The Therapeutic Potential of Amphetamine-like Psychostimulants
Abstract
:1. Introduction
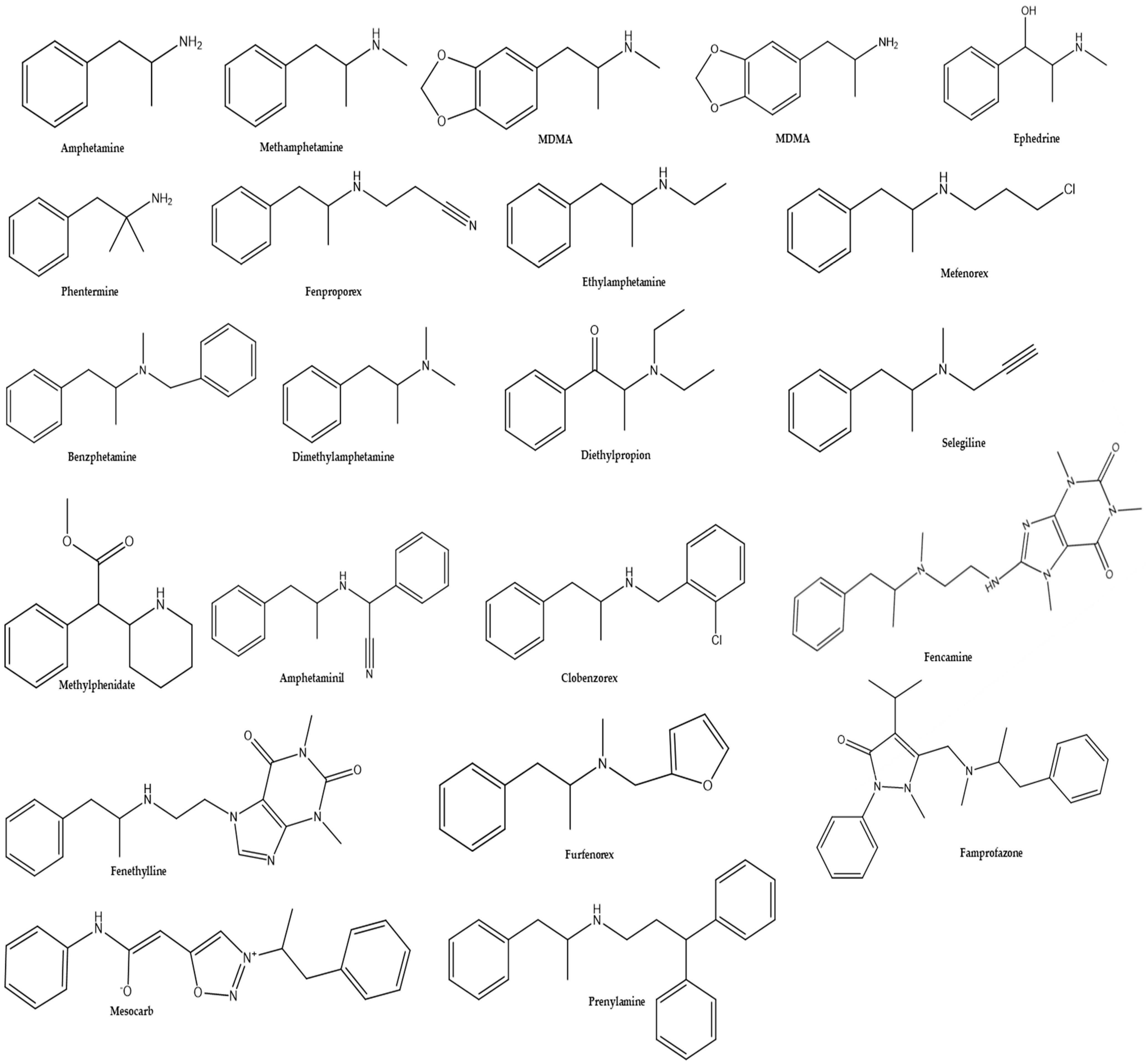
2. Brief History
3. Toxicokinetics
4. Therapeutic Applications
4.1. Lisdexamphetamine Dimesylate
4.2. Mixed Amphetamine Salts
4.3. 3,4-Methylenedioxymethamphetamine (MDMA)
4.4. Amphetamine, Phentermine, Dexamphetamine, and Dextroamphetamine
| Amphetamine-like Compounds Used | Dosage (mg) | Study Conditions | Type of Study | Duration (Weeks/Months) | Participants (Ages) | Results | Reference |
|---|---|---|---|---|---|---|---|
| MAS | 12.5 to 50 mg | 4 weeks of dose optimization; 11 months of dose maintenance; and a 30-day (±5 days) follow-up period | Phase 3, multicentered; open-label extension of two Phase 3 studies | 12 months | 505 Adults (between 18 and 55 years of age) | Long-term safety and tolerability; long-term effectiveness in the treatment of ADHD symptoms for up to 12 months. | [55] |
| LDX | 10; 15; 20; | Four periods: screening and washout; dose optimization (6 weeks); dose maintenance (2 weeks); and safety follow-up (1 week) | Phase 2, multicentered; open-label dose-optimization study | ~11 months | Children (between 4 and 5 years of age) | Safety and tolerability were consistent with its known effects in older children and adolescents with ADHD; the titration scheme used was well tolerated and conferred treatment benefits. | [46] |
| MAS | 25, 50, and 75 | 2-week screening phase; 1- to 4-week washout phase; 6-week forced-dose double-blind treatment phase; 30-day (±5 days) follow-up period | Randomized; placebo; controlled, double-blind, forced-dose study | 7 months | Adults (between 18 and 55 years of age) | All triple-bead MAS doses assessed (25, 50, and 75 mg) were statistically superior to the placebo treatment in reducing ADHD symptoms in adults; there was no statistical evidence of a dose–response relationship for efficacy; the short-term safety and tolerability profiles of the triple-bead MAS were like other long-acting stimulants. | [54] |
| LDX | 20; 30 and 70 mg/d. | 10-week crossover trial with 2 double-blind treatment periods consisting of 4 weeks each and an intervening 2-week single-blind placebo washout | Randomized placebo; controlled, crossover trial | 27 months | 38 Adult (between 18 and 60 years of age) | Significant improvements after LDX vs. placebo were showed for comorbid sluggish cognitive tempo in adults with ADHD. | [47] |
| LDX | 5, 10, 15, 20, or 30 | long-term study including four periods: screening and washout; dose optimization; dose maintenance; safety follow-up | Phase 3, open-label; multicentered | 52 weeks | 113 children (between 4 and 5 years of age) | At doses between 5 and 30 mg/d, the treatment was found to be safe and well tolerated; no new safety signals were identified. The efficacy profile was consistent, with robust improvements in ADHD. | [44] |
| LDX | 10-, 20-, and 30-mg doses | Screening and washout (1–4 weeks); fixed-dose titration (3 weeks); dose maintenance (3 weeks); safety follow-up (1 week) | Phase 3 randomized; double-blind; multicentered, parallel group; PBO-controlled; fixed-dose study | ~13 months | Children (between 4 and 5 years of age) | LDX was generally well tolerated; no new safety signals were identified. | [45] |
| LDX | from 30 to 70 mg daily | N.A. | Randomized; placebo; controlled trial | 12 weeks | 58 children and young adults (between 6 and 25 years of age) | Active treatment significantly improved the inattentive, hyperactive/impulsive, and emotional lability subscales. | [48] |
| amphetamine extended-release oral suspension | 2.5–20 mg/day | 1–4 weeks of screening; 5-week open-label period | Phase 3, randomized; dose-optimized; double-blind, placebo; controlled; laboratory classroom assessment | 10 weeks | 99 children (between 6 and 12 years of age) | Robust and consistent effects beginning early in the morning and continuing throughout the day in the treatment of symptoms. | [67] |
| d-ATS | 5, 10, 15, and, 20 mg; (equivalent to approved doses of 4.5 mg/9 h, 9 mg/9 h, 13.5 mg/9 h, and 18 mg/9 h, respectively | Open-label dose-optimization period. Randomized, crossover double-blind treatment period | Open-label dose-optimization period preceded a randomized, crossover double-blind treatment | 7 weeks | 110 children and adolescents (between 6 and 17 years of age) | d-ATS was effective in the treatment of ADHD in children and adolescents; d-ATS represents an important innovation for the known population of patients with ADHD who respond better to amphetaminethan methylphenidate. | [69] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADD | Attention deficit disorder |
| ADHD | Attention deficit hyperactivity disorder |
| Amphetaminil | α-methylphenethyl(amino)phenylacetonitrile |
| BD | Bipolar disorder |
| Benzphetamine | N-benzyl-N,-dimethylphenethylamine |
| CBD | Cannabidiol |
| Clobenzorex | N-(2-chlorobenzyl)-amphetamine |
| CNS | Central nervous system |
| COMT | Catechol-O-methyl transferase |
| CUD | Cocaine use disorder |
| CYP | Cytocrome P450 |
| d-ATS | Dextroamphetamine transdermal system |
| DAT | Dopamine transporter deficiencies |
| EMCDDA | European Monitoring Centre for Drugs and Drug Addiction |
| EROS | Extended-release oral suspension |
| FC | Functional connectivity |
| FDA | Food and Drug Administration |
| LC-NE | Locus coeruleus–norepinephrine |
| LDX | Lisdexamphetamine Dimesylate |
| LSD | Lysergic acid diethylamide |
| LTI | Life-threatening illness |
| MAS | Mixed amphetamine salts |
| MDA | 3,4-methylenedioxyamphetamine |
| MDMA | 3,4-methylenedioxymethamphetamine |
| NNT | Number needed to treat |
| PFC | Prefrontal cortex |
| PTG | Posttraumatic growth |
| PTSD | Post-traumatic stress disorder |
| PWS | Prader–Willi syndrome |
| SCT | Sluggish cognitive tempo |
| TEAE | Treatment-emergent adverse events |
| THC | Tetrahydrocannabinol |
| UNODC | United Nations Office on Drugs and Crime |
References
- Busch, F.N. Problem-Focused Psychodynamic Psychotherapies. Psychiatr. Serv. 2021, 72, 1461–1463. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.C.; Schwartz, A.C.; Kaslow, N.J. Evidence-Based Psychotherapy: Advantages and Challenges. Neurotherapeutics 2017, 14, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Ray, L.A.; Meredith, L.R.; Kiluk, B.D.; Walthers, J.; Carroll, K.M.; Magill, M. Combined Pharmacotherapy and Cognitive Behavioral Therapy for Adults with Alcohol or Substance Use Disorders: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2020, 3, e208279. [Google Scholar] [CrossRef] [PubMed]
- Barnett, B.S.; Morris, N.P.; Suzuki, J. Addressing In-Hospital Illicit Substance Use. Lancet Psychiatry 2021, 8, 17–18. [Google Scholar] [CrossRef]
- Reiff, C.M.; Richman, E.E.; Nemeroff, C.B.; Carpenter, L.L.; Widge, A.S.; Rodriguez, C.I.; Kalin, N.H.; McDonald, W.M. Psychedelics and Psychedelic-Assisted Psychotherapy. Am. J. Psychiatry 2020, 177, 391–410. [Google Scholar] [CrossRef]
- Trope, A.; Anderson, B.T.; Hooker, A.R.; Glick, G.; Stauffer, C.; Woolley, J.D. Psychedelic-Assisted Group Therapy: A Systematic Review Alexander. Psychoact. Drugs 2019, 51, 174–188. [Google Scholar] [CrossRef]
- Sessa, B. MDMA and PTSD Treatment: “PTSD: From Novel Pathophysiology to Innovative Therapeutics”. Neurosci. Lett. 2017, 649, 176–180. [Google Scholar] [CrossRef]
- Cramer, H.; Anheyer, D.; Saha, F.J.; Dobos, G. Yoga for Posttraumatic Stress Disorder—A Systematic Review and Meta-Analysis. BMC Psychiatry 2018, 18, 72. [Google Scholar] [CrossRef]
- Lowe, H.; Toyang, N.; Steele, B.; Valentine, H.; Grant, J.; Ali, A.; Ngwa, W.; Gordon, L. The Therapeutic Potential of Psilocybin. Molecules 2021, 26, 2948. [Google Scholar] [CrossRef]
- Davis, A.K.; Barrett, F.S.; May, D.G.; Cosimano, M.P.; Sepeda, N.D.; Johnson, M.W.; Finan, P.H.; Griffiths, R.R. Effects of Psilocybin-Assisted Therapy on Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2021, 78, 481–489. [Google Scholar] [CrossRef]
- Simão, A.Y.; Antunes, M.; Cabral, E.; Oliveira, P.; Rosendo, L.M.; Brinca, A.T.; Alves, E.; Marques, H.; Rosado, T.; Passarinha, L.A.; et al. An Update on the Implications of New Psychoactive Substances in Public Health. Int. J. Environ. Res. Public Health 2022, 19, 4869. [Google Scholar] [CrossRef]
- Vargas, A.S.; Luís, Â.; Barroso, M.; Gallardo, E.; Pereira, L. Psilocybin as a New Approach to Treat Depression and Anxiety in the Context of Life-Threatening Diseases-A Systematic Review and Meta-Analysis of Clinical Trials. Biomedicines 2020, 8, 331. [Google Scholar] [CrossRef]
- Gonçalves, J.; Rosado, T.; Soares, S.; Simão, A.; Caramelo, D.; Luís, Â.; Fernández, N.; Barroso, M.; Gallardo, E.; Duarte, A. Cannabis and Its Secondary Metabolites: Their Use as Therapeutic Drugs, Toxicological Aspects, and Analytical Determination. Medicines 2019, 6, 31. [Google Scholar] [CrossRef]
- Antunes, M.; Barroso, M.; Gallardo, E. Analysis of Cannabinoids in Biological Specimens: An Update. Int. J. Environ. Res. Public Health 2023, 20, 2312. [Google Scholar] [CrossRef] [PubMed]
- Bakouni, H.; McAnulty, C.; Tatar, O.; Socias, M.E.; Le Foll, B.; Lim, R.; Ahamad, K.; Jutras-Aswad, D. Associations of Methadone and Buprenorphine-Naloxone Doses with Unregulated Opioid Use, Treatment Retention, and Adverse Events in Prescription-Type Opioid Use Disorders: Exploratory Analyses of the OPTIMA Study. Am. J. Addict. 2023, 32, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Toljan, K.; Vrooman, B. Low-Dose Naltrexone (LDN)-Review of Therapeutic Utilization. Med. Sci. 2018, 6, 82. [Google Scholar] [CrossRef]
- Younger, J.; Parkitny, L.; McLain, D. The Use of Low-Dose Naltrexone (LDN) as a Novel Anti-Inflammatory Treatment for Chronic Pain. Clin. Rheumatol. 2014, 33, 451–459. [Google Scholar] [CrossRef]
- Costa, V.M. Pharmacology and Toxicology of Amphetamine-Type Stimulants. Future Pharmacol. 2023, 3, 515–516. [Google Scholar]
- United Nations Office on Drugs and Crime (ONUDC). Drug Market Trends: Cocaine Amphetamine-Type Stimulants; ONUDC: Vienna, Austria, 2021. [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). European Drug Report: Trends and Developments; EMCDDA: Lisbon, Portugal, 2021. [Google Scholar]
- Carvalho, M.; Carmo, H.; Costa, V.M.; Capela, J.P.; Pontes, H.; Remião, F.; Carvalho, F.; De Lourdes Bastos, M. Toxicity of Amphetamines: An Update. Arch. Toxicol. 2012, 86, 1167–1231. [Google Scholar] [CrossRef]
- The Drugs of Misuse. In Auricular Acupuncture & Addiction; Churchill Livingstone: London, UK, 2009; pp. 93–135.
- Morelli, M.; Tognotti, E. Brief History of the Medical and Non-Medical Use of Amphetamine-like Psychostimulants. Exp. Neurol. 2021, 342, 113754. [Google Scholar] [CrossRef]
- Pryor, K.O.; Storer, K.P. Drugs for Neuropsychiatric Disorders. In Pharmacology and Physiology for Anesthesia: Foundations and Clinical Application; Elsevier: Amsterdam, The Netherlands, 2018; pp. 241–269. ISBN 9780323481106. [Google Scholar]
- Richards, J.R.; Hamidi, S.; Grant, C.D.; Wang, C.G.; Tabish, N.; Turnipseed, S.D.; Derlet, R.W. Methamphetamine Use and Emergency Department Utilization: 20 Years Later. J. Addict. 2017, 2017, 4050932. [Google Scholar] [CrossRef] [PubMed]
- What Is the History of MDMA?|National Institute on Drug Abuse (NIDA). Available online: https://nida.nih.gov/publications/research-reports/mdma-ecstasy-abuse/what-is-the-history-of-mdma (accessed on 14 July 2023).
- Naranjo, C.; Shulgin, A.T.; Sargent, T. Evaluation of 3,4-Methylenedioxyamphetamine (MDA) as an Adjunct to Psychotherapy. Pharmacology 1978, 17, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, L.; Watson, M.R.; Serrano, A.; Campoli, M.; Kaltman, S.I.; Talisman, N.; Green, B.L. Primary Care Providers’ Perspectives on Prescribing Antidepressant Medication to Latino Immigrant Patients: A Preliminary Study. J. Nerv. Ment. Dis. 2020, 208, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, N. Amphetamine-Type Stimulants: The Early History of Their Medical and Non-Medical Uses. Int. Rev. Neurobiol. 2015, 120, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, K.C.; Rossato, L.G.; Fröehlich, P.E.; Limberger, R.P. Amphetamine-Type Medicines: A Review of Pharmacokinetics, Pharmacodynamics, and Toxicological Aspects. Curr. Clin. Pharmacol. 2013, 8, 350–357. [Google Scholar] [CrossRef]
- Simão, A.Y.; Antunes, M.; Marques, H.; Rosado, T.; Soares, S.; Gonçalves, J.; Barroso, M.; Gallardo, E. Amphetamine in Biological Specimens: Impact and Implications for Public Health. In Handbook of Substance Misuse and Addictions: From Biology to Public Health; Springer: Berlin/Heidelberg, Germany, 2022; pp. 2003–2027. [Google Scholar] [CrossRef]
- Kraemer, T.; Maurer, H.H. Toxicokinetics of Amphetamines: Metabolism and Toxicokinetic Data of Designer Drugs, Amphetamine, Methamphetamine, and Their N-Alkyl Derivatives. Ther. Drug Monit. 2002, 24, 277–289. [Google Scholar] [CrossRef]
- Chung, H.; Choe, S. Amphetamine-Type Stimulants in Drug Testing. Mass Spectrom. Lett. 2019, 10, 1–10. [Google Scholar]
- Cody, J.T.; Valtier, S. Amphetamine, Clobenzorex, and 4-Hydroxyclobenzorex Levels Following Multidose Administration of Clobenzorex. J. Anal. Toxicol. 2001, 25, 158–165. [Google Scholar] [CrossRef]
- Amphetaminil. Available online: https://www.chemeurope.com/en/encyclopedia/Amphetaminil.html (accessed on 14 July 2023).
- Musshoff, F. Illegal or Legitimate Use? Precursor Compounds to Amphetamine and Methamphetamine. Drug Metab. Rev. 2001, 32, 15–44. [Google Scholar] [CrossRef]
- Stăcescu, S.; Hancu, G.; Podar, D.; Todea, Ș.; Tero-Vescan, A. A Historical Overview Upon the Use of Amphetamine Derivatives in the Treatment of Obesity. J. Pharm. Care 2019, 7, 72–79. [Google Scholar] [CrossRef]
- Clark, S. Diethylpropion. In xPharm: The Comprehensive Pharmacology Reference; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–4. [Google Scholar] [CrossRef]
- Pagán, A.F.; Huizar, Y.P.; Short, T.R.; Gotcher, Z.; Schmidt, A.T. Adult Attention-Deficit/Hyperactivity Disorder: A Narrative Review of Biological Mechanisms, Treatments, and Outcomes. Curr. Neurol. Neurosci. Rep. 2023, 23, 451–460. [Google Scholar] [CrossRef]
- Koevoet, D.; Deschamps, P.K.H.; Kenemans, J.L. Catecholaminergic and Cholinergic Neuromodulation in Autism Spectrum Disorder: A Comparison to Attention-Deficit Hyperactivity Disorder. Front. Neurosci. 2022, 16, 1078586. [Google Scholar] [CrossRef] [PubMed]
- Laudani, S.; Torrisi, S.A.; Alboni, S.; Bastiaanssen, T.F.S.; Benatti, C.; Rivi, V.; Moloney, R.D.; Fuochi, V.; Furneri, P.M.; Drago, F.; et al. Gut Microbiota Alterations Promote Traumatic Stress Susceptibility Associated with P-Cresol-Induced Dopaminergic Dysfunctions. Brain. Behav. Immun. 2023, 107, 385–396. [Google Scholar] [CrossRef]
- Westphal, A.J.; Ballard, M.E.; Rodriguez, N.; Vega, T.A.; D’Esposito, M.; Kayser, A.S. Working Memory, Cortical Dopamine Tone, and Frontoparietal Brain Recruitment in Post-Traumatic Stress Disorder: A Randomized Controlled Trial. Transl. Psychiatry 2021, 11, 389. [Google Scholar] [CrossRef]
- Ross, J.A.; Van Bockstaele, E.J. The Role of Catecholamines in Modulating Responses to Stress: Sex-Specific Patterns, Implications, and Therapeutic Potential for Post-Traumatic Stress Disorder and Opiate Withdrawal. Eur. J. Neurosci. 2020, 52, 2429–2465. [Google Scholar] [CrossRef]
- Childress, A.C.; Lloyd, E.; Johnson, S.A.; Gunawardhana, L.; Arnold, V. A Long-Term, Open-Label Safety and Tolerability Study of Lisdexamfetamine Dimesylate in Children Aged 4-5 Years with Attention-Deficit/Hyperactivity Disorder. J. Child Adolesc. Psychopharmacol. 2022, 32, 98–106. [Google Scholar] [CrossRef]
- Childress, A.C.; Lloyd, E.; Jacobsen, L.; Gunawardhana, L.; Johnson, S.A.; Findling, R.L. Efficacy and Safety of Lisdexamfetamine in Preschool Children With Attention-Deficit/Hyperactivity Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2022, 61, 1423–1434. [Google Scholar] [CrossRef]
- Childress, A.C.; Findling, R.L.; Wu, J.; Kollins, S.H.; Wang, Y.; Martin, P.; Robertson, B. Lisdexamfetamine Dimesylate for Preschool Children with Attention-Deficit/Hyperactivity Disorder. J. Child Adolesc. Psychopharmacol. 2020, 30, 128–136. [Google Scholar] [CrossRef]
- Adler, L.A.; Leon, T.L.; Sardoff, T.M.; Krone, B.; Faraone, S.V.; Silverstein, M.J.; Newcorn, J.H. A Placebo-Controlled Trial of Lisdexamfetamine in the Treatment of Comorbid Sluggish Cognitive Tempo and Adult ADHD. J. Clin. Psychiatry 2021, 77, 22–27. [Google Scholar] [CrossRef]
- Wang, Y.; Kessel, E.; Lee, S.; Hong, S.; Raffanello, E.; Hulvershorn, L.A.; Margolis, A.; Peterson, B.S.; Posner, J. Causal Effects of Psychostimulants on Neural Connectivity: A Mechanistic, Randomized Clinical Trial. J. Child Psychol. Psychiatry Allied Discip. 2022, 63, 1381–1391. [Google Scholar] [CrossRef]
- Acheson, L.S.; Ezard, N.; Lintzeris, N.; Dunlop, A.; Brett, J.; Rodgers, C.; Gill, A.; Christmass, M.; McKetin, R.; Farrell, M.; et al. Trial Protocol of an Open Label Pilot Study of Lisdexamfetamine for the Treatment of Acute Methamphetamine Withdrawal. PLoS ONE 2022, 17, e0275371. [Google Scholar] [CrossRef]
- Acheson, L.S.; Ezard, N.; Lintzeris, N.; Dunlop, A.; Brett, J.; Rodgers, C.; Gill, A.; Christmass, M.; McKetin, R.; Farrell, M.; et al. Lisdexamfetamine for the Treatment of Acute Methamphetamine Withdrawal: A Pilot Feasibility and Safety Trial. Drug Alcohol Depend. 2022, 241, 109692. [Google Scholar] [CrossRef]
- Ezard, N.; Clifford, B.; Dunlop, A.; Bruno, R.; Carr, A.; Liu, Z.; Siefried, K.J.; Lintzeris, N. Safety and Tolerability of Oral Lisdexamfetamine in Adults with Methamphetamine Dependence: A Phase-2 Dose-Escalation Study. BMJ Open 2021, 11, e044696. [Google Scholar] [CrossRef]
- Mariani, J.J.; Choi, C.J.; Pavlicova, A.; Brooks, D.J.; Grabowski, J.; Levin, F.R. Open-Label Pilot Study of Lisdexamfetamine for Cocaine Use Disorder. Am. J. Drug Alchol Abus. 2021, 47, 402–409. [Google Scholar] [CrossRef]
- Preddy, J.; Smith-Wade, S.; Houghton, K. Lisdexamphetamine as a Novel Therapy for Hyperphagia in Prader-Willi Syndrome. J. Paediatr. Child Health 2023, 59, 570–572. [Google Scholar] [CrossRef]
- Frick, G.; Yan, B.; Adler, L.A. Triple-Bead Mixed Amphetamine Salts (SHP465) in Adults With ADHD: Results of a Phase 3, Double-Blind, Randomized, Forced-Dose Trial. J. Atten. Disord. 2020, 24, 402–413. [Google Scholar] [CrossRef]
- Adler, L.A.; Frick, G.; Yan, B. A Long-Term, Open-Label, Safety Study of Triple-Bead Mixed Amphetamine Salts (SHP465) in Adults With ADHD. J. Atten. Disord. 2020, 24, 434–446. [Google Scholar] [CrossRef]
- Al Awami, R.; Albanna, A. Sedation after a Trial of Mixed Amphetamine Salts in a Boy with Attention-Deficit/Hyperactivity Disorder. Am. J. Case Rep. 2020, 21, e928269-1–e928269-4. [Google Scholar] [CrossRef]
- Armstrong, C.; Kapolowicz, M.R. Mixed Amphetamine Salts without a Mood Stabilizer for Treating Comorbid Attention-Deficit Hyperactivity Disorder and Bipolar Disorder: Two Case Reports. Mil. Med. 2023, 188, e1316–e1319. [Google Scholar] [CrossRef]
- Blevins, D.; Carpenter, K.M.; Martinez, D.; Mariani, J.J.; Levin, F.R. An Adaptive Clinical Trial Design for Cocaine Use Disorder: Extended-Release Amphetamine Salts for Early Behavioral Intervention Non-Responders. Contemp. Clin. Trials 2020, 98, 106187. [Google Scholar] [CrossRef]
- Levin, F.R.; Mariani, J.J.; Pavlicova, M.; Choi, C.J.; Mahony, A.L.; Brooks, D.J.; Bisaga, A.; Dakwar, E.; Carpenter, K.M.; Naqvi, N.; et al. Extended Release Mixed Amphetamine Salts and Topiramate for Cocaine Dependence: A Randomized Clinical Replication Trial with Frequent Users. Drug Alcohol Depend. 2020, 14, 535–562. [Google Scholar] [CrossRef]
- Gorman, I.; Belser, A.B.; Jerome, L.; Hennigan, C.; Shechet, B.; Hamilton, S.; Yazar-Klosinski, B.; Emerson, A.; Feduccia, A.A. Posttraumatic Growth After MDMA-Assisted Psychotherapy for Posttraumatic Stress Disorder. J. Trauma. Stress 2020, 33, 161–170. [Google Scholar] [CrossRef]
- Jerome, L.; Feduccia, A.A.; Wang, J.B.; Hamilton, S.; Yazar-Klosinski, B.; Emerson, A.; Mithoefer, M.C.; Doblin, R. Long-Term Follow-up Outcomes of MDMA-Assisted Psychotherapy for Treatment of PTSD: A Longitudinal Pooled Analysis of Six Phase 2 Trials. Psychopharmacology 2020, 237, 2485–2497. [Google Scholar] [CrossRef]
- Mitchell, J.M.; Bogenschutz, M.; Lilienstein, A.; Harrison, C.; Kleiman, S.; Parker-Guilbert, K.; Ot’alora, G.M.; Garas, W.; Paleos, C.; Gorman, I.; et al. MDMA-Assisted Therapy for Severe PTSD: A Randomized, Double-Blind, Placebo-Controlled Phase 3 Study. Nat. Med. 2021, 27, 1025–1033. [Google Scholar] [CrossRef]
- Nicholasa, C.R.; Wang, J.B.; Coker, A.; Mitchell, J.M.; Klaire, S.S.; Yazar-Klosinski, B.; Emerson, A.; Brown, R.T.; Doblin, R. The effects of MDMA-assisted therapy on alcohol and substance use in a phase 3 trial for treatment of severe PTSD. Drug Alcohol Depend. 2022, 233, 109356. [Google Scholar] [CrossRef]
- Ponte, L.; Jerome, L.; Hamilton, S.; Mithoefer, M.C.; Yazar-Klosinski, B.B.; Vermetten, E.; Feduccia, A.A. Sleep Quality Improvements After MDMA-Assisted Psychotherapy for the Treatment of Posttraumatic Stress Disorder. J. Trauma. Stress 2021, 34, 851–863. [Google Scholar] [CrossRef]
- Jovanovic, T.; Yasinski, C.; Jarboe, K.; Rakofsky, J.; Rauch, S. A Randomized Controlled Trial of 3,4-Methylenedioxymethamphetamine (MDMA) and Fear Extinction Retention in Healthy Adults. Psychopharmacology 2022, 36, 368–377. [Google Scholar] [CrossRef]
- Wolfson, P.E.; Andries, J.; Feduccia, A.A.; Jerome, L.; Wang, J.B.; Williams, E.; Carlin, S.C.; Sola, E.; Hamilton, S.; Yazar-Klosinski, B.; et al. MDMA-Assisted Psychotherapy for Treatment of Anxiety and Other Psychological Distress Related to Life-Threatening Illnesses: A Randomized Pilot Study. Sci. Rep. 2020, 10, 20442. [Google Scholar] [CrossRef]
- Faraone, S.V.; Childress, A.C.; Gomeni, R.; Rafla, E.; Kando, J.C.; Dansie, L.; Naik, P.; Pardo, A. Efficacy of Amphetamine Extended-Release Oral Suspension in Children with Attention-Deficit/Hyperactivity Disorder: Effect Size Across the Day. J. Child Adolesc. Psychopharmacol. 2023, 33, 14–19. [Google Scholar] [CrossRef]
- Swerdlow, N.R.; Bhakta, S.G.; Talledo, J.; Benster, L.; Kotz, J.; Vinogradov, S.; Molina, J.L.; Light, G.A. Auditory Discrimination and Frequency Modulation Learning in Schizophrenia Patients: Amphetamine within-Subject Dose Response and Time Course. Psychol. Med. 2021, 53, 140–148. [Google Scholar] [CrossRef]
- Cutler, A.J.; Suzuki, K.; Starling, B.; Balakrishnan, K.; Komaroff, M.; Meeves, S.; Castelli, M.; Childress, A. D-Amphetamine Transdermal System in Treatment of Children and Adolescents with Attention-Deficit/Hyperactivity Disorder: Secondary Endpoint Results and Post Hoc Effect Size Analyses from a Pivotal Trial. J. Child Adolesc. Psychopharmacol. 2023, 33, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Blanken, P.; Nuijten, M.; van den Brink, W.; Hendriks, V.M. Clinical Effects beyond Cocaine Use of Sustained-Release Dexamphetamine for the Treatment of Cocaine Dependent Patients with Comorbid Opioid Dependence: Secondary Analysis of a Double-Blind, Placebo-Controlled Randomized Trial. Addiction 2020, 115, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Palis, H.; MacDonald, S.; Jun, J.; Oviedo-Joekes, E. Use of Sustained Release Dextroamphetamine for the Treatment of Stimulant Use Disorder in the Setting of Injectable Opioid Agonist Treatment in Canada: A Case Report. Harm Reduct. J. 2021, 18, 57. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Cruz, M.; Kammar-García, A.; Huerta-Cruz, J.C.; del Carmen Carrasco-Portugal, M.; Barranco-Garduño, L.M.; Rodríguez-Silverio, J.; González, H.I.R.; Reyes-García, J.G. Three- And Six-Month Efficacy and Safety of Phentermine in a Mexican Obese Population. Int. J. Clin. Pharmacol. Ther. 2021, 59, 539–548. [Google Scholar] [CrossRef]
- Pérez-Cruz, E.; Guevara-Cruz, M.; Ortiz-Gutiérrez, S.; Luna-Camacho, Y.; Guzmán-Aguilar, R.; Briceño-Sáenz, G.; González-Salazar, L.; Flores-López, A. Effect of Phentermine on Hepatic Steatosis in Bariatric Surgery: A Pilot Study. Med. Princ. Pract. 2022, 31, 254–261. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pires, B.; Rosendo, L.M.; Brinca, A.T.; Simão, A.Y.; Barroso, M.; Rosado, T.; Gallardo, E. The Therapeutic Potential of Amphetamine-like Psychostimulants. Life 2023, 13, 2180. https://doi.org/10.3390/life13112180
Pires B, Rosendo LM, Brinca AT, Simão AY, Barroso M, Rosado T, Gallardo E. The Therapeutic Potential of Amphetamine-like Psychostimulants. Life. 2023; 13(11):2180. https://doi.org/10.3390/life13112180
Chicago/Turabian StylePires, Bruno, Luana M. Rosendo, Ana Teresa Brinca, Ana Y. Simão, Mário Barroso, Tiago Rosado, and Eugenia Gallardo. 2023. "The Therapeutic Potential of Amphetamine-like Psychostimulants" Life 13, no. 11: 2180. https://doi.org/10.3390/life13112180
APA StylePires, B., Rosendo, L. M., Brinca, A. T., Simão, A. Y., Barroso, M., Rosado, T., & Gallardo, E. (2023). The Therapeutic Potential of Amphetamine-like Psychostimulants. Life, 13(11), 2180. https://doi.org/10.3390/life13112180









