Progression-Free Survival Efficacy in Refractory/Relapsed Multiple Myeloma among Elderly Patients: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Risk of Bias Assessment
2.5. Data Extraction
2.6. Data Synthesis and Statistical Analysis
3. Results
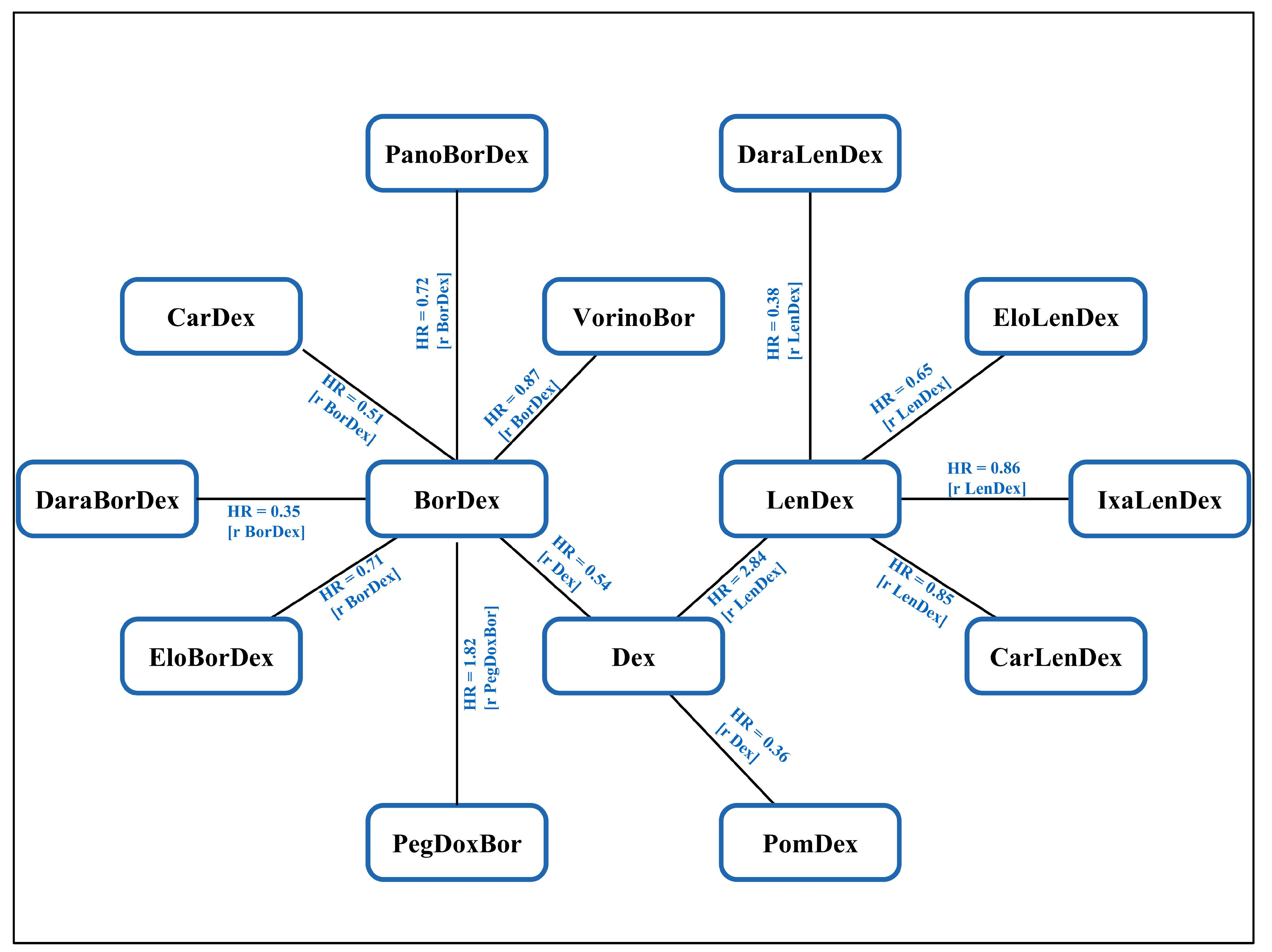
3.1. Network Meta-Analysis
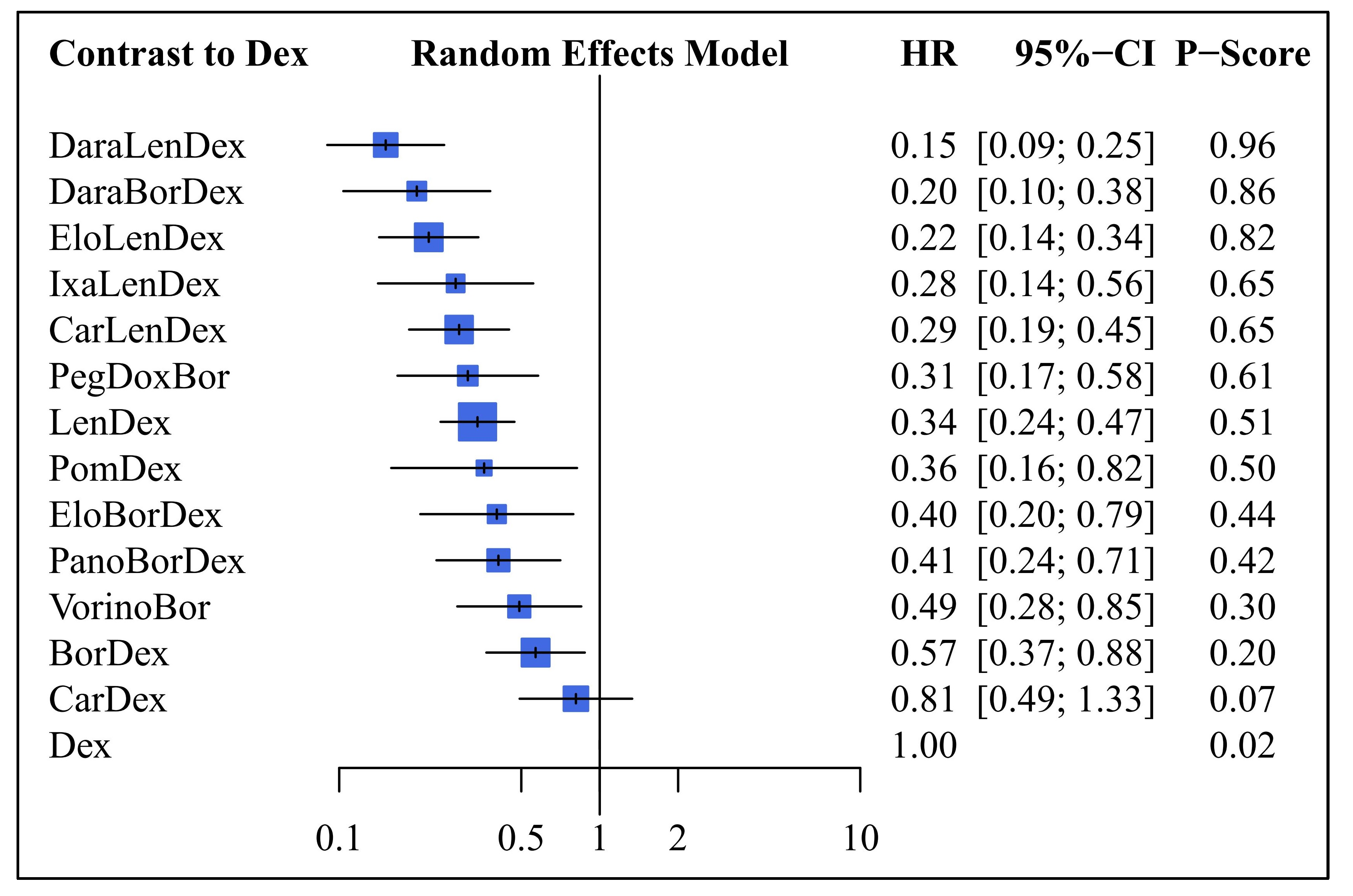
3.2. Progression Free Survival
4. Discussion
4.1. Overview and Key Findings
4.2. Significance and Consistency with Prior Studies
4.3. Frailty Considerations and Comprehensive Assessments
4.4. Elderly Criteria and Treatment Optimization Debate
4.5. Rationale for Dexamethasone as Reference
4.6. Underlying Assumptions in NMA
4.7. Emergence of New Therapeutic Modalities
4.8. Study Limitations and Data Sources
4.9. Concluding Remarks and Clinical Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sabattini, E.; Bacci, F.; Sagramoso, C.; Pileri, S.A. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: An overview. Pathologica 2010, 102, 83–87. [Google Scholar] [PubMed]
- Palumbo, A.; Anderson, K. Multiple myeloma. N. Engl. J. Med. 2011, 364, 1046–1060. [Google Scholar] [CrossRef]
- Pozzi, S.; Marcheselli, L.; Bari, A.; Liardo, E.V.; Marcheselli, R.; Luminari, S.; Quaresima, M.; Cirilli, C.; Ferri, P.; Federico, M.; et al. Survival of multiple myeloma patients in the era of novel therapies confirms the improvement in patients younger than 75 years: A population-based analysis. Br. J. Haematol. 2013, 163, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Bringhen, S.; Ludwig, H.; Dimopoulos, M.A.; Blade, J.; Mateos, M.V.; Rosinol, L.; Boccadoro, M.; Cavo, M.; Lokhorst, H.; et al. Personalized therapy in multiple myeloma according to patient age and vulnerability: A report of the European Myeloma Network (EMN). Blood 2011, 118, 4519–4529. [Google Scholar] [CrossRef] [PubMed]
- Cronin, K.A.; Ries, L.A.; Edwards, B.K. The Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute. Cancer 2014, 120 (Suppl. 23), 3755–3757. [Google Scholar] [CrossRef] [PubMed]
- Cottini, F.; Anderson, K. Novel therapeutic targets in multiple myeloma. Clin. Adv. Hematol. Oncol. 2015, 13, 236–248. [Google Scholar] [PubMed]
- Liu, X.; He, C.K.; Meng, X.; He, L.; Li, K.; Liang, Q.; Shao, L.; Liu, S. Bortezomib-based vs non-bortezomib-based post-transplantation treatment in multiple myeloma patients: A systematic review and meta-analysis of Phase III randomized controlled trials. Onco Targets Ther. 2015, 8, 1459–1469. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Rajkumar, S.V.; Dispenzieri, A.; Lacy, M.Q.; Hayman, S.R.; Buadi, F.K.; Zeldenrust, S.R.; Dingli, D.; Russell, S.J.; Lust, J.A.; et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood 2008, 111, 2516–2520. [Google Scholar] [CrossRef]
- Palumbo, A.; Bringhen, S.; Mateos, M.V.; Larocca, A.; Facon, T.; Kumar, S.K.; Offidani, M.; McCarthy, P.; Evangelista, A.; Lonial, S.; et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: An International Myeloma Working Group report. Blood 2015, 125, 2068–2074. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef]
- Parmar, M.K.; Torri, V.; Stewart, L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat. Med. 1998, 17, 2815–2834. [Google Scholar] [CrossRef]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Available online: http://handbook.cochrane.org/ (accessed on 1 December 2022).
- Roberts, C.; Torgerson, D.J. Understanding controlled trials: Baseline imbalance in randomised controlled trials. BMJ 1999, 319, 185. [Google Scholar] [CrossRef] [PubMed]
- Review Manager (RevMan) [Computer Program]. Version 5.3. Available online: https://revman.cochrane.org/ (accessed on 30 June 2023).
- Hjorth, M.; Hjertner, O.; Knudsen, L.M.; Gulbrandsen, N.; Holmberg, E.; Pedersen, P.T.; Andersen, N.F.; Andreasson, B.; Billstrom, R.; Carlson, K.; et al. Thalidomide and dexamethasone vs. bortezomib and dexamethasone for melphalan refractory myeloma: A randomized study. Eur. J. Haematol. 2012, 88, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, R.Z.; Gercheva, L.; Williams, C.; Sutherland, H.; Robak, T.; Masszi, T.; Goranova-Marinova, V.; Dimopoulos, M.A.; Cavenagh, J.D.; Spicka, I.; et al. A phase 2, randomized, double-blind, placebo-controlled study of siltuximab (anti-IL-6 mAb) and bortezomib versus bortezomib alone in patients with relapsed or refractory multiple myeloma. Am. J. Hematol. 2015, 90, 42–49. [Google Scholar] [CrossRef]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Salanti, G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: Many names, many benefits, many concerns for the next generation evidence synthesis tool. Res. Synth. Methods 2012, 3, 80–97. [Google Scholar] [CrossRef]
- Rücker, G.; Krahn, U.; König, J.; Efthimiou, O.; Davies, A.; Papakonstantinou, T.; Schwarzer, G.; netmeta: Network Meta-Analysis Using Frequentist Methods. R Package Version 0.7-0. Available online: http://cran.at.r-project.org/web/packages/netmeta/ (accessed on 1 December 2022).
- Leyva, F.; Plummer, C.J. National Institute for Health and Care Excellence 2014 guidance on cardiac implantable electronic devices: Health economics reloaded. Europace 2015, 17, 339–342. [Google Scholar] [CrossRef][Green Version]
- Krahn, U.; Binder, H.; Konig, J. A graphical tool for locating inconsistency in network meta-analyses. BMC Med. Res. Methodol. 2013, 13, 35. [Google Scholar] [CrossRef]
- Jackson, D.; White, I.R.; Riley, R.D. Quantifying the impact of between-study heterogeneity in multivariate meta-analyses. Stat. Med. 2012, 31, 3805–3820. [Google Scholar] [CrossRef]
- Chaimani, A.; Higgins, J.P.; Mavridis, D.; Spyridonos, P.; Salanti, G. Graphical tools for network meta-analysis in STATA. PLoS ONE 2013, 8, e76654. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.P.; Trikalinos, T.; Cappelleri, J.C.; Daw, J.; Andes, S.; Eldessouki, R.; Salanti, G. Indirect treatment comparison/network meta-analysis study questionnaire to assess relevance and credibility to inform health care decision making: An ISPOR-AMCP-NPC Good Practice Task Force report. Value Health 2014, 17, 157–173. [Google Scholar] [CrossRef]
- Salanti, G.; Del Giovane, C.; Chaimani, A.; Caldwell, D.M.; Higgins, J.P. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE 2014, 9, e99682. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.D.; Higgins, J.P.; Deeks, J.J. Interpretation of random effects meta-analyses. BMJ 2011, 342, d549. [Google Scholar] [CrossRef] [PubMed]
- Rucker, G.; Schwarzer, G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med. Res. Methodol. 2015, 15, 58. [Google Scholar] [CrossRef] [PubMed]
- van Beurden-Tan, C.H.Y.; Franken, M.G.; Blommestein, H.M.; Uyl-de Groot, C.A.; Sonneveld, P. Systematic Literature Review and Network Meta-Analysis of Treatment Outcomes in Relapsed and/or Refractory Multiple Myeloma. J. Clin. Oncol. 2017, 35, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.; Siegel, D.S.; Lonial, S.; Qi, J.; Hajek, R.; Facon, T.; Rosinol, L.; Williams, C.; Blacklock, H.; Goldschmidt, H.; et al. Vorinostat or placebo in combination with bortezomib in patients with multiple myeloma (VANTAGE 088): A multicentre, randomised, double-blind study. Lancet Oncol. 2013, 14, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, R.Z.; Nagler, A.; Sonneveld, P.; Blade, J.; Hajek, R.; Spencer, A.; San Miguel, J.; Robak, T.; Dmoszynska, A.; Horvath, N.; et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: Combination therapy improves time to progression. J. Clin. Oncol. 2007, 25, 3892–3901. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Moreau, P.; Palumbo, A.; Joshua, D.; Pour, L.; Hajek, R.; Facon, T.; Ludwig, H.; Oriol, A.; Goldschmidt, H.; et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): A randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016, 17, 27–38. [Google Scholar] [CrossRef]
- San-Miguel, J.F.; Hungria, V.T.; Yoon, S.S.; Beksac, M.; Dimopoulos, M.A.; Elghandour, A.; Jedrzejczak, W.W.; Gunther, A.; Nakorn, T.N.; Siritanaratkul, N.; et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: A multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 2014, 15, 1195–1206. [Google Scholar] [CrossRef]
- Palumbo, A.; Chanan-Khan, A.; Weisel, K.; Nooka, A.K.; Masszi, T.; Beksac, M.; Spicka, I.; Hungria, V.; Munder, M.; Mateos, M.V.; et al. Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 375, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.G.; Sonneveld, P.; Schuster, M.W.; Irwin, D.; Stadtmauer, E.A.; Facon, T.; Harousseau, J.L.; Ben-Yehuda, D.; Lonial, S.; San Miguel, J.F.; et al. Safety and efficacy of bortezomib in high-risk and elderly patients with relapsed multiple myeloma. Br. J. Haematol. 2007, 137, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Weisel, K.; Doyen, C.; Dimopoulos, M.; Yee, A.; Lahuerta, J.J.; Martin, A.; Travers, K.; Druyts, E.; Toor, K.; Abildgaard, N.; et al. A systematic literature review and network meta-analysis of treatments for patients with untreated multiple myeloma not eligible for stem cell transplantation. Leuk. Lymphoma 2017, 58, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.M.; Chen, C.; Niesvizky, R.; Wang, M.; Belch, A.; Stadtmauer, E.A.; Siegel, D.; Borrello, I.; Rajkumar, S.V.; Chanan-Khan, A.A.; et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N. Engl. J. Med. 2007, 357, 2133–2142. [Google Scholar] [CrossRef]
- Stewart, A.K.; Rajkumar, S.V.; Dimopoulos, M.A.; Masszi, T.; Spicka, I.; Oriol, A.; Hajek, R.; Rosinol, L.; Siegel, D.S.; Mihaylov, G.G.; et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N. Engl. J. Med. 2015, 372, 142–152. [Google Scholar] [CrossRef]

| Trial Name/First Author | Number of Patients | Treatment Arm A | Treatment Arm B | Cut-Off Level of Age | Primary Objective |
|---|---|---|---|---|---|
| CASTOR (NCT02136134) | 241 | DaraBorDex | BorDex | 65 | PFS |
| ELOQUENT-2 (NCT01239797) | 370 | EloLenDex | LenDex | 65 | PFS |
| MM-003 (NCT01311687) | 36 | PomDex | Dex | 75 | PFS |
| PANORAMA1 (NCT01023308) | 323 | PanoBorDex | BorDex | 65 | PFS |
| POLLUX (NCT02076009) | 296 | DaraLenDex | LenDex | 65 | PFS |
| ENDEAVOR (NCT01568866) | 496 | CarDex | BorDex | 65 | PFS |
| VANTAGE 088 (NCT00773747) | 256 | VorinoBor | Bor | 65 | PFS |
| ASPIRE (NCT01080391) | 393 | CarLenDex | LenDex | 65 | PFS |
| Orlowski (NCT00103506) | 250 | Bor | PegDoxBor | 65 | TTP |
| Jakubowiak (NCT01478048) | 85 | EloBorDex | BorDex | 65 | PFS |
| MM-009 (NCT00056160) MM-010 (NCT00424047) | 314 | Dex | Dex | 65 | PFS |
| APEX (NCT00048230) | 245 | Bor | Dex | 65 | TTP |
| Tourmaline-MM1(NCT01564537) | 32 | IxaLenDex | LenDex | 65 | PFS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.-L.; Lin, C.; Ho, C.-L.; Huang, T.-C.; Wu, Y.-Y.; Jhou, H.-J.; Chen, P.-H.; Lee, C.-H. Progression-Free Survival Efficacy in Refractory/Relapsed Multiple Myeloma among Elderly Patients: A Systematic Review. Life 2023, 13, 2259. https://doi.org/10.3390/life13122259
Yang T-L, Lin C, Ho C-L, Huang T-C, Wu Y-Y, Jhou H-J, Chen P-H, Lee C-H. Progression-Free Survival Efficacy in Refractory/Relapsed Multiple Myeloma among Elderly Patients: A Systematic Review. Life. 2023; 13(12):2259. https://doi.org/10.3390/life13122259
Chicago/Turabian StyleYang, Tung-Lung, Chin Lin, Ching-Liang Ho, Tzu-Chuan Huang, Yi-Ying Wu, Hong-Jie Jhou, Po-Huang Chen, and Cho-Hao Lee. 2023. "Progression-Free Survival Efficacy in Refractory/Relapsed Multiple Myeloma among Elderly Patients: A Systematic Review" Life 13, no. 12: 2259. https://doi.org/10.3390/life13122259
APA StyleYang, T.-L., Lin, C., Ho, C.-L., Huang, T.-C., Wu, Y.-Y., Jhou, H.-J., Chen, P.-H., & Lee, C.-H. (2023). Progression-Free Survival Efficacy in Refractory/Relapsed Multiple Myeloma among Elderly Patients: A Systematic Review. Life, 13(12), 2259. https://doi.org/10.3390/life13122259







