Preparing Size-Controlled Liposomes Modified with Polysaccharide Derivatives for pH-Responsive Drug Delivery Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
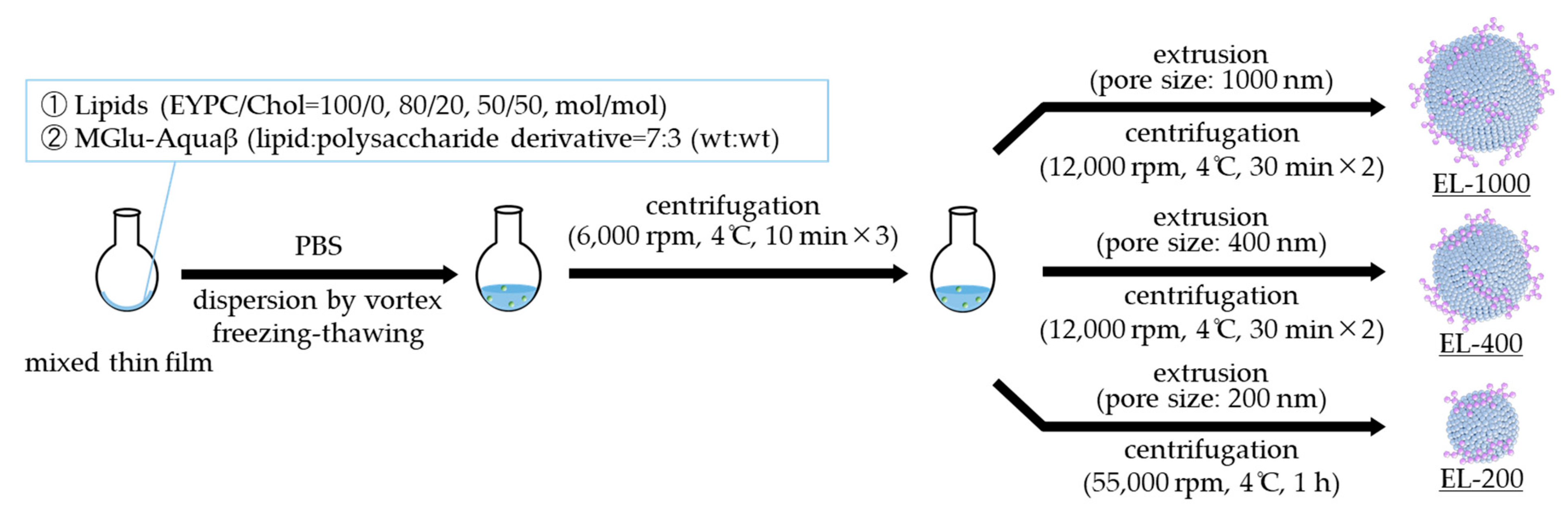
2.2. Preparation of Polysaccharide-Derivative-Modified Liposomes
2.3. Characterization of Polysaccharide-Derivative-Modified Liposomes
2.4. pH-Responsive Properties of Polysaccharide-Derivative-Modified Liposomes
3. Results and Discussion
3.1. Preparation of Size-Controlled Polysaccharide-Derivative-Modified Liposomes
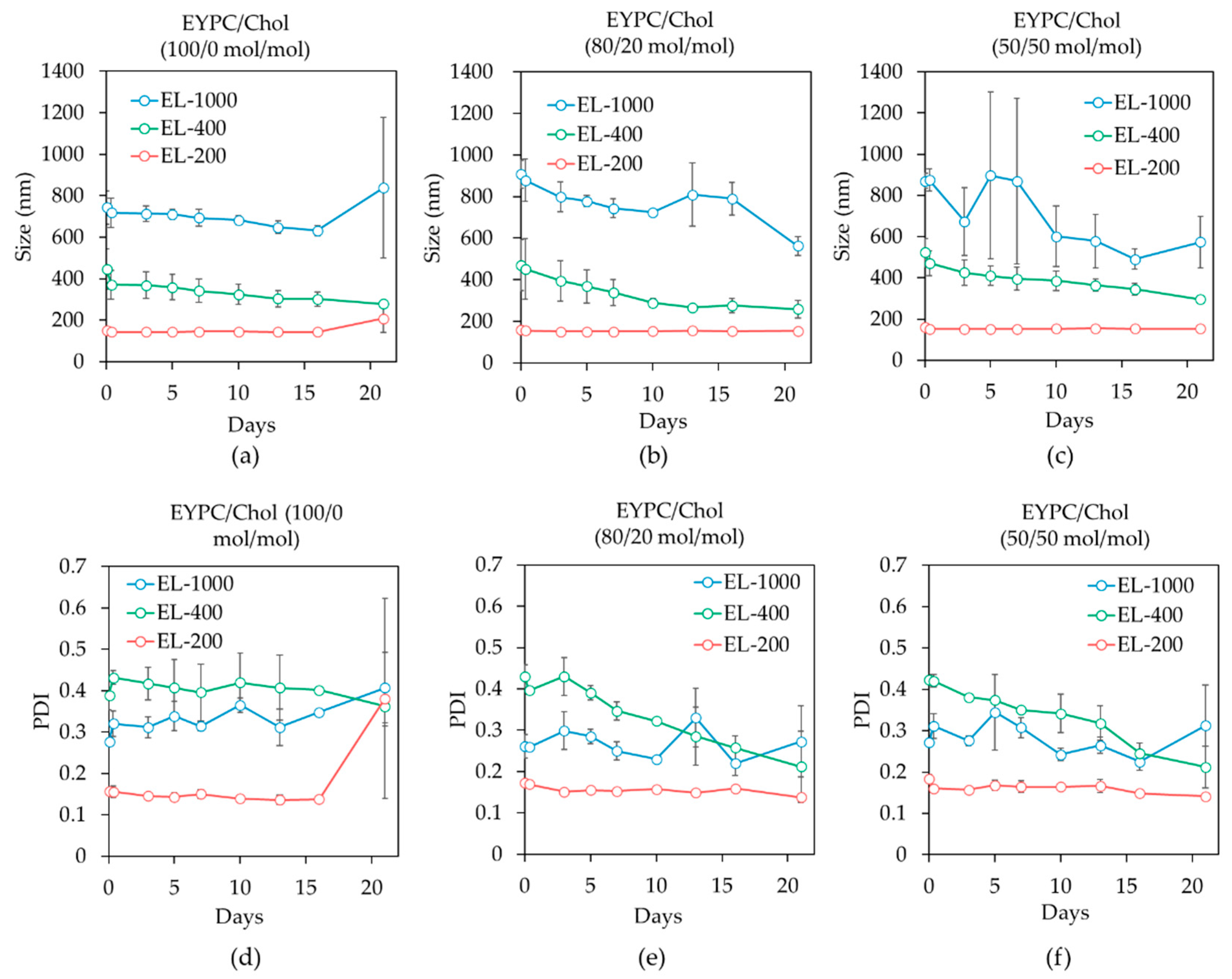
3.2. Cholesterol Content Effects on Liposome Stability
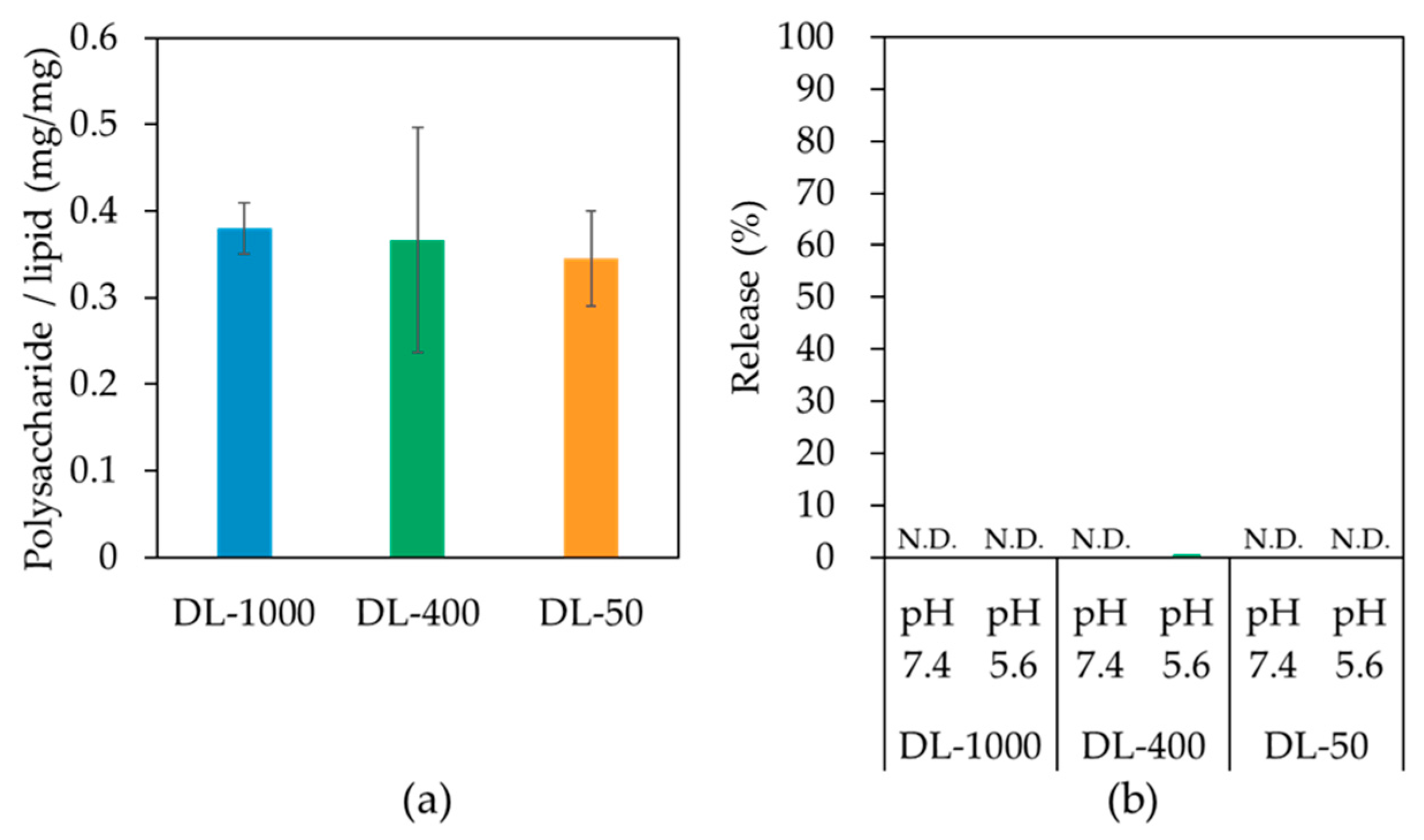
3.3. Liposome Size Effects on pH-Responsive Properties
3.4. Lipid Composition Effects
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pham, S.H.; Choi, Y.; Choi, J. Stimuli-Responsive Nanomaterials for Application in Antitumor Therapy and Drug Delivery. Pharmaceutics 2020, 12, 630. [Google Scholar] [CrossRef] [PubMed]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, Composition, Types, and Clinical Applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Gracia, E.; López-Camacho, A.; Higuera-Ciapara, I.; Velázquez-Fernández, J.B.; Vallejo-Cardona, A.A. Nanomedicine Review: Clinical Developments in Liposomal Applications. Cancer Nanotechnol. 2019, 10, 11. [Google Scholar] [CrossRef]
- Liu, Y.; Castro Bravo, K.M.; Liu, J. Targeted Liposomal Drug Delivery: A Nanoscience and Biophysical Perspective. Nanoscale Horiz. 2021, 6, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Baranov, M.V.; Kumar, M.; Sacanna, S.; Thutupalli, S.; van den Bogaart, G. Modulation of Immune Responses by Particle Size and Shape. Front. Immunol. 2020, 11, 607945. [Google Scholar] [CrossRef] [PubMed]
- Abdul Ghaffar, K.; Kumar Giddam, A.; Zaman, M.; Skwarczynski, M.; Toth, I. Liposomes as Nanovaccine Delivery Systems. Curr. Top. Med. Chem. 2014, 14, 1194–1208. [Google Scholar] [CrossRef]
- Oussoren, C.; Zuidema, J.; Crommelin, D.J.A.; Storm, G. Lymphatic Uptake and Biodistribution of Liposomes after Subcutaneous Injection. II. Influence of Liposomal Size, Lipid Composition and Lipid Dose. Biochim. Biophys. Acta Biomembr. 1997, 1328I, 261–272. [Google Scholar] [CrossRef]
- Nagayasu, A.; Shimooka, T.; Kiwada, H. Effect of Vesicle Size on in Vivo Release of Daunorubicin from Hydrogenated Egg Phosphatidylcholine-Based Liposomes into Blood Circulation. Biol. Pharm. Bull. 1995, 18, 1020–1023. [Google Scholar] [CrossRef]
- Matsuura-Sawada, Y.; Maeki, M.; Uno, S.; Wada, K.; Tokeshi, M. Controlling Lamellarity and Physicochemical Properties of Liposomes Prepared Using a Microfluidic Device. Biomater. Sci. 2023, 11, 2419–2426. [Google Scholar] [CrossRef]
- Grazia Calvagno, M.; Celia, C.; Paolino, D.; Cosco, D.; Iannone, M.; Castelli, F.; Doldo, P.; Fresta, M. Effects of Lipid Composition and Preparation Conditions on Physical-Chemical Properties, Technological Parameters and In Vitro Biological Activity of Gemcitabine-Loaded Liposomes. Curr. Drug Deliv. 2007, 4, 89–101. [Google Scholar] [CrossRef]
- Düzgüneş, N.; Wilschut, J.; Hong, K.; Fraley, R.; Perry, C.; Friend, D.S.; James, T.L.; Papahadjopoulos, D. Physicochemical Characterization of Large Unilamellar Phospholipid Vesicles Prepared by Reverse-Phase Evaporation. Biochim. Biophys. Acta 1983, 732, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Hossann, M.; Wang, T.; Wiggenhorn, M.; Schmidt, R.; Zengerle, A.; Winter, G.; Eibl, H.; Peller, M.; Reiser, M.; Issels, R.D.; et al. Size of Thermosensitive Liposomes Influences Content Release. J. Control. Release 2010, 147, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Allemailem, K.S.; Almatroodi, S.A.; Almatroudi, A.; Rahmani, A.H. Recent Strategies towards the Surface Modification of Liposomes: An Innovative Approach for Different Clinical Applications. 3 Biotech 2020, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.X.; Huang, L.; Gauthier, M.; Yang, G.; Wang, Q. Recent Advances in Liposome Surface Modification for Oral Drug Delivery. Nanomedicine 2016, 11, 1169–1185. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Nie, W.; Dai, L.; Luo, R.; Lin, D.; Zhang, M.; Zhang, J.; Gao, F. Recent Advances in Natural Polysaccharides-Based Controlled Release Nanosystems for Anti-Cancer Phototherapy. Carbohydr. Polym. 2023, 301, 120311. [Google Scholar] [CrossRef]
- De Leo, V.; Milano, F.; Agostiano, A.; Catucci, L. Recent Advancements in Polymer/Liposome Assembly for Drug Delivery: From Surface Modifications to Hybrid Vesicles. Polymers 2021, 13, 1027. [Google Scholar] [CrossRef]
- Sihorkar, V.; Vyas, S.P. Potential of Polysaccharide Anchored Liposomes in Drug Delivery, Targeting and Immunization. J. Pharm. Pharm. Sci. 2001, 4, 138–158. [Google Scholar]
- Li, Z.; Paulson, A.T.; Gillb, T.A. Encapsulation of Bioactive Salmon Protein Hydrolysates with Chitosan-Coated Liposomes. J. Funct. Foods 2015, 19, 733–743. [Google Scholar] [CrossRef]
- Bai, C.; Peng, H.; Xiong, H.; Liu, Y.; Zhao, L.; Xiao, X. Carboxymethylchitosan-Coated Proliposomes Containing Coix Seed Oil: Characterisation, Stability and in Vitro Release Evaluation. Food Chem. 2011, 129, 1695–1702. [Google Scholar] [CrossRef]
- Takechi-Haraya, Y.; Sakai-Kato, K.; Abe, Y.; Kawanishi, T.; Okuda, H.; Goda, Y. Atomic Force Microscopic Analysis of the Effect of Lipid Composition on Liposome Membrane Rigidity. Langmuir 2016, 32, 6074–6082. [Google Scholar] [CrossRef] [PubMed]
- Farzaneh, H.; Ebrahimi Nik, M.; Mashreghi, M.; Saberi, Z.; Jaafari, M.R.; Teymouri, M. A Study on the Role of Cholesterol and Phosphatidylcholine in Various Features of Liposomal Doxorubicin: From Liposomal Preparation to Therapy. Int. J. Pharm. 2018, 551, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Hąc-Wydro, K.; Jędrzejek, K.; Dynarowicz-Łątka, P. Effect of Saturation Degree on the Interactions between Fatty Acids and Phosphatidylcholines in Binary and Ternary Langmuir Monolayers. Colloids Surf. B 2009, 72, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Yuba, E.; Tajima, N.; Yoshizaki, Y.; Harada, A.; Hayashi, H.; Kono, K. Dextran Derivative-Based pH-Sensitive Liposomes for Cancer Immunotherapy. Biomaterials 2014, 35, 3091–3101. [Google Scholar] [CrossRef] [PubMed]
- Yanagihara, S.; Kasho, N.; Sasaki, K.; Shironaka, N.; Kitayama, Y.; Yuba, E.; Harada, A. pH-Sensitive Branched β-Glucan-Modified Liposomes for Activation of Antigen Presenting Cells and Induction of Antitumor Immunity. J. Mater. Chem. B 2021, 9, 7713–7724. [Google Scholar] [CrossRef]
- Yuba, E.; Yamaguchi, A.; Yoshizaki, Y.; Harada, A.; Kono, K. Bioactive Polysaccharide-Based pH-Sensitive Polymers for Cytoplasmic Delivery of Antigen and Activation of Antigen-Specific Immunity. Biomaterials 2017, 120, 32–45. [Google Scholar] [CrossRef]
- Okubo, M.; Miyazaki, M.; Yuba, E.; Harada, A. Chondroitin Sulfate-Based pH-Sensitive Polymer-Modified Liposomes for Intracellular Antigen Delivery and Induction of Cancer Immunity. Bioconjug. Chem. 2019, 30, 1518–1529. [Google Scholar] [CrossRef]
- Miyazaki, M.; Yuba, E.; Hayashi, H.; Harada, A.; Kono, K. Hyaluronic Acid-Based pH-Sensitive Polymer-Modified Liposomes for Cell-Specific Intracellular Drug Delivery Systems. Bioconjug. Chem. 2018, 29, 44–55. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A. Methods of Liposomes Preparation: Formation and Control Factors of Versatile Nanocarriers for Biomedical and Nanomedicine Application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef]
- Shepherd, S.J.; Issadore, D.; Mitchell, M.J. Microfluidic Formulation of Nanoparticles for Biomedical Applications. Biomaterials 2021, 274, 120826. [Google Scholar] [CrossRef]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.-I.; Lee, Y.C. Carbohydrate Analysis by a Phenol–Sulfuric Acid Method in Microplate Format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Monsigny, M.; Petit, C.; Roche, A.C. Colorimetric Determination of Neutral Sugars by a Resorcinol Sulfuric Acid Micromethod. Anal. Biochem. 1988, 175, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.S. Total Carbohydrate by Phenol-Sulfuric Acid Method. In Food Analysis Laboratory Manual, 3rd ed.; Nielsen, S.S., Ed.; Springer: Cham, Switzerland, 2019; pp. 137–141. [Google Scholar]
- Rao, P.; Pattabiraman, T.N. Reevaluation of the Phenol-Sulfuric Acid Reaction for the Estimation of Hexoses and Pentoses. Anal. Biochem. 1989, 181, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Daleke, D.L.; Hong, K.; Papahadjopoulos, D. Endocytosis of Liposomes by Macrophages: Binding, Acidification and Leakage of Liposomes Monitored by a New Fluorescence Assay. Biochim. Biophys. Acta 1990, 1024, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, Y.; Onohara, E.; Kojima, N.; Kuroda, Y. Importance of Particle Size of Oligomannose-Coated Liposomes for Induction of Th1 Immunity. Int. Immunopharmacol. 2021, 99, 108068. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.-Z.; Chen, W.-Y.; Tasi, L.-M.; Yang, S.-P. Microcalorimetric and Shear Studies on the Effects of Cholesterol on the Physical Stability of Lipid Vesicles. Colloids Surf. A 2000, 172, 57–67. [Google Scholar] [CrossRef]
- Zhao, L.; Temelli, F.; Curtis, J.M.; Chen, L. Preparation of Liposomes Using Supercritical Carbon Dioxide Technology: Effects of Phospholipids and Sterols. Food Res. Int. 2015, 77, 63–72. [Google Scholar] [CrossRef]
- Doskocz, J.; Dałek, P.; Foryś, A.; Trzebicka, B.; Przybyło, M.; Mesarec, L.; Iglič, A.; Langner, M. The Effect of Lipid Phase on Liposome Stability upon Exposure to the Mechanical Stress. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183361. [Google Scholar] [CrossRef]
- Müller, R.H.; Jacobs, C.; Kayser, O. Nanosuspensions as Particulate Drug Formulations in Therapy. Rationale for Development and What We Can Expect for the Future. Adv. Drug Deliv. Rev. 2001, 47, 3–19. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, W.; Zou, L.; Liu, W.; Liu, C.; Liang, R.; Chen, J. Storage Stability and Skin Permeation of Vitamin C Liposomes Improved by Pectin Coating. Colloids Surf. B Biointerfaces 2014, 117, 330–337. [Google Scholar] [CrossRef]
- Smistad, G.; Nyström, B.; Zhu, K.; Grønvold, M.K.; Røv-Johnsen, A.; Hiorth, M. Liposomes Coated with Hydrophobically Modified Hydroxyethyl Cellulose: Influence of Hydrophobic Chain Length and Degree of Modification. Colloids Surf. B 2017, 156, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Serfis, A.B.; Brancato, S.; Fliesler, S.J. Comparative Behavior of Sterols in Phosphatidylcholine-Sterol Monolayer Films. Biochim. Biophys. Acta Biomembr. 2001, 1511, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Haldar, S. Interactions between Cholesterol and Lipids in Bilayer Membranes. Role of Lipid Headgroup and Hydrocarbon Chain–backbone Linkage. Biochim. Biophys. Acta Biomembr. 2000, 1467, 39–53. [Google Scholar] [CrossRef]
- Hatzakis, N.S.; Bhatia, V.K.; Larsen, J.; Madsen, K.L.; Bolinger, P.-Y.; Kunding, A.H.; Castillo, J.; Gether, U.; Hedegård, P.; Stamou, D. How Curved Membranes Recruit Amphipathic Helices and Protein Anchoring Motifs. Nat. Chem. Biol. 2009, 5, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Morata, L.; Giannotti, M.I.; Sanz, F. Influence of Cholesterol on the Phase Transition of Lipid Bilayers: A Temperature-Controlled Force Spectroscopy Study. Langmuir 2012, 28, 12851–12860. [Google Scholar] [CrossRef]
- Gürsoy, A.; Kut, E.; Ozkirimli, S. Co-Encapsulation of Isoniazid and Rifampicin in Liposomes and Characterization of Liposomes by Derivative Spectroscopy. Int. J. Pharm. 2004, 271, 115–123. [Google Scholar] [CrossRef]
- Jo, S.-M.; Lee, H.Y.; Kim, J.-C. Glucose-Sensitivity of Liposomes Incorporating Conjugates of Glucose Oxidase and poly(N-Isopropylacrylamide-Co-Methacrylic Acid-Co-Octadecylacrylate). Int. J. Biol. Macromol. 2009, 45, 421–426. [Google Scholar] [CrossRef]
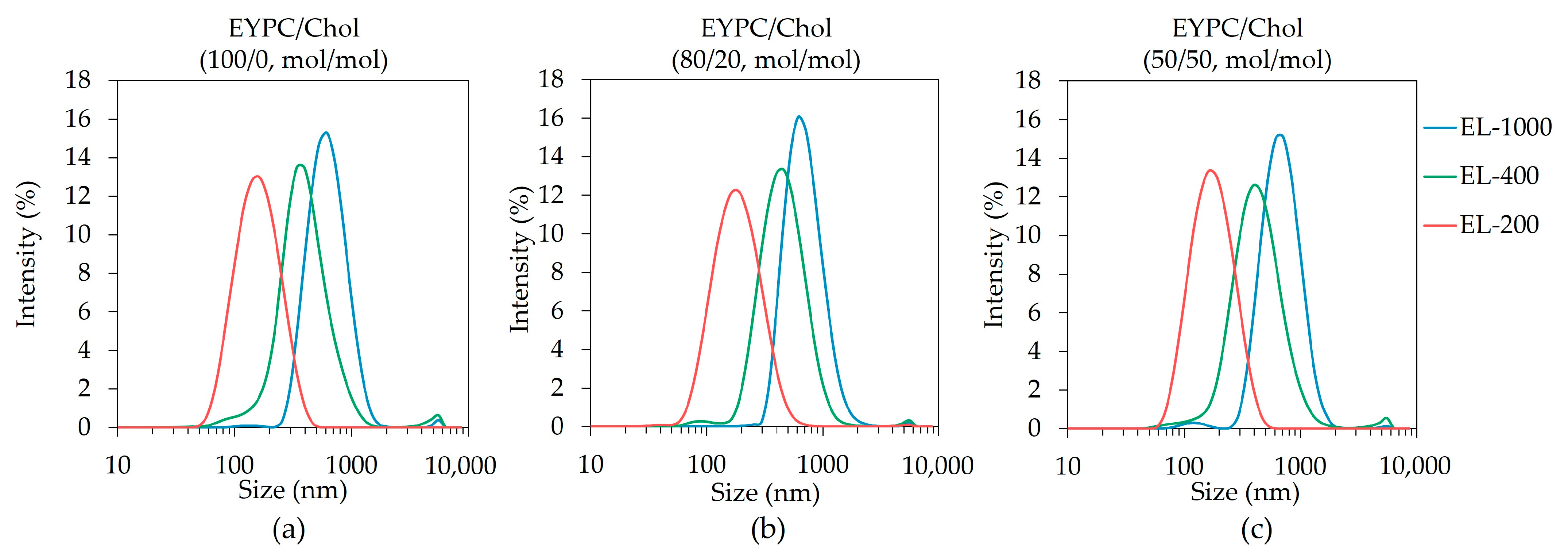


| Liposome | Pore Size of Membrane for Extrusion | EYPC/Chol (100/0, mol/mol) | EYPC/Chol (80/20, mol/mol) | EYPC/Chol (50/50, mol/mol) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Size (nm) | PDI | ξ-Potential (mV) | Size (nm) | PDI | ξ-Potential (mV) | Size (nm) | PDI | ξ-Potential (mV) | ||
| EL-1000 | 1000 nm | 588.5 ± 9.5 | 0.284 | −31.7 | 655.8 ± 31.2 | 0.226 | −31.3 | 630.1 ± 18.6 | 0.278 | −30.1 |
| EL-400 | 400 nm | 346.4 ± 25.6 | 0.237 | −29.6 | 402.8 ± 5.8 | 0.248 | −16.1 | 384.5 ± 11.1 | 0.296 | −24.8 |
| EL-200 | 200 nm | 142.6 ± 5.6 | 0.168 | −29.3 | 162.2 ± 6.6 | 0.185 | −15.0 | 155.5 ± 4.1 | 0.169 | −36.0 |
| Liposome | Pore Size of Membrane for Extrusion | Size (nm) | PDI |
|---|---|---|---|
| DL-1000 | 1000 nm | 1019.4 ± 137.2 | 0.298 |
| DL-400 | 400 nm | 560.2 ± 39.9 | 0.243 |
| DL-50 | 50 nm | 104.6 ± 3.3 | 0.181 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yanagihara, S.; Kitayama, Y.; Yuba, E.; Harada, A. Preparing Size-Controlled Liposomes Modified with Polysaccharide Derivatives for pH-Responsive Drug Delivery Applications. Life 2023, 13, 2158. https://doi.org/10.3390/life13112158
Yanagihara S, Kitayama Y, Yuba E, Harada A. Preparing Size-Controlled Liposomes Modified with Polysaccharide Derivatives for pH-Responsive Drug Delivery Applications. Life. 2023; 13(11):2158. https://doi.org/10.3390/life13112158
Chicago/Turabian StyleYanagihara, Shin, Yukiya Kitayama, Eiji Yuba, and Atsushi Harada. 2023. "Preparing Size-Controlled Liposomes Modified with Polysaccharide Derivatives for pH-Responsive Drug Delivery Applications" Life 13, no. 11: 2158. https://doi.org/10.3390/life13112158






