2020, 181, 1489–1501.e15. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.X.; Tang, X.J.; Shi, Q.L.; Li, Q.; Deng, H.J.; Yuan, J.; Hu, J.L.; Xu, W.; Zhang, Y.; Lv, F.J.; et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef]
- Elgellaie, A.; Thomas, S.J.; Kaelle, J.; Bartschi, J.; Larkin, T. Pro-inflammatory cytokines IL-1α, IL-6 and TNF-α in major depressive disorder: Sex-specific associations with psychological symptoms. Eur. J. Neurosci. 2023, 57, 1913–1928. [Google Scholar] [CrossRef]
- Gallais, F.; Velay, A.; Nazon, C.; Wendling, M.J.; Partisani, M.; Sibilia, J.; Candon, S.; Fafi-Kremer, S. Intrafamilial exposure to SARS-CoV-2 associated with cellular immune response without seroconversion, France. Emerg. Infect Dis. 2021, 27, 113–121. [Google Scholar] [CrossRef]
- Varnaitė, R.; García, M.; Glans, H.; Maleki, K.T.; Sandberg, J.T.; Tynell, J.; Christ, W.; Lagerqvist, N.; Asgeirsson, H.; Ljunggren, H.G.; et al. Expansion of SARS-CoV-2-specific antibody-secreting cells and generation of neutralizing antibodies in hospitalized COVID-19 patients. J. Immunol. 2020, 205, 2437–2446. [Google Scholar] [CrossRef]
- Lechner-Scott, J.; Levy, M.; Hawkes, C.; Yeh, A.; Giovannoni, G. Long COVID or post COVID-19 syndrome. Mult. Scler. Relat. Disord. 2021, 55, 103268. [Google Scholar] [CrossRef]
- Venkatesan, P. NICE guideline on long COVID. Lancet Respir. Med. 2021, 9, 129. [Google Scholar] [CrossRef]
- Wang, E.Y.; Mao, T.; Klein, J.; Dai, Y.; Huck, J.D.; Jaycox, J.R.; Liu, F.; Zhou, T.; Israelow, B.; Wong, P.; et al. Diverse functional autoantibodies in patients with COVID-19. Nature 2021, 595, 283–288. [Google Scholar] [CrossRef]
- Stein, S.R.; Ramelli, S.C.; Grazioli, A.; Chung, J.Y.; Singh, M.; Yinda, C.K.; Winkler, C.W.; Sun, J.; Dickey, J.M.; Ylaya, K.; et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature 2022, 612, 758–763. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, C.; Tang, L.; Hong, Z.; Zhou, J.; Dong, X.; Yin, H.; Xiao, Q.; Tang, Y.; Qu, X.; et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020, 5, 434–435. [Google Scholar] [CrossRef]
- Hirabara, S.M.; Serdan, T.D.A.; Gorjao, R.; Masi, L.N.; Pithon-Curi, T.C.; Covas, D.T.; Curi, R.; Durigon, E.L. SARS-CoV-2 variants: Differences and potential of immune evasion. Front. Cell. Infect Microbiol. 2022, 11, 781429. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Pant, A.B. Long Covid: Untangling the complex syndrome and the search for therapeutics. Viruses 2022, 15, 42. [Google Scholar] [CrossRef] [PubMed]
- Tay, M.Z.; Rouers, A.; Fong, S.W.; Goh, Y.S.; Chan, Y.H.; Chang, Z.W.; Xu, W.; Tan, C.W.; Chia, W.N.; Torres-Ruesta, A.; et al. Decreased memory B cell frequencies in COVID-19 delta variant vaccine breakthrough infection. EMBO Mol. Med. 2022, 14, e15227. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Ding, C.; Li, J.; Wang, Y.; Guo, H.; Lu, Z.; Wang, J.; Zheng, C.; Jin, T.; Gao, Y.; et al. Characteristics of patients with coronavirus disease (COVID-19) confirmed using an IgM-IgG antibody test. J. Med. Virol. 2020, 92, 2004–2010. [Google Scholar] [CrossRef]
- Wiech, M.; Chroscicki, P.; Swatler, J.; Stepnik, D.; De Biasi, S.; Hampel, M.; Brewinska-Olchowik, M.; Maliszewska, A.; Sklinda, K.; Durlik, M.; et al. Remodeling of T cell dynamics during Long COVID is dependent on severity of SARS-CoV-2 infection. Front. Immunol. 2022, 13, 886431. [Google Scholar] [CrossRef]
- Zuo, J.; Dowell, A.C.; Pearce, H.; Verma, K.; Long, H.M.; Begum, J.; Aiano, F.; Amin-Chowdhury, Z.; Hoschler, K.; Brooks, T.; et al. Robust SARS-CoV-2-specific T cell immunity is maintained at 6 months following primary infection. Nat. Immunol. 2021, 22, 620–626. [Google Scholar] [CrossRef]
- Zimmermann, P.; Pittet, L.F.; Curtis, N. How common is Long COVID in children and adolescents? Pediatr. Infect Dis. J. 2021, 40, e482–e487. [Google Scholar] [CrossRef] [PubMed]
- Rodda, L.B.; Netland, J.; Shehata, L.; Pruner, K.B.; Morawski, P.A.; Thouvenel, C.D.; Takehara, K.K.; Eggenberger, J.; Hemann, E.A.; Waterman, H.R.; et al. Functional SARS-CoV-2 specific immune memory persists after mild COVID-19. Cell 2021, 184, 169–183.e17. [Google Scholar] [CrossRef]
- Goel, R.R.; Apostolidis, S.A.; Painter, M.M.; Mathew, D.; Pattekar, A.; Kuthuru, O.; Gouma, S.; Hicks, P.; Meng, W.; Rosenfeld, A.M.; et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals following mRNA vaccination. Sci. Immunol. 2021, 6, eabi6950. [Google Scholar] [CrossRef] [PubMed]
- Michelen, M.; Manoharan, L.; Elkheir, N.; Cheng, V.; Dagens, A.; Hastie, C.; O’Hara, M.; Suett, J.; Dahmash, D.; Bugaeva, P.; et al. Characterising long COVID: A living systematic review. BMJ Glob. Health 2021, 6, e005427. [Google Scholar] [CrossRef] [PubMed]
- Mateus, J.; Grifoni, A.; Tarke, A.; Sidney, J.; Ramirez, S.I.; Dan, J.M.; Burger, Z.C.; Rawlings, S.A.; Smith, D.M.; Phillips, E.; et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science 2020, 370, 89–94. [Google Scholar] [CrossRef]
- Thompson, M.G.; Burgess, J.L.; Naleway, A.L.; Tyner, H.; Yoon, S.K.; Meece, J.; Olsho, L.E.W.; Caban-Martinez, A.J.; Fowlkes, A.L.; Lutrick, K.; et al. Prevention and attenuation of COVID-19 with the BNT162b2 and mRNA-1273 vaccines. N. Engl. J. Med. 2021, 385, 320–329. [Google Scholar] [CrossRef]
- Asadi-Pooya, A.A.; Nemati, H.; Shahisavandi, M.; Akbari, A.; Emami, A.; Lotfi, M.; Rostamihosseinkhani, M.; Barzegar, Z.; Kabiri, M.; Zeraatpisheh, Z.; et al. Long COVID in children and adolescents. World J. Pediatr. 2021, 17, 495–499. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Rahmati, M.; Yon, D.K.; Lee, S.W.; Udeh, R.; McEVoy, M.; Kim, M.S.; Gyasi, R.M.; Oh, H.; López Sánchez, G.F.; Jacob, L.; et al. New-onset type 1 diabetes in children and adolescents as postacute sequelae of SARS-CoV-2 infection: A systematic review and meta-analysis of cohort studies. J. Med. Virol. 2023, 95, e28833. [Google Scholar] [CrossRef]
- Fernández-de-Las-Peñas, C.; Palacios-Ceña, D.; Gómez-Mayordomo, V.; Cuadrado, M.L.; Florencio, L.L. Defining post-COVID symptoms (Post-acute COVID, long COVID, persistent post-COVID): An Integrative Classification. Int. J. Environ. Res. Public Health 2021, 18, 2621. [Google Scholar] [CrossRef]
- Rathore, S.; Dahiya, P. Drug repurposing using computational tools and preventive strategies for COVID-19 and future pandemics. J. Health Manag. 2023, 24, 43–52. [Google Scholar]
- Wu, L.P.; Wang, N.C.; Chang, Y.H.; Tian, X.Y.; Na, D.Y.; Zhang, L.Y.; Zheng, L.; Lan, T.; Wang, L.F.; Liang, G.D. Duration of antibody responses after severe acute respiratory syndrome. Emerg. Infect Dis. 2007, 13, 1562–1564. [Google Scholar] [CrossRef]
- Chams, N.; Chams, S.; Badran, R.; Shams, A.; Araji, A.; Raad, M.; Mukhopadhyay, S.; Stroberg, E.; Duval, E.J.; Barton, L.M.; et al. COVID-19: A multidisciplinary review. Front. Public Health 2020, 8, 383. [Google Scholar] [CrossRef]
- Lai, C.K.C.; Lam, W. Laboratory testing for the diagnosis of COVID-19. Biochem. Biophys. Res. Commun. 2021, 538, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Rogers, T.F.; Zhao, F.; Huang, D.; Beutler, N.; Burns, A.; He, W.T.; Limbo, O.; Smith, C.; Song, G.; Woehl, J.; et al. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science 2020, 369, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Majumder, J.; Minko, T. Recent developments on therapeutic and diagnostic approaches for COVID-19. AAPS J. 2021, 23, 14. [Google Scholar] [CrossRef]
- Gavriatopoulou, M.; Ntanasis-Stathopoulos, I.; Korompoki, E.; Fotiou, D.; Migkou, M.; Tzanninis, I.G.; Psaltopoulou, T.; Kastritis, E.; Terpos, E.; Dimopoulos, M.A. Emerging treatment strategies for COVID-19 infection. Clin. Exp. Med. 2021, 21, 167–179. [Google Scholar] [CrossRef]
- Wada, H.; Shiraki, K.; Shimpo, H.; Shimaoka, M.; Iba, T.; Suzuki-Inoue, K. Thrombotic mechanism involving platelet activation, hypercoagulability and hypofibrinolysis in coronavirus disease 2019. Int. J. Mol. Sci. 2023, 24, 7975. [Google Scholar] [CrossRef] [PubMed]
- Manzur-Pineda, K.; O’Neil, C.F.; Bornak, A.; Lalama, M.J.; Shao, T.; Kang, N.; Kennel-Pierre, S.; Tabbara, M.; Velazquez, O.C.; Rey, J. COVID-19-related thrombotic complications experience before and during delta wave. J. Vasc. Surg. 2022, 76, 1374–1382.e1. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.; de Mingo Pulido, Á.; Ruffell, B. Dendritic cells and their role in immunotherapy. Front. Immunol. 2020, 11, 924. [Google Scholar] [CrossRef] [PubMed]
- Morse, M.A.; Nair, S.K.; Mosca, P.J.; Hobeika, A.C.; Clay, T.M.; Deng, Y.; Boczkowski, D.; Proia, A.; Neidzwiecki, D.; Clavien, P.A.; et al. Immunotherapy with autologous, human dendritic cells transfected with carcinoembryonic antigen mRNA. Cancer Investig. 2003, 21, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Recovery Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in hospitalized patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Salama, C.; Han, J.; Yau, L.; Reiss, W.G.; Kramer, B.; Neidhart, J.D.; Criner, G.J.; Kaplan-Lewis, E.; Baden, R.; Pandit, L.; et al. Tocilizumab in patients hospitalized with COVID-19 pneumonia. N. Engl. J. Med. 2021, 384, 20–30. [Google Scholar] [CrossRef]
- Carod-Artal, F.J. Post-COVID-19 syndrome: Epidemiology, diagnostic criteria and pathogenic mechanisms involved. Rev. Neurol. 2021, 72, 384–396. [Google Scholar] [CrossRef]
- Chow, K.W.; Pham, N.V.; Ibrahim, B.M.; Hong, K.; Saab, S. Autoimmune Hepatitis-like syndrome following COVID-19 vaccination: A systematic review of the literature. Dig. Dis. Sci. 2022, 67, 4574–4580. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil extracellular traps in COVID-19. JCI Insight 2020, 5, e138999. [Google Scholar] [CrossRef]
- Chen, C.; Amelia, A.; Ashdown, G.W.; Mueller, I.; Coussens, A.K.; Eriksson, E.M. Risk surveillance and mitigation: Autoantibodies as triggers and inhibitors of severe reactions to SARS-CoV-2 infection. Mol. Med. 2021, 27, 160. [Google Scholar] [CrossRef]
- Vuille-Lessard, É.; Montani, M.; Bosch, J.; Semmo, N. Autoimmune hepatitis triggered by SARS-CoV-2 vaccination. J. Autoimmun. 2021, 123, 102710. [Google Scholar] [CrossRef]
- Woodruff, M.C.; Ramonell, R.P.; Nguyen, D.C.; Cashman, K.S.; Saini, A.S.; Haddad, N.S.; Ley, A.M.; Kyu, S.; Howell, J.C.; Ozturk, T.; et al. Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nat. Immunol. 2020, 21, 1506–1516. [Google Scholar] [CrossRef]
- Cao, T.; Liu, L.; To, K.K.; Lim, C.Y.; Zhou, R.; Ming, Y.; Kwan, K.Y.; Yu, S.; Chan, C.Y.; Zhou, B.; et al. Mitochondrial regulation of acute extrafollicular B-cell responses to COVID-19 severity. Clin. Transl. Med. 2022, 12, e1025. [Google Scholar] [CrossRef]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 370, 4585. [Google Scholar] [CrossRef] [PubMed]
- Meffre, E.; Iwasaki, A. Interferon deficiency can lead to severe COVID. Nature 2020, 587, 374–376. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.L.; Kamceva, M.; Rao, S.A.; Linos, E. Cutaneous manifestations of COVID-19: A preliminary review. J. Am. Acad. Dermatol. 2020, 83, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.S.; Caricchio, R.; Casanova, J.L.; Combes, A.J.; Diamond, B.; Fox, S.E.; Hanauer, D.A.; James, J.A.; Kanthi, Y.; Ladd, V.; et al. The intersection of COVID-19 and autoimmunity. J. Clin. Investig. 2021, 131, e154886. [Google Scholar] [CrossRef]
- Fagyas, M.; Nagy, B., Jr.; Ráduly, A.P.; Mányiné, I.S.; Mártha, L.; Erdősi, G.; Sipka, S., Jr.; Enyedi, E.; Szabó, A.Á.; Pólik, Z.; et al. The majority of severe COVID-19 patients develop anti-cardiac autoantibodies. Geroscience 2022, 44, 2347–2360. [Google Scholar] [CrossRef]
- Cunningham, M.W. Molecular mimicry, autoimmunity, and infection: The cross-reactive antigens of group A Streptococci and their Sequelae. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef]
- Mobasheri, L.; Nasirpour, M.H.; Masoumi, E.; Azarnaminy, A.F.; Jafari, M.; Esmaeili, S.A. SARS-CoV-2 triggering autoimmune diseases. Cytokine 2022, 154, 155873. [Google Scholar] [CrossRef]
- Vahabi, M.; Ghazanfari, T.; Sepehrnia, S. Molecular mimicry, hyperactive immune system, and SARS-CoV-2 are three prerequisites of the autoimmune disease triangle following COVID-19 infection. Int. Immunopharmacol. 2022, 112, 109183. [Google Scholar] [CrossRef]
- Peng, M.Y.; Liu, W.C.; Zheng, J.Q.; Lu, C.L.; Hou, Y.C.; Zheng, C.M.; Song, J.Y.; Lu, K.C.; Chao, Y.C. Immunological aspects of SARS-CoV-2 infection and the putative beneficial role of Vitamin-D. Int. J. Mol. Sci. 2021, 22, 5251. [Google Scholar] [CrossRef]
- Zhou, Y.; Han, T.; Chen, J.; Hou, C.; Hua, L.; He, S.; Guo, Y.; Zhang, S.; Wang, Y.; Yuan, J.; et al. Clinical and autoimmune characteristics of severe and critical cases of COVID-19. Clin. Transl. Sci. 2020, 13, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Groff, D.; Sun, A.; Ssentongo, A.E.; Ba, D.M.; Parsons, N.; Poudel, G.R.; Lekoubou, A.; Oh, J.S.; Ericson, J.E.; Ssentongo, P.; et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection. JAMA Netw. Open. 2021, 4, e2128568. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Murthy, S.; Diaz, J.V.; Slutsky, A.S.; Villar, J.; Angus, D.C.; Annane, D.; Azevedo, L.C.P.; Berwanger, O.; Cavalcanti, A.B.; et al. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA 2020, 324, 1330–1341. [Google Scholar] [CrossRef] [PubMed]
- Bartone, P.T.; McDonald, K.; Hansma, B.J.; Solomon, J. Hardiness moderates the effects of COVID-19 stress on anxiety and depression. J. Affect Disord. 2022, 317, 236–244. [Google Scholar] [CrossRef]
- Polastri, M.; Nava, S.; Clini, E.; Vitacca, M.; Gosselink, R. COVID-19 and pulmonary rehabilitation: Preparing for phase three. Eur. Respir. J. 2020, 55, 2001822. [Google Scholar] [CrossRef]
- Spruit, M.A.; Singh, S.J.; Garvey, C.; ZuWallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.; et al. An official American thoracic society/European respiratory society statement: Key concepts and advances in pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64. [Google Scholar] [CrossRef]
- Holland, A.E.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F.; et al. An official European respiratory society/American thoracic society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1428–1446. [Google Scholar] [CrossRef]
- Larson, J.L.; Covey, M.K.; Wirtz, S.E. Cycle ergometer and inspiratory muscle training in chronic obstructive pulmonary disease. J. Cardiopulm. Rehabil. 2000, 20, 7. [Google Scholar] [CrossRef]
- Nici, L.; Donner, C.; Wouters, E.; Zuwallack, R.; Ambrosino, N.; Bourbeau, J.; Carone, M.; Celli, B.; Engelen, M.; Fahy, B.; et al. American thoracic society/European respiratory society statement on pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2006, 173, 1390–1413. [Google Scholar] [CrossRef]
- Mazza, M.G.; De Lorenzo, R.; Conte, C.; Poletti, S.; Vai, B.; Bollettini, I.; Melloni, E.M.T.; Furlan, R.; Ciceri, F.; Rovere-Querini, P.; et al. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav. Immun. 2020, 89, 594–600. [Google Scholar] [CrossRef]
- Rogers, J.P.; Chesney, E.; Oliver, D.; Pollak, T.A.; McGuire, P.; Fusar-Poli, P.; Zandi, M.S.; Lewis, G.; David, A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 2020, 7, 611–627. [Google Scholar] [CrossRef]
- Hampshire, A.; Trender, W.; Chamberlain, S.R. Cognitive deficits in people who have recovered from COVID-19 relative to controls: An N=84,285 online study. medRxiv 2021. [Google Scholar]
- Zheng, K.I.; Feng, G.; Liu, W.Y.; Targher, G.; Byrne, C.D.; Zheng, M.H. Extrapulmonary complications of COVID-19: A multisystem disease? J. Med. Virol. 2021, 93, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Barker-Davies, R.M.; O’Sullivan, O.; Senaratne, K.P.P.; Baker, P.; Cranley, M.; Dharm-Datta, S.; Ellis, H.; Goodall, D.; Gough, M.; Lewis, S.; et al. The Stanford Hall consensus statement for post-COVID-19 rehabilitation. Br. J. Sports Med. 2020, 54, 949–959. [Google Scholar] [PubMed]
- Negrini, F.; Ferrario, I.; Mazziotti, D. Neuromuscular and balance symptoms as late effects after COVID-19: A case series. Eur. J. Phys. Rehabil. Med. 2021, 57, 306–309. [Google Scholar]
- Lee, M.J.; Sayers, A.E.; Drake, T.M.; Singh, P.; Bradburn, M.; Wilson, T.R.; Murugananthan, A.; Walsh, C.J.; Fearnhead, N.S.; NASBO Steering Group; et al. Malnutrition, nutritional interventions and clinical outcomes of patients with acute small bowel obstruction: Results from a national, multicentre, prospective audit. BMJ Open 2019, 9, 029235. [Google Scholar] [CrossRef]
- Lai, H.Y.; Chang, H.T.; Lin, Y.W.; Huang, S.H.; Hsu, C.Y. Nutritional intervention and the recovery of COVID-19 patients. J. Formos. Med. Assoc. 2021, 120, 1333–1334. [Google Scholar]
- Calder, P.C.; Carr, A.C.; Gombart, A.F.; Eggersdorfer, M. Optimal nutritional status for a well-functioning. immune system is an important factor to protect against viral infections. Nutrients 2020, 12, 1181. [Google Scholar] [CrossRef]
- World Health Organization. Water, Sanitation, Hygiene, and Waste Management for SARS-CoV-2, the Virus that Causes COVID-19. 2020. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-IPC-WASH-2020.4 (accessed on 29 July 2020).
- Shah, K.; Saxena, D.; Mavalankar, D. Secondary impact of COVID-19 on nutrition transition and food security in India. Front. Public Health 2020, 8, 89699. [Google Scholar]
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in Post-COVID-19 syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef]
- Naliboff, B.D.; Wu, S.M.; Schieffer, B.; Bolus, R.; Pham, Q.; Baria, A. A randomized trial of 2 prescription strategies for opioid-treated chronic low back pain: Results from the coordinated opioid use reduction with buprenorphine (CORB) study. Pain 2021, 162, 1631–1642. [Google Scholar] [CrossRef]
- Chauvet-Gélinier, J.C.; Novella, J.L.; Jolly, D. Impact of major depression on chronic medical illness. Encephale 2010, 36, D59–D70. [Google Scholar]
- Alsubaie, M.M.; Abbott, R.; Dunn, B.; Dickens, C.; Keil, T.F.; Henley, W.; Kuyken, W. Mechanisms of action in mindfulness-based cognitive therapy (MBCT) and mindfulness-based stress reduction (MBSR) in people with physical and/or psychological conditions: A systematic review. Clin. Psychol. Rev. 2017, 55, 74–91. [Google Scholar] [CrossRef] [PubMed]
- Dennis, C.L. Peer support within a health care context: A concept analysis. Int. J. Nurs. Stud. 2003, 40, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Carfì, A.; Bernabei, R.; Landi, F. Persistent symptoms in patients after acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Jimenez, J.L.; Prather, K.A.; Tufekci, Z.; Fisman, D.; Schooley, R. Ten scientific reasons in support of airborne transmission of SARS-CoV-2. Lancet 2020, 397, 1603–1605. [Google Scholar]
- Mazza, M.G.; Palladini, M.; De Lorenzo, R.; Magnaghi, C.; Poletti, S.; Furlan, R.; Ciceri, F.; Rovere-Querini, P.; Benedetti, F.; COVID-19 BioB Outpatient Clinic Study Group. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: Effect of inflammatory biomarkers at three-month follow-up. Brain Behav. Immun. 2020, 88, 105–109. [Google Scholar] [CrossRef]
- Menni, C.; Valdes, A.M.; Freidin, M.B.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Ganesh, S.; Varsavsky, T.; Cardoso, M.J.; El-Sayed Moustafa, J.S.; et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat. Med. 2021, 27, 703–709. [Google Scholar] [CrossRef]
- Arnold, D.T.; Hamilton, F.W.; Milne, A.; Morley, A.J.; Viner, J.; Attwood, M.; Noel, A.; Gunning, S.; Hatrick, J.; Hamilton, S.; et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: Results from a prospective UK cohort. Thorax 2021, 76, 399–401. [Google Scholar] [CrossRef]
- Chang, W.T.; Toh, H.S.; Liao, C.T.; Yu, W.L. Comprehensive review of cardiac involvement in COVID-19. Circulation 2021, 143, 409–428. [Google Scholar] [CrossRef]
- Tsutsui, M.; Gerayeli, F.; Sin, D.D. Pulmonary Rehabilitation in a Post-COVID-19 World: Telerehabilitation as a New Standard in Patients with COPD. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Guo, L.; Tian, F.; Dai, T.; Xing, X.; Zhao, J.; Li, Q. Rehabilitation in patients with COVID-19: A systematic review. J. Med. Virol. 2021, 93, 323–335. [Google Scholar] [CrossRef]
- Feng, X.; Liu, Z.; He, X.; Wang, X.; Yuan, C.; Huang, L.; Song, R.; Wu, Y. Malnutrition, nutritional interventions and clinical outcomes of COVID-19: A systematic review and meta-analysis. J. Clin. Med. 2021, 10, 1668. [Google Scholar] [CrossRef]
- Shacham, M.; Hamama-Raz, Y.; Kolerman, R.; Mijiritsky, O.; Ben-Ezra, M.; Mijiritsky, E. Psychological and psychosocial factors associated with Long COVID-19. Int. J. Environ. Res. Public Health 2021, 18, 2857. [Google Scholar] [CrossRef]
- Ahmed, H.; Patel, K.; Greenwood, D.C.; Halpin, S.; Lewthwaite, P.; Salawu, A.; Eyre, L.; Breen, A.; O’Connor, R.; Jones, A.; et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: A systematic review and meta-analysis. J. Rehabil. Med. 2020, 52, 00063. [Google Scholar] [CrossRef] [PubMed]
- Graham, E.L.; Clark, J.R.; Orban, Z.S.; Lim, P.H.; Szymanski, A.L.; Taylor, C.; DiBiase, R.M.; Jia, D.T.; Balabanov, R.; Ho, S.U.; et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized COVID-19 “Long Haulers”. Ann. Clin. Transl. Neurol. 2021, 8, 1073–1085. [Google Scholar] [CrossRef] [PubMed]
- Taquet, M.; Geddes, J.R.; Husain, M.; Luciano, S.; Harrison, P.J. 6-month neurological and psychiatric outcomes in 236,379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psychiatry 2021, 8, 416–427. [Google Scholar] [CrossRef]
- Halpin, S.J.; McIvor, C.; Whyatt, G.; Adams, A.; Harvey, O.; McLean, L.; Walshaw, C.; Kemp, S.; Corrado, J.; Singh, R.; et al. Postdischarge Symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. J. Med. Virol. 2021, 93, 1013–1022. [Google Scholar] [CrossRef]
- Rossen, L.M.; Branum, A.M.; Ahmad, F.B.; Sutton, P.; Anderson, R.N. Excess deaths associated with COVID-19, by age and race and ethnicity—United States. MMWR Morb. Mortal Wkly. Rep. 2020, 69, 1522–1527. [Google Scholar] [CrossRef]
- Townsend, L.; Dowds, J.; O’Brien, K.; Sheill, G.; Dyer, A.H.; O’Kelly, B.; Hynes, J.P.; Mooney, A.; Dunne, J.; Ni Cheallaigh, C.; et al. Persistent poor health post-COVID-19 is not associated with respiratory complications or initial disease severity. Ann. Amer. Thorac. Soc. 2021, 18, 997–1003. [Google Scholar] [CrossRef]
- Weinreich, D.M.; Sivapalasingam, S.; Norton, T.; Ali, S.; Gao, H.; Bhore, R.; Musser, B.J.; Soo, Y.; Rofail, D.; Im, J.; et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N. Engl. J. Med. 2021, 384, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the treatment of COVID-19—Final report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Gonzalez-Rojas, Y.; Juarez, E. Early treatment for COVID-19 with SARS-CoV-2 neutralizing antibody Sotrovimab. N. Engl. J. Med. 2021, 385, 1941–1950. [Google Scholar] [CrossRef]
- Gottlieb, R.L.; Nirula, A.; Chen, P.; Boscia, J.; Heller, B.; Morris, J.; Huhn, G.; Cardona, J.; Mocherla, B.; Stosor, V.; et al. Effect of Bamlanivimab as monotherapy or in combination with Etesevimab on viral load in patients with mild to moderate COVID-19: A randomized clinical trial. JAMA 2021, 325, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Gentry, C.A.; Nguyen, P.; Thind, S.K.; Kurdgelashvili, G.; Williams, R.J. Characteristics and outcomes of US Veterans at least 65 years of age at high risk of severe SARS-CoV-2 infection with or without receipt of oral antiviral agents. J. Infect. 2023, 86, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Nirula, A.; Heller, B.; Gottlieb, R.L.; Boscia, J.; Morris, J.; Huhn, G.; Cardona, J.; Mocherla, B.; Stosor, V.; et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19. N. Engl. J. Med. 2021, 384, 229–237. [Google Scholar] [CrossRef]
- Gottlieb, A.B.; Armstrong, A.W.; FitzGerald, O.; Gladman, D.D. Prologue: Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) 2022 Annual Meeting. J. Rheumatol. 2023, jrheum.2023-0496. [Google Scholar] [CrossRef]
- Camou, F.; Tinevez, C.; Beguet-Yachine, M.; Bellecave, P.; Ratiarison, D.; Tumiotto, C.; Lafarge, X.; Guisset, O.; Mourissoux, G.; Lafon, M.E.; et al. Feasibility of convalescent plasma therapy in severe COVID-19 patients with persistent SARS-CoV-2 viremia. J. Med. Virol. 2021, 93, 5594–5598. [Google Scholar] [CrossRef]
- Wen, W.; Chen, C.; Tang, J.; Wang, C.; Zhou, M.; Cheng, Y.; Zhou, X.; Wu, Q.; Zhang, X.; Feng, Z.; et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: A meta-analysis. Ann. Med. 2022, 54, 516–523. [Google Scholar] [CrossRef]
- Gottlieb, R.L.; Vaca, C.E.; Paredes, R.; Mera, J.; Webb, B.J.; Perez, G.; Oguchi, G.; Ryan, P.; Nielsen, B.U.; Brown, M.; et al. Early Remdesivir to Prevent Progression to Severe COVID-19 in Outpatients. N. Engl. J. Med. 2022, 386, 305–315. [Google Scholar] [CrossRef]
- Spinner, C.D.; Gottlieb, R.L.; Criner, G.J.; Arribas López, J.R.; Cattelan, A.M.; Soriano Viladomiu, A.; Ogbuagu, O.; Malhotra, P.; Mullane, K.M.; Castagna, A.; et al. Effect of Remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: A randomized clinical trial. JAMA 2020, 324, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Parkar, J.; Ansari, A.; Vora, A.; Talwar, D.; Tiwaskar, M.; Patil, S.; Barkate, H. Role of favipiravir in the treatment of COVID-19. Int. J. Infect Dis. 2021, 102, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, A.; Dahiya, P. COVID-19 pandemic: Assessment of current strategies and socio-economic impact. J. Health Manag. 2020, 24, 466–477. [Google Scholar] [CrossRef]
- Piechotta, V.; Chai, K.L.; Valk, S.J.; Kimber, C.; Monsef, I.; Doree, C.; Wood, E.M.; Lamikanra, A.A.; Roberts, D.J.; McQuilten, Z.; et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: A living systematic review. Cochrane Database Syst Rev. 2020, 7, CD013600. [Google Scholar] [CrossRef] [PubMed]
- Leng, Z.; Zhu, R.; Hou, W.; Feng, Y.; Yang, Y.; Han, Q.; Shan, G.; Meng, F.; Du, D.; Wang, S.; et al. Transplantation of ACE2- mesenchymal stem cells improves the outcome of patients with COVID-19 Pneumonia. Aging Dis. 2020, 11, 216–228. [Google Scholar] [CrossRef]
- Shang, L.; Zhao, J.; Hu, Y.; Du, R.; Cao, B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet 2020, 395, 683–684. [Google Scholar] [CrossRef]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, 924–944, Erratum in Lancet 2023, 401, 644. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, V.; Shannon, C.P.; Wei, X.S.; Xiang, X.; Wang, X.; Wang, Z.H.; Tebbutt, S.J.; Kollmann, T.R.; Fish, E.N. Interferon-α2b Treatment for COVID-19. Front. Immunol. 2020, 11, 1061. [Google Scholar] [CrossRef]
- Tripathi, D.; Sodani, M.; Gupta, P.K.; Kulkarni, S. Host directed therapies: COVID-19 and beyond. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100058. [Google Scholar] [CrossRef]
- Sharun, K.; Tiwari, R.; Dhama, J.; Dhama, K. Dexamethasone to combat cytokine storm in COVID-19: Clinical trials and preliminary evidence. Int. J. Surg. 2020, 82, 179–181. [Google Scholar] [CrossRef]
- Annane, D. Corticosteroids for COVID-19. J. Intensive Med. 2021, 1, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Kasiri, H.; Ghazaiean, M.; Rouhani, N.; Naderi-Behdani, F.; Ghazaeian, M.; Ghodssi-Ghassemabadi, R. The effects of colchicine on hospitalized COVID-19 patients: A randomized, double-blind, placebo-controlled clinical trial. J. Investig. Med. 2023, 71, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Castillejos-López, M.; Torres-Espíndola, L.M.; Huerta-Cruz, J.C.; Flores-Soto, E.; Romero-Martinez, B.S.; Velázquez-Cruz, R.; Higuera-Iglesias, A.; Camarena, Á.; Torres-Soria, A.K.; Salinas-Lara, C.; et al. Ivermectin: A Controversial Focal Point during the COVID-19 Pandemic. Life 2022, 12, 1384. [Google Scholar] [CrossRef] [PubMed]
- Fact Sheet for Healthcare Providers: Emergency Use Authorization for Bebtelovimab Highlights of Emergency Use Authorization (EUA). These Highlights of the EUA Do Not Include All the Information Needed to Use Bebtelovimab under the EUA. Published on 2 May 2022. Available online: https://www.fda.gov/media/156152/download (accessed on 5 November 2022).
- Raman, B.; Bluemke, D.A.; Lüscher, T.F.; Neubauer, S. Long COVID: Post-acute sequelae of COVID-19 with a cardiovascular focus. Eur. Heart J. 2022, 43, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Singh, A.; Singh, R.; Misra, A. Molnupiravir in COVID-19: A systematic review of literature. Diabetes Metab. Syndr. 2021, 15, 102329. [Google Scholar] [CrossRef]
- Khetpal, V.; Berkowitz, J.; Vijayakumar, S.; Choudhary, G.; Mukand, J.A.; Rudolph, J.L.; Wu, W.C.; Erqou, S. Long-term cardiovascular manifestations and complications of COVID-19: Spectrum and approach to diagnosis and management. Rhode Isl. Med. J. 2022, 105, 16–22. [Google Scholar]
- Rosas, I.O.; Bräu, N.; Waters, M.; Go, R.C.; Hunter, B.D.; Bhagani, S.; Skiest, D.; Aziz, M.S.; Cooper, N.; Douglas, I.S.; et al. Tocilizumab in hospitalized patients with severe COVID-19 Pneumonia. N. Engl. J. Med. 2021, 384, 1503–1516. [Google Scholar] [CrossRef]
- Rubin, E.J.; Longo, D.L.; Baden, L.R. Interleukin-6 receptor inhibition in COVID-19—Cooling the inflammatory soup. N. Engl. J. Med. 2021, 384, 1564–1565. [Google Scholar] [CrossRef]
- Joyner, M.J.; Wright, R.S.; Fairweather, D.; Senefeld, J.W.; Bruno, K.A.; Klassen, S.A.; Carter, R.E.; Klompas, A.M.; Wiggins, C.C.; Shepherd, J.R.; et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J. Clin. Investig. 2020, 130, 4791–4797. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Haynes, B.F. A new vaccine to battle COVID-19. N. Engl. J. Med. 2021, 384, 470–471. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Li, J.; Song, H.; Han, H.; Wang, Y.; Yin, B.; Farmer, D.L.; Murthy, N.; Wang, A. In utero delivery of mRNA to the heart, diaphragm and muscle with lipid nanoparticles. Bioact. Mater. 2023, 25, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Ryzhikov, A.B.; Ryzhikov, E.A.; Bogryantseva, M.P.; Usova, S.V.; Nechaeva, E.A.; Danilenko, E.D.; Pyankov, S.A.; Gudymo, A.S.; Moiseeva, A.A.; Onkhonova, G.S.; et al. Assessment of safety and prophylactic efficacy of the EpiVacCorona peptide vaccine for COVID-19 prevention (Phase III). Vaccines 2023, 11, 998. [Google Scholar] [CrossRef] [PubMed]
- Chohan, H.K.; Jamal, A.; Mubeen, M.; Khan, M.U.; Junaid, M.; Chohan, M.K.; Imran, A.; Aslam, A.; Anwar, A.; Hashmi, A.A.; et al. The common systemic and local adverse effects of the Sinovac COVID-19 vaccine: An observational study from Pakistan. Cureus 2023, 15, e38564. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Vaccines. In Drugs and Lactation Database (LactMed®); National Institute of Child Health and Human Development: Bethesda, MD, USA, 2023.
- Longueira, Y.; Ojeda, D.S.; Battistelli, R.B.A.; Sanchez, L.; Oviedo Rouco, S.; Albano, D.; Guevara, E.; Valls, V.; Pando, M.A.; Gamarnik, A.V. SARS-CoV-2-Specific IgG and IgA response in maternal blood and breastmilk of vaccinated naïve and convalescent lactating participants. Front. Immunol. 2022, 13, 909995. [Google Scholar] [CrossRef]
- Matthay, M.A.; Calfee, C.S.; Zhuo, H.; Thompson, B.T.; Wilson, J.G.; Levitt, J.E.; Rogers, A.J.; Gotts, J.E.; Wiener-Kronish, J.P.; Bajwa, E.K.; et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): A randomised phase 2a safety trial. Lancet Respir. Med. 2019, 7, 154–162. [Google Scholar] [CrossRef]
- Kreuzberger, N.; Hirsch, C.; Andreas, M.; Böhm, L.; Bröckelmann, P.J.; Di Cristanziano, V.; Golinski, M.; Hausinger, R.I.; Mellinghoff, S.; Lange, B.; et al. Immunity after COVID-19 vaccination in people with higher risk of compromised immune status: A scoping review. Cochrane Database Syst. Rev. 2022, 8, CD015021. [Google Scholar] [CrossRef]
- Abbott, T.R.; Dhamdhere, G.; Liu, Y.; Lin, X.; Goudy, L.; Zeng, L.; Chemparathy, A.; Chmura, S.; Heaton, N.S.; Debs, R.P.; et al. Development of CRISPR as an antiviral strategy to combat SARS-CoV-2 and influenza. Cell 2020, 181, 865–876.e12. [Google Scholar] [CrossRef]
- Al-Saud, H.; Al-Romaih, K.; Bakheet, R.; Mahmoud, L.; Al-Harbi, N.; Alshareef, I.; Judia, S.B.; Aharbi, L.; Alzayed, A.; Jabaan, A.; et al. Automated SARS-CoV-2 RNA extraction from patient nasopharyngeal samples using a modified DNA extraction kit for high throughput testing. Ann. Saudi Med. 2020, 40, 373–381. [Google Scholar] [CrossRef]
- Ayanoğlu, F.B.; Elçin, A.E.; Elçin, Y.M. Bioethical issues in genome editing by CRISPR-Cas9 tec technology. Turk. J. Biol. 2020, 44, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Batra, L.; Malik, M.T. Insights of Novel Coronavirus (SARS-CoV-2) disease outbreak, management and treatment. AIMS Microbiol. 2020, 6, 183. [Google Scholar] [CrossRef] [PubMed]
| Treatment | Description | Key Factors | Survival Rate | Mortality Rate | References |
|---|---|---|---|---|---|
| Symptomatic management | Taking care of specific symptoms, including pain, exhaustion, and cough | Symptom relief | NA | 2–7% | [117] |
| Rehabilitation therapy | Enhancing function and quality of life via physical and occupational therapies | Function improvement, quality of life | 78% | NA | [118] |
| Pulmonary rehabilitation | Exercise-based program to improve respiratory symptoms and lung function | Respiratory symptoms, lung function improvement | 81% | NA | [119] |
| Cognitive behavioural therapy (CBT) | Psychotherapy helps strengthen coping mechanisms and address mental health problems | Coping mechanisms, mental health | 50–75% | NA | [120] |
| Pharmacological interventions | Medications that address long-COVID-19 symptoms or consequences | Symptom management, disease consequences | 95% | 13% | [117] |
| Multidisciplinary clinics | Comprehensive treatment and coordination for long-COVID-19 patients provided by specialized clinics | Coordination, specialized care | NA | 37.5% | [121] |
| Respiratory support | Breathing issues can be treated with oxygen therapy, inhalers, or other respiratory therapies | Breathing issues | 59% | 50–97% | [118] |
| Cardiac management | Cardiovascular problems, such as myocarditis and arrhythmias, are monitored and treated | Cardiovascular problems | NA | 24.2% | [116] |
| Mental health support | Support groups, psychotherapy, and counseling to treat psychological problems and distress | Psychological well-being | 90% | 8.5% | [7] |
| Rehabilitation for specific symptoms | Targeted treatments for symptoms, such as fatigue after exercise or mental fog | Specific symptom management | 87% | 13% | [122] |
| Medications | To treat long-COVID-19 symptoms, such as pain, exhaustion, or inflammation, different drugs may be administered | Symptom management | 69–72% | 1.83% | [24] |
| Pulmonary rehabilitation | Programs of disciplined exercises and breathing drills to enhance lung capacity and stamina | Lung capacity, stamina improvement | 73% | 7.3% | [123] |
| Cognitive rehabilitation | Rehabilitation programs that focus on memory issues, brain fog, and cognitive deficits | Memory issues, cognitive deficits | NA | 15.1% | [124] |
| Physical therapy | Techniques used in physical therapy to treat joint pain, musculoskeletal complaints, and physical restrictions | Joint pain, musculoskeletal issues | 55–60% | 3–5% | [125] |
| Nutritional support | Personalized food regimens and nutritional treatments to aid in recuperation and enhance general well-being | Nutrition, general well-being | NA | 15–20% | [126] |
| Psychological support | Therapy sessions, counseling, and mental health care provided to address anxiety, despair, and emotional well-being | Mental health, emotional well-being | NA | 25% | [13] |
| Challenges in Treating Long COVID-19 | Description | Outcomes | Key Factors | Limitations | References |
|---|---|---|---|---|---|
| Guidelines for treatment that are not standardized | Not having a standardized track of treatment guidelines makes it difficult to provide consistent care | Individualized therapy strategies are necessary due to symptom variability | Lack of standardized treatment guidelines for long COVID-19 | Inconsistent care and potential for ineffective treatments | [127] |
| Symptoms that are complex and multidimensional | Long COVID-19 presents with a variety of intricate symptoms affecting multiple body systems | Interdisciplinary care is necessary to effectively treat the diverse symptoms | Complex and diverse symptomatology of long COVID-19 | Challenges in providing comprehensive care and addressing all symptoms | [24] |
| Reduced knowledge of pathophysiology | The exact causes of long COVID-19 are poorly understood | Additional research is needed to understand the interactions between viral persistence, immune dysregulation, and tissue damage | Limited understanding of the patho-physiological mechanisms underlying long COVID-19 | Difficulties in developing targeted therapies and treatments | [128] |
| Lack of long-term follow-up studies | Few studies examine the long-term effects and progression of long COVID-19 | Long-term follow up is necessary to understand symptom duration, progression, and therapy effectiveness | Limited availability of longitudinal data on the natural course and outcomes of long COVID-19 | Challenges in predicting long-term outcomes and optimizing treatment plans | [129] |
| Psychosocial and mental support | Addressing mental health effects is crucial for comprehensive long-COVID-19 care | Integrated mental health support should be included in the treatment strategies | Impact of long COVID-19 on mental health and psychosocial well-being | Difficulties in providing holistic care and managing mental health aspects | [130] |
| Availability of specialized care | Accessing specialized care for long COVID-19 is challenging due to limited resources | Ensuring fair access to specialized clinics and healthcare practitioners is crucial | Scarcity of specialized clinics and lengthy waiting lists for long-COVID-19 care | Inequitable access to specialized care and potential delays in treatment | [115] |
| Lack of specific therapies | There are no specific therapies or medications currently available for long COVID-19 | Limited targeted treatment options make symptom management challenging | Absence of approved therapies for long COVID-19 | Difficulties in managing symptoms and promoting recovery | [131] |
| Heterogeneity and individual variability | Long COVID-19 manifests differently in individuals and presents a wide spectrum of symptoms | Individualized approaches are necessary to address the variability and diverse symptoms | Variability in symptom presentations and outcomes among individuals with long COVID-19 | Challenges in tailoring treatment plans and interventions for each patient | [24] |
| Effect on quality of life | Long COVID-19 negatively affects physical, cognitive, and psychosocial aspects of life | Treatment goals include addressing the impact of long COVID-19 on overall well-being | Long COVID-19 can have a significant negative impact on an individual’s quality of life | Challenges in improving quality of life and restoring functional abilities | [129] |
| Insufficient rehabilitation programs | There is a lack of specialized rehabilitation programs for long COVID-19 | Access to comprehensive and specialized rehabilitation treatments is necessary for optimal recovery | Limited availability of rehabilitation programs tailored to the needs of individuals with long COVID-19 | Difficulties in optimizing recovery and restoring functional abilities | [132] |
| Long-term follow-up and monitoring sessions | Long-term monitoring and follow-up sessions are crucial to provide ongoing assistance to those with chronic COVID-19 | Establishing post-COVID-19 care programs for long-term symptom management and support | Long-term monitoring and follow-up sessions are necessary to track symptom development and address new problems | Challenges in establishing long-term care programs and ensuring continuity of care | [133] |
| Patient support and education | Providing accurate information and support to patients is essential for their well-being | Educating patients about long COVID-19, self-care techniques, and available services are important | Offering patients the information and resources they need to actively engage in their recovery process | Challenges in disseminating accurate and up-to-date information to patients | [134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohan, A.; Iyer, V.A.; Kumar, D.; Batra, L.; Dahiya, P. Navigating the Post-COVID-19 Immunological Era: Understanding Long COVID-19 and Immune Response. Life 2023, 13, 2121. https://doi.org/10.3390/life13112121
Mohan A, Iyer VA, Kumar D, Batra L, Dahiya P. Navigating the Post-COVID-19 Immunological Era: Understanding Long COVID-19 and Immune Response. Life. 2023; 13(11):2121. https://doi.org/10.3390/life13112121
Chicago/Turabian StyleMohan, Aditi, Venkatesh Anand Iyer, Dharmender Kumar, Lalit Batra, and Praveen Dahiya. 2023. "Navigating the Post-COVID-19 Immunological Era: Understanding Long COVID-19 and Immune Response" Life 13, no. 11: 2121. https://doi.org/10.3390/life13112121
APA StyleMohan, A., Iyer, V. A., Kumar, D., Batra, L., & Dahiya, P. (2023). Navigating the Post-COVID-19 Immunological Era: Understanding Long COVID-19 and Immune Response. Life, 13(11), 2121. https://doi.org/10.3390/life13112121









