Predicting Bone Adaptation in Astronauts during and after Spaceflight
Abstract
:1. Introduction
2. Methods
2.1. Participants and HR-pQCT Image Acquisition
2.2. Image Pre-Processing
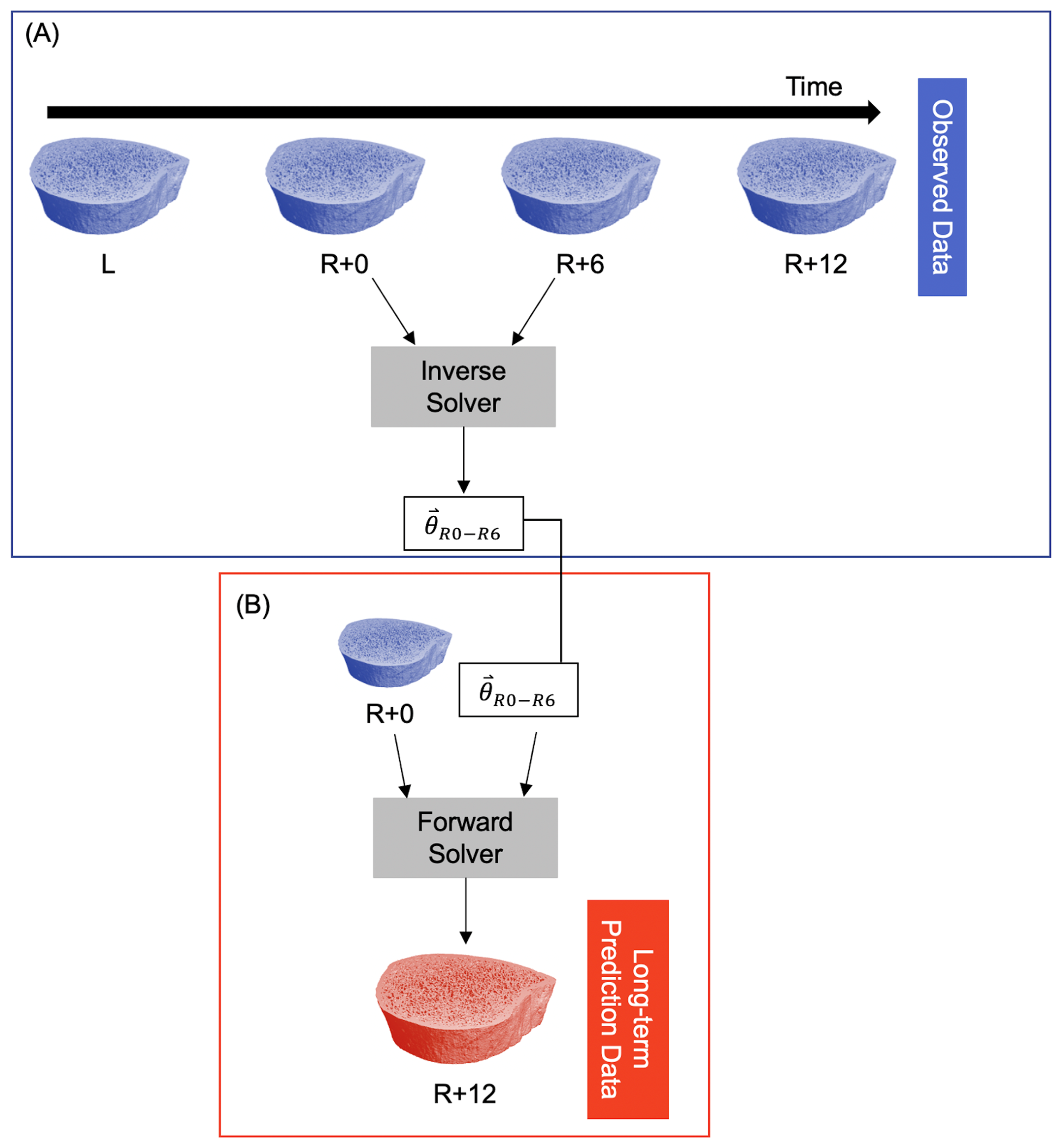
2.3. Computational Technique
2.4. Morphometry and Surface Metrics
2.5. Statistical Analysis
3. Results
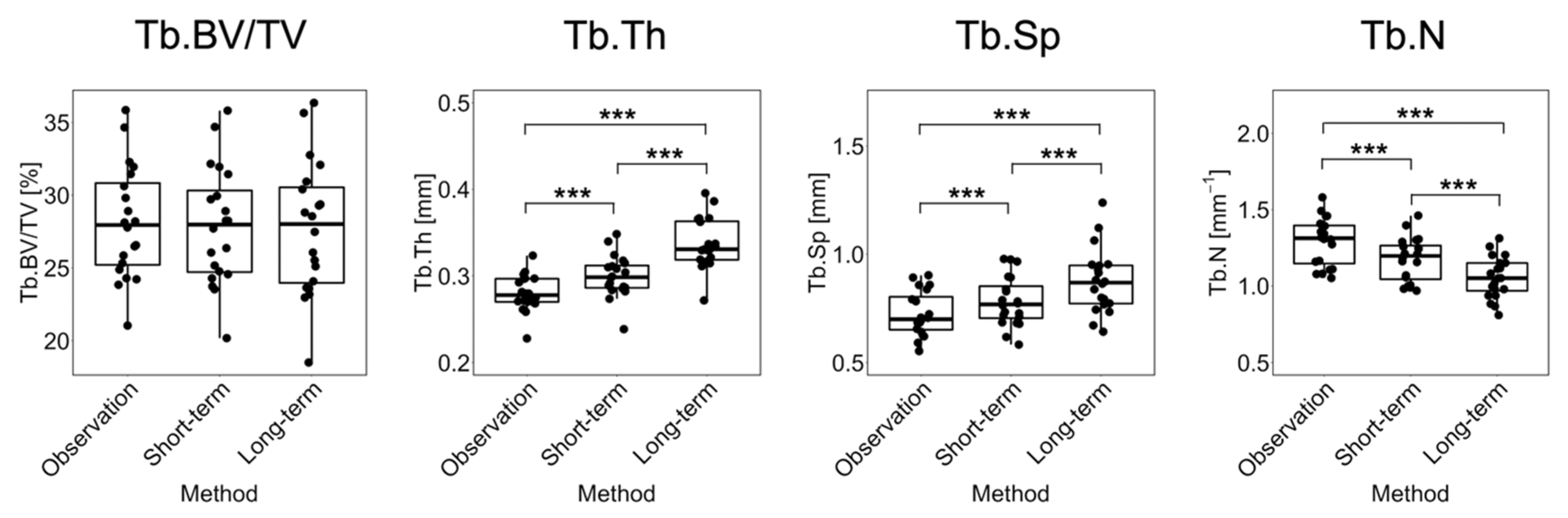
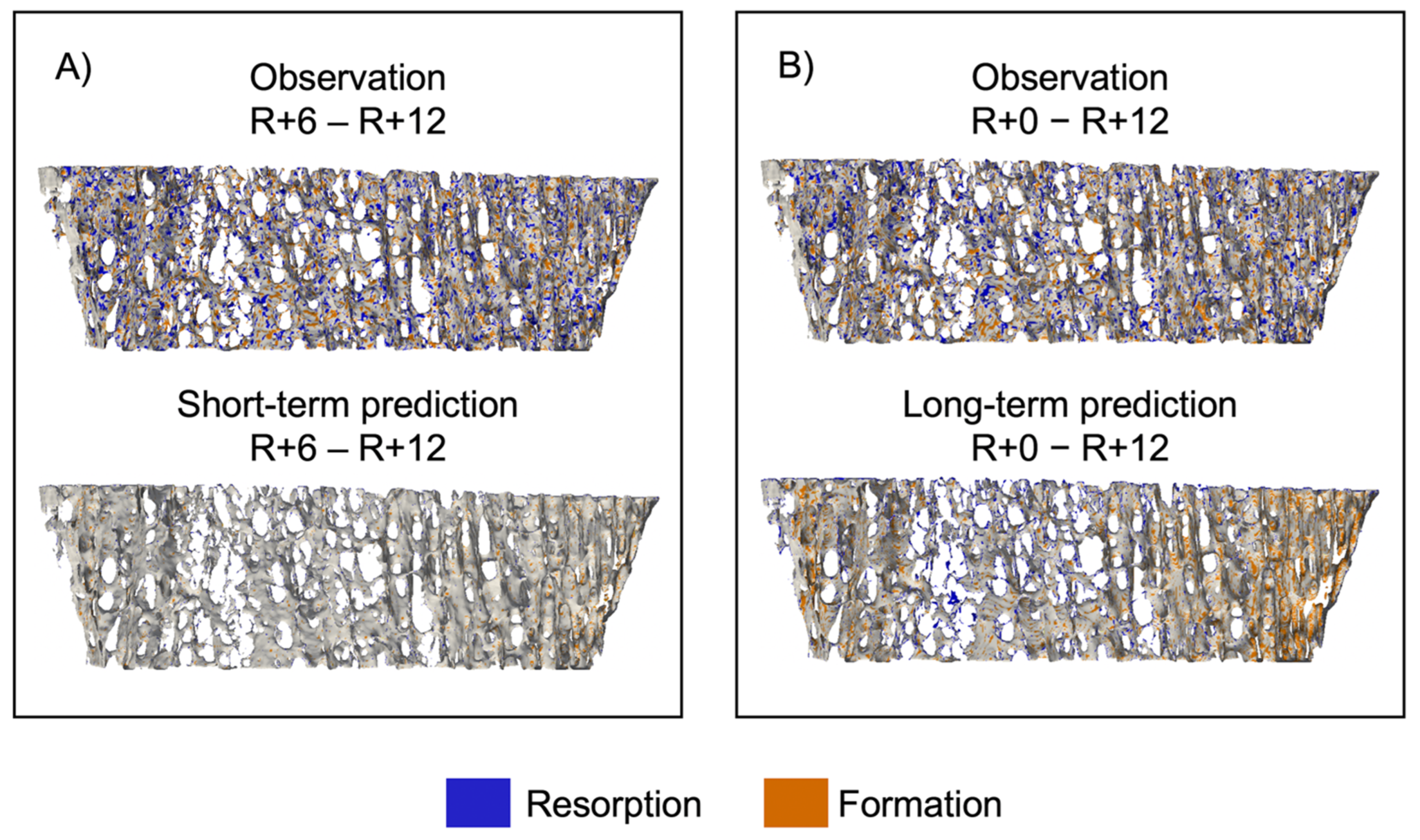
3.1. Short-Term Prediction
3.2. Long-Term Prediction
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vico, L.; van Rietbergen, B.; Vilayphiou, N.; Linossier, M.-T.; Locrelle, H.; Normand, M.; Zouch, M.; Gerbaix, M.; Bonnet, N.; Novikov, V.; et al. Cortical and Trabecular Bone Microstructure Did Not Recover at Weight-Bearing Skeletal Sites and Progressively Deteriorated at Non-Weight-Bearing Sites During the Year Following International Space Station Missions. J. Bone Miner. Res. 2017, 32, 2010–2021. [Google Scholar] [CrossRef]
- Hannan, M.T.; Felson, D.T.; Dawson-Hughes, B.; Tucker, K.L.; Cupples, L.A.; Wilson, P.W.F.; Kiel, D.P. Risk factors for longitudinal bone loss in elderly men and women: The Framingham Osteoporosis Study. J. Bone Miner. Res. 2000, 15, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Robling, A.G.; Turner, C.H. Mechanical signaling for bone modeling and remodeling. Crit. Rev. Eukaryot. Gene Expr. 2009, 19, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Zérath, E. Effects of microgravity on bone and calcium homeostasis. Adv. Space Res. 1998, 21, 1049–1058. [Google Scholar] [CrossRef]
- Özçivici, E. Effects of spaceflight on cells of bone marrow origin. Turkish J. Hematol. Tology 2013, 30, 1–7. [Google Scholar] [CrossRef]
- Smith, S.M.; Abrams, S.A.; Davis-Street, J.E.; Heer, M.; O’brien, K.O.; Wastney, M.E.; Zwart, S.R. Fifty Years of Human Space Travel: Implications for Bone and Calcium Research. Annu. Rev. Nutr. 2014, 34, 377–400. [Google Scholar] [CrossRef]
- Schneider, S.M.; Amonette, W.E.; Blazine, K.; Bentley, J.; Lee, S.M.C.; Loehr, J.A.; Moore, A.D.; Rapley, M.; Mulder, E.R.; Smith, S.M. Training with the International Space Station Interim Resistive Exercise Device. Med. Sci. Sports Exerc. 2003, 35, 1935–1945. [Google Scholar] [CrossRef] [PubMed]
- Sibonga, J.D.; Cavanagh, P.R.; Lang, T.F.; LeBlanc, A.D.; Schneider, V.S.; Shackelford, L.C.; Smith, S.M.; Vico, L. Adaptation of the skeletal system during long-duration spaceflight. Clin. Rev. Bone Miner. Metab. 2007, 5, 249–261. [Google Scholar] [CrossRef]
- Loehr, J.A.; Lee, S.M.C.; English, K.L.; Sibonga, J.; Smith, S.M.; Spiering, B.A.; Hagan, R.D. Musculoskeletal adaptations to training with the advanced resistive exercise device. Med. Sci. Sports Exerc. 2011, 43, 146–156. [Google Scholar] [CrossRef]
- Smith, S.M.; Heer, M.A.; Shackelford, L.C.; Sibonga, J.D.; Ploutz-Snyder, L.; Zwart, S.R. Benefits for bone from resistance exercise and nutrition in long-duration spaceflight: Evidence from biochemistry and densitometry. J. Bone Miner. Res. 2012, 27, 1896–1906. [Google Scholar] [CrossRef]
- LeBlanc, A.; Matsumoto, T.; Jones, J.; Shapiro, J.; Lang, T.; Shackelford, L.; Smith, S.M.; Evans, H.; Spector, E.; Ploutz-Snyder, R.; et al. Bisphosphonates as a supplement to exercise to protect bone during long-duration spaceflight. Osteoporos. Int. 2013, 24, 2105–2114. [Google Scholar] [CrossRef] [PubMed]
- Sibonga, J.; Matsumoto, T.; Jones, J.; Shapiro, J.; Lang, T.; Shackelford, L.; Smith, S.M.; Young, M.; Keyak, J.; Kohri, K.; et al. Resistive exercise in astronauts on prolonged spaceflights provides partial protection against spaceflight-induced bone loss. Bone 2019, 128, 112037. [Google Scholar] [CrossRef] [PubMed]
- Vico, L.; Collet, P.; Guignandon, A.; Lafage-Proust, M.H.; Thomas, T.; Rehailia, M.; Alexandre, C. Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. Lancet 2000, 355, 1607–1611. [Google Scholar] [CrossRef]
- Lang, T.F.; Leblanc, A.D.; Evans, H.J.; Lu, Y. Adaptation of the proximal femur to skeletal reloading after long-duration spaceflight. J. Bone Miner. Res. 2006, 21, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Jee, W.S.S. The Skeletal Tissues, Histology: Cell & Tissue Biology; Urban & Schwarzenberg: Baltimore, MA, USA, 1989; pp. 211–259. [Google Scholar]
- Kostenuik, P. On the Evolution and Contemporary Roles of Bone Remodeling. In Marcus and Feldman’s Osteoporosis. Two Vol. Set Vol. 1, 4th ed.; Marcus, R., Dempster, D.W., Cauley, J.A., Feldman, D., Luckey, M., Eds.; Elsevier Science & Technology: Amsterdam, The Netherlands, 2013; pp. 873–914. [Google Scholar]
- Frost, H.M. Bone “mass” and the “mechanostat”: A proposal. Anat. Rec. 1987, 219, 1–9. [Google Scholar] [CrossRef]
- Martin, R.B. Toward a unifying theory of bone remodeling. Bone 2000, 26, 1–6. [Google Scholar] [CrossRef]
- Meade, J.B.; Cowin, S.C.; Klawitter, J.J.; Van Buskirk, W.C.; Skinner, H.B. Bone remodeling due to continuously applied loads. Calcif. Tissue Int. 1984, 36, S25–S30. [Google Scholar] [CrossRef]
- Parfitt, A.M. Skeletal Heterogeneity and the Purposes of Bone Remodeling: Implications for the Understanding of Osteoporosis, In Osteoporos, 4th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 855–872. [Google Scholar] [CrossRef]
- Frost, H.M. Tetracycline-based histological analysis of bone remodeling. Calcif. Tissue Res. 1969, 3, 211–237. [Google Scholar] [CrossRef] [PubMed]
- Sims, N.A.; Morris, H.A.; Moore, R.J.; Durbridge, T.C. Increased bone resorption precedes increased bone formation in the ovariectomized rat. Calcif. Tissue Int. 1996, 59, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Boyd, S.K.; Davison, P.; Müller, R.; Gasser, J.A. Monitoring individual morphological changes over time in ovariectomized rats by in vivo micro-computed tomography. Bone 2006, 39, 854–862. [Google Scholar] [CrossRef]
- Hart, R.T.; Davy, D.T.; Heiple, K.G. Mathematical modeling and numerical solutions for functionally dependent bone remodeling. Calcif. Tissue Int. 1984, 36, 104–109. [Google Scholar] [CrossRef]
- Huiskes, R.; Ruimerman, R.; van Lenthe, G.H.; Janssen, J.D. Effects of mechanical forces on maintenance and adaptation of form in trabecular bone. Nature 2000, 405, 704–706. [Google Scholar] [CrossRef]
- Adachi, T.; Tsubota, K.I.; Tomita, Y.; Scott, J.H. Trabecular surface remodeling simulation for cancellous bone using microstructural voxel finite element models. J. Biomech. Eng. 2001, 123, 403–409. [Google Scholar] [CrossRef]
- Müller, R. Long-term prediction of three-dimensional bone architecture in simulations of pre-, peri- and post-menopausal microstructural bone remodeling. Osteoporos. Int. 2005, 16, S25–S35. [Google Scholar] [CrossRef] [PubMed]
- Schulte, F.A.; Zwahlen, A.; Lambers, F.M.; Kuhn, G.; Ruffoni, D.; Betts, D.; Webster, D.J.; Müller, R. Strain-adaptive in silico modeling of bone adaptation—A computer simulation validated by in vivo micro-computed tomography data. Bone 2013, 52, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Besler, B.A.; Gabel, L.; Burt, L.A.; Forkert, N.D.; Boyd, S.K. Bone adaptation as level set motion. In Computational Methods and Clinical Applications in Musculoskeletal Imaging, MSKI 2018. Lecture Notes in Computer Science; Vrtovec, T., Yao, J., Zheng, G., Pozo, J., Eds.; Springer: Cham, Switerland, 2019; pp. 58–72. [Google Scholar] [CrossRef]
- Kemp, T.D.; Besler, B.A.; Boyd, S.K. An inverse technique to identify participant-specific bone adaptation from serial CT measurements. Int. J. Numer. Method. Biomed. Eng. 2021, 37, e3515. [Google Scholar] [CrossRef]
- Gabel, L.; Liphardt, A.-M.; Hulme, P.A.; Heer, M.; Zwart, S.R.; Sibonga, J.D.; Smith, S.M.; Boyd, S. Pre-flight exercise and bone metabolism predict unloading-induced bone loss due to spaceflight. Br. J. Sport. Med. 2022, 56, 196–203. [Google Scholar] [CrossRef]
- Gabel, L.; Liphardt, A.M.; Hulme, P.A.; Heer, M.; Zwart, S.R.; Sibonga, J.D.; Smith, S.M.; Boyd, S.K. Incomplete recovery of bone strength and trabecular microarchitecture at the distal tibia 1 year after return from long duration spaceflight. Sci. Rep. 2022, 12, 9446. [Google Scholar] [CrossRef]
- Whittier, D.E.; Boyd, S.K.; Burghardt, A.J.; Paccou, J.; Ghasem-Zadeh, A.; Chapurlat, R.; Engelke, K. Guidelines for the assessment of bone density and microarchitecture in vivo using high-resolution peripheral quantitative computed tomography. Osteoporos. Int. 2020, 31, 1607–1627. [Google Scholar] [CrossRef] [PubMed]
- Pialat, J.B.; Burghardt, A.J.; Sode, M.; Link, T.M.; Majumdar, S. Visual grading of motion induced image degradation in high resolution peripheral computed tomography: Impact of image quality on measures of bone density and micro-architecture. Bone 2012, 50, 111–118. [Google Scholar] [CrossRef]
- Burghardt, A.J.; Buie, H.R.; Laib, A.; Majumdar, S.; Boyd, S.K. Reproducibility of direct quantitative measures of cortical bone microarchitecture of the distal radius and tibia by HR-pQCT. Bone 2010, 47, 519–528. [Google Scholar] [CrossRef]
- Kemp, T.D.; de Bakker, C.M.J.; Gabel, L.; Hanley, D.A.; Billington, E.O.; Burt, L.A.; Boyd, S.K. Longitudinal bone microarchitectural changes are best detected using image registration. Osteoporos. Int. 2020, 31, 1995–2005. [Google Scholar] [CrossRef]
- Mattes, D.; Haynor, D.R.; Vesselle, H.; Lewellyn, T.K.; Eubank, W. Nonrigid multimodality image registration. In Medical Imaging 2001: Image Processing; SPIE: San Diego, CA, USA, 2001; Volume 4322. [Google Scholar] [CrossRef]
- Unser, M. Splines: A perfect fit for signal and image processing. IEEE Signal Process. Mag. 1999, 16, 22–38. [Google Scholar] [CrossRef]
- Osher, S.; Sethian, J.A. Fronts propagating with curvature-dependent speed: Algorithms based on Hamilton-Jacobi formulations. J. Comput. Phys. 1988, 79, 12–49. [Google Scholar] [CrossRef]
- Sethian, J.A. Level Set Methods and Fast Marching Methods, 2nd ed.; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Maurer, C.R.; Qi, R.; Raghavan, V. A linear time algorithm for computing exact Euclidean distance transforms of binary images in arbitrary dimensions. IEEE Trans. Pattern Anal. Mach. Intell. 2003, 25, 265–270. [Google Scholar] [CrossRef]
- Kingma, D.P.; Ba, J.L. Adam: A method for stochastic optimization. arXiv 2014, arXiv:1412.6980v9. [Google Scholar]
- Waarsing, J.H.; Day, J.S.; Van Der Linden, J.C.; Ederveen, A.G.; Spanjers, C.; De Clerck, N.; Sasov, A.; Verhaar, J.A.N.; Weinans, H. Detecting and tracking local changes in the tibiae of individual rats: A novel method to analyse longitudinal in vivo micro-CT data. Bone 2004, 34, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, J.E.M.; Van Rietbergen, B.; Huiskes, R.; Ito, K. Effects of PTH treatment on tibial bone of ovariectomized rats assessed by in vivo micro-CT. Osteoporos. Int. 2009, 20, 1823–1835. [Google Scholar] [CrossRef] [PubMed]
- Schulte, F.A.; Lambers, F.M.; Kuhn, G.; Müller, R. In vivo micro-computed tomography allows direct three-dimensional quantification of both bone formation and bone resorption parameters using time-lapsed imaging. Bone 2011, 48, 433–442. [Google Scholar] [CrossRef]
- Hildebrand, T.; Rüegsegger, P. A new method for the model-independent assessment of thickness in three-dimensional images. J. Microsc. 1997, 185, 67–75. [Google Scholar] [CrossRef]
- Dice, L.R. Measures of the Amount of Ecologic Association between Species. Ecology 1945, 26, 297–302. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Int. J. Nurs. Stud. 2010, 47, 931–936. [Google Scholar] [CrossRef]
- Glüer, C.C. Monitoring skeletal changes by radiological techniques. J. Bone Miner. Res. 1999, 14, 1952–1962. [Google Scholar] [CrossRef] [PubMed]
- Bonnick, S.L.; Johnston, C.C.; Kleerekoper, M.; Lindsay, R.; Miller, P.; Sherwood, L.; Siris, E. Importance of precision in bone density measurements. J. Clin. Densitom. 2001, 4, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Warden, S.J.; Liu, Z.; Fuchs, R.K.; van Rietbergen, B.; Moe, S.M. Reference data and calculators for second-generation HR-pQCT measures of the radius and tibia at anatomically standardized regions in White adults. Osteoporos. Int 2022, 33, 791–806. [Google Scholar] [CrossRef]

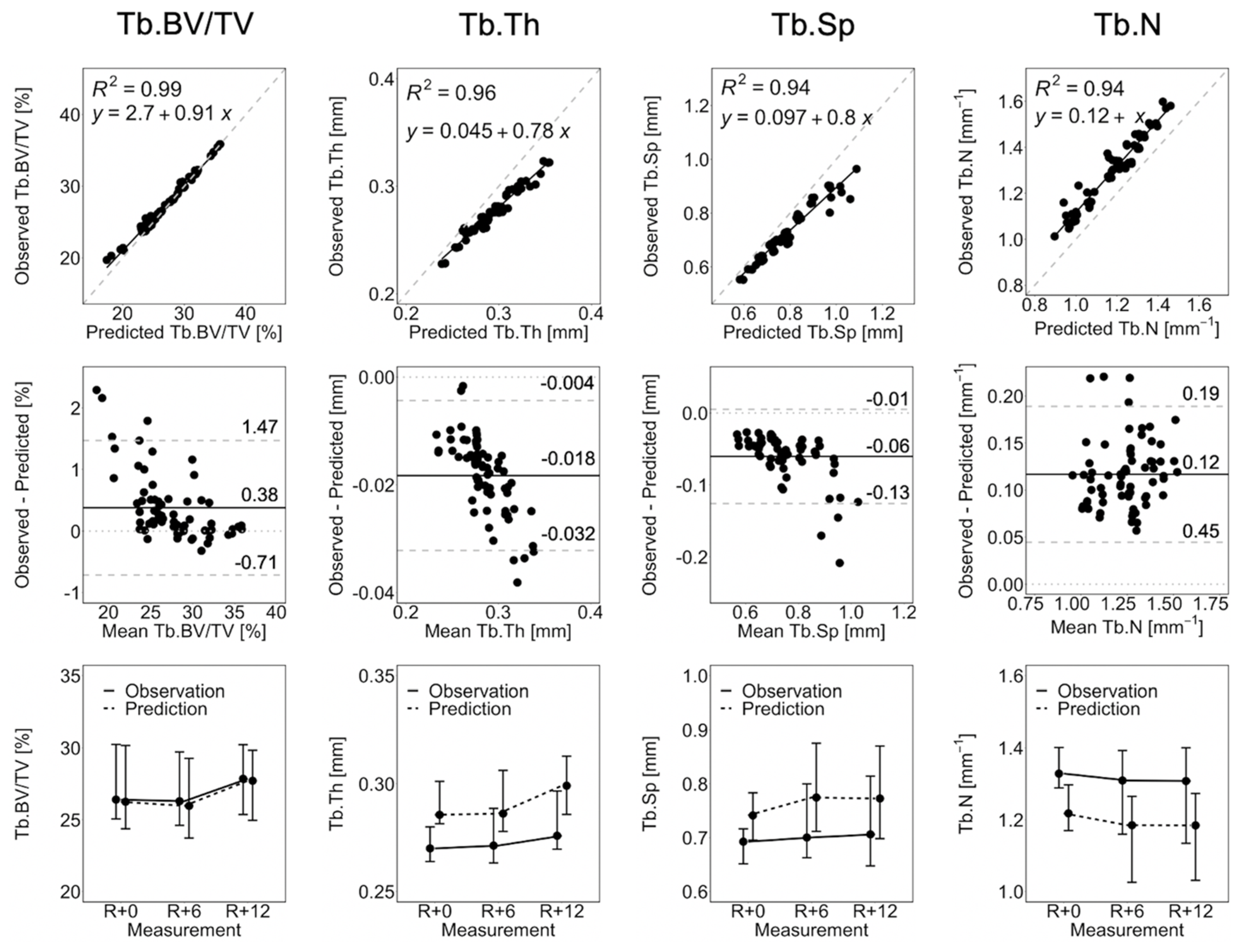
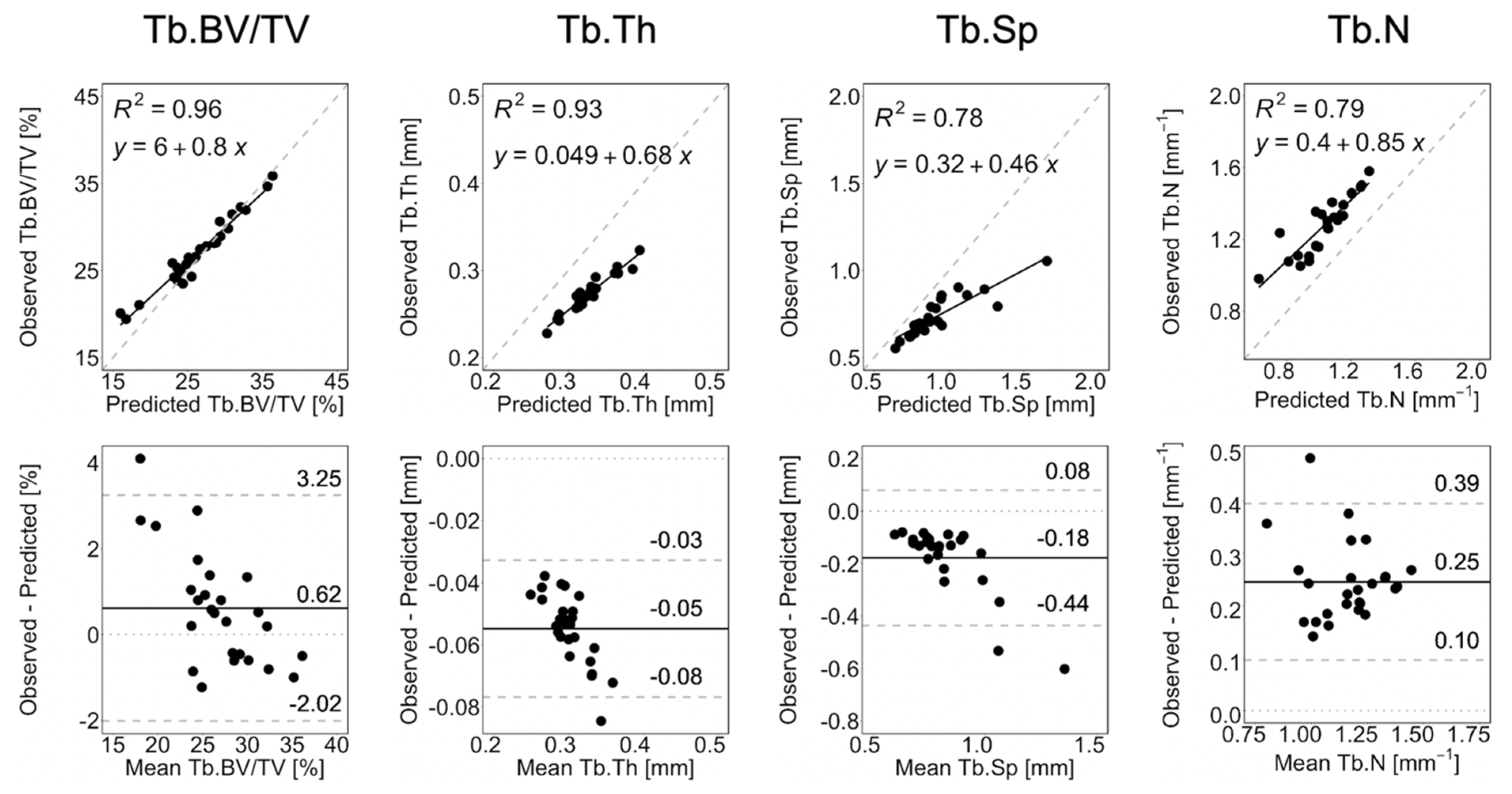
| Parameter | Error R+0 [%] | Error R+6 [%] | Error R+12 [%] | p-Value |
|---|---|---|---|---|
| Tb.BV/TV | 1.6 ± 2.0 | 2.5 ± 3.2 | 1.0 ± 1.3 | 0.393 |
| Tb.Th | 6.2 ± 2.6 | 6.2 ± 1.8 | 7.2 ± 2.3 | <0.0001 |
| Tb.Sp | 7.4 ± 2.1 | 9.1 ± 5.2 | 8.2 ± 3.0 | <0.0001 |
| Tb.N | 8.6 ± 2.3 | 9.4 ± 3.6 | 8.9 ± 2.1 | <0.0001 |
| L - R+0 | R+0 - R+6 | R+6 - R+12 | |||||
|---|---|---|---|---|---|---|---|
| Parameter | Observation | Short-Term Prediction | Observation | Short-Term Prediction | Observation | Short-Term Prediction | p-Value |
| BFR [] | 0.12 (0.11, 0.16) | 0.04 (0.02, 0.04) | 0.12 (0.12, 0.14) | 0.04 (0.03, 0.04) | 0.11 (0.10, 0.13) | 0.04 (0.03, 0.04) | <0.0001 |
| BRR [] | 0.13 (0.12, 0.17) | 0.05 (0.04, 0.07) | 0.12 (0.11, 0.13) | 0.04 (0.03, 0.05) | 0.11 (0.10, 0.13) | 0.04 (0.03, 0.05) | <0.0001 |
| MS [] | 25.5 (24.29, 27.90) | 5.73 (4.42, 6.32) | 27.82 (25.83, 32.60) | 6.36 (5.77, 7.60) | 27.09 (25.12, 28.60) | 6.23 (5.60, 7.26) | <0.0001 |
| ES [] | 28.13 (26.41, 30.94) | 9.27 (8.33, 12.12) | 26.49 (25.26, 29.24) | 7.49 (6.58, 10.55) | 26.32 (25.59, 28.24) | 8.18 (6.87, 9.52) | <0.0001 |
| MAR [] | 0.73 (0.69, 0.92) | 0.72 (0.66, 0.88) | 0.75 (0.71, 0.77) | 0.73 (0.67, 0.75) | 0.71 (0.66, 0.74) | 0.67 (0.64, 0.73) | 0.477 |
| MRR [] | 0.73 (0.70, 0.93) | 0.73 (0.67, 0.89) | 0.74 (0.71. 0.77) | 0.74 (0.69, 0.76) | 0.69 (0.66, 0.74) | 0.68 (0.64, 0.73) | 0.162 |
| R+0 | R+6 | R+12 | |
|---|---|---|---|
| Dice coefficient | 0.86 (0.84, 0.88) | 0.87 (0.85, 0.88) | 0.87 (0.86, 0.88) |
| 41.6 (38.6, 46.2) | 40.6 (37.5, 48.4) | 40.0 (37.5, 43.2) |
| Parameter | Observation | Long-Term Prediction | p-Value |
|---|---|---|---|
| ] | 0.06 (0.06, 0.07) | 0.04 (0.04, 0.05) | <0.0001 |
| ] | 0.06 (0.05, 0.06) | 0.04 (0.03, 0.05) | 0.0008 |
| ] | 28.15 (27.06, 33.62) | 18.74 (17.11, 21.17) | <0.0001 |
| ] | 26.32 (25.48, 28.56) | 20.35 (17.20, 25.11) | 0.0004 |
| ] | 0.35 (0.34, 0.38) | 0.35 (0.33, 0.35) | 0.0174 |
| ] | 0.35 (0.34, 0.37) | 0.35 (0.33, 0.35) | 0.0174 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kemp, T.D.; Besler, B.A.; Gabel, L.; Boyd, S.K. Predicting Bone Adaptation in Astronauts during and after Spaceflight. Life 2023, 13, 2183. https://doi.org/10.3390/life13112183
Kemp TD, Besler BA, Gabel L, Boyd SK. Predicting Bone Adaptation in Astronauts during and after Spaceflight. Life. 2023; 13(11):2183. https://doi.org/10.3390/life13112183
Chicago/Turabian StyleKemp, Tannis D., Bryce A. Besler, Leigh Gabel, and Steven K. Boyd. 2023. "Predicting Bone Adaptation in Astronauts during and after Spaceflight" Life 13, no. 11: 2183. https://doi.org/10.3390/life13112183
APA StyleKemp, T. D., Besler, B. A., Gabel, L., & Boyd, S. K. (2023). Predicting Bone Adaptation in Astronauts during and after Spaceflight. Life, 13(11), 2183. https://doi.org/10.3390/life13112183







