The Effect of Mechanical Stress on Hyaluronan Fragments’ Inflammatory Cascade: Clinical Implications
Abstract
:1. Introduction
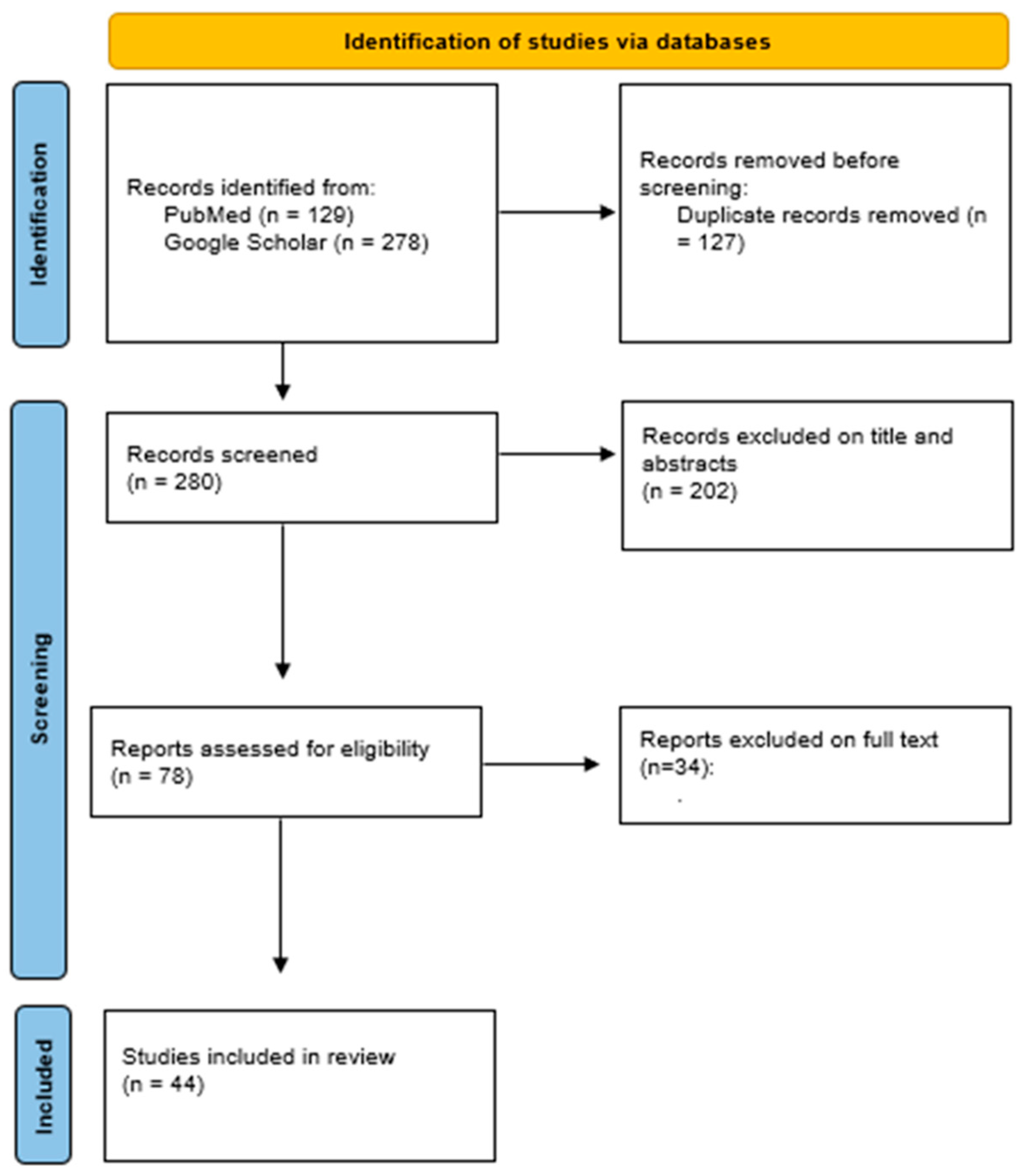
2. Materials and Methods
3. Results
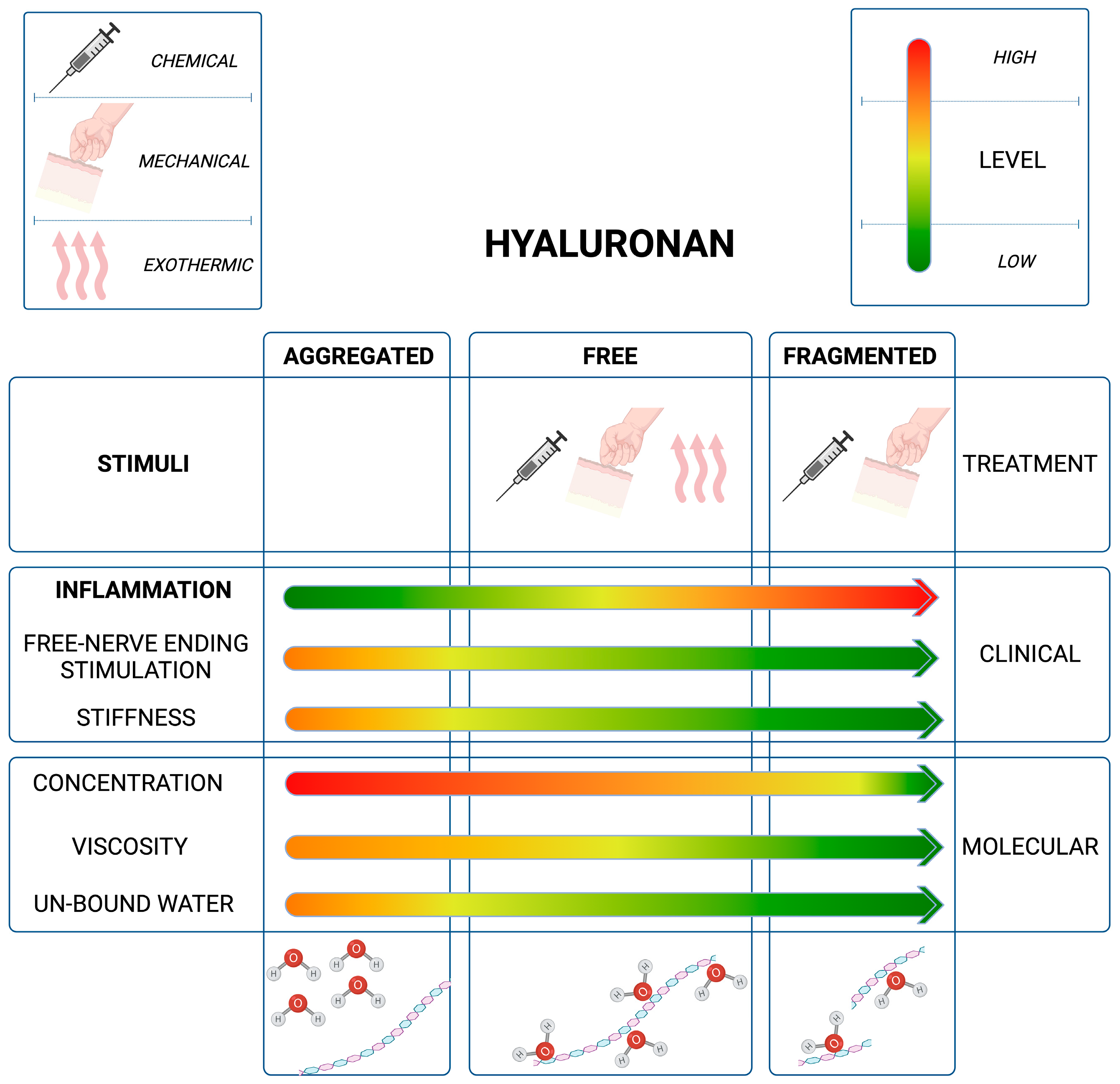
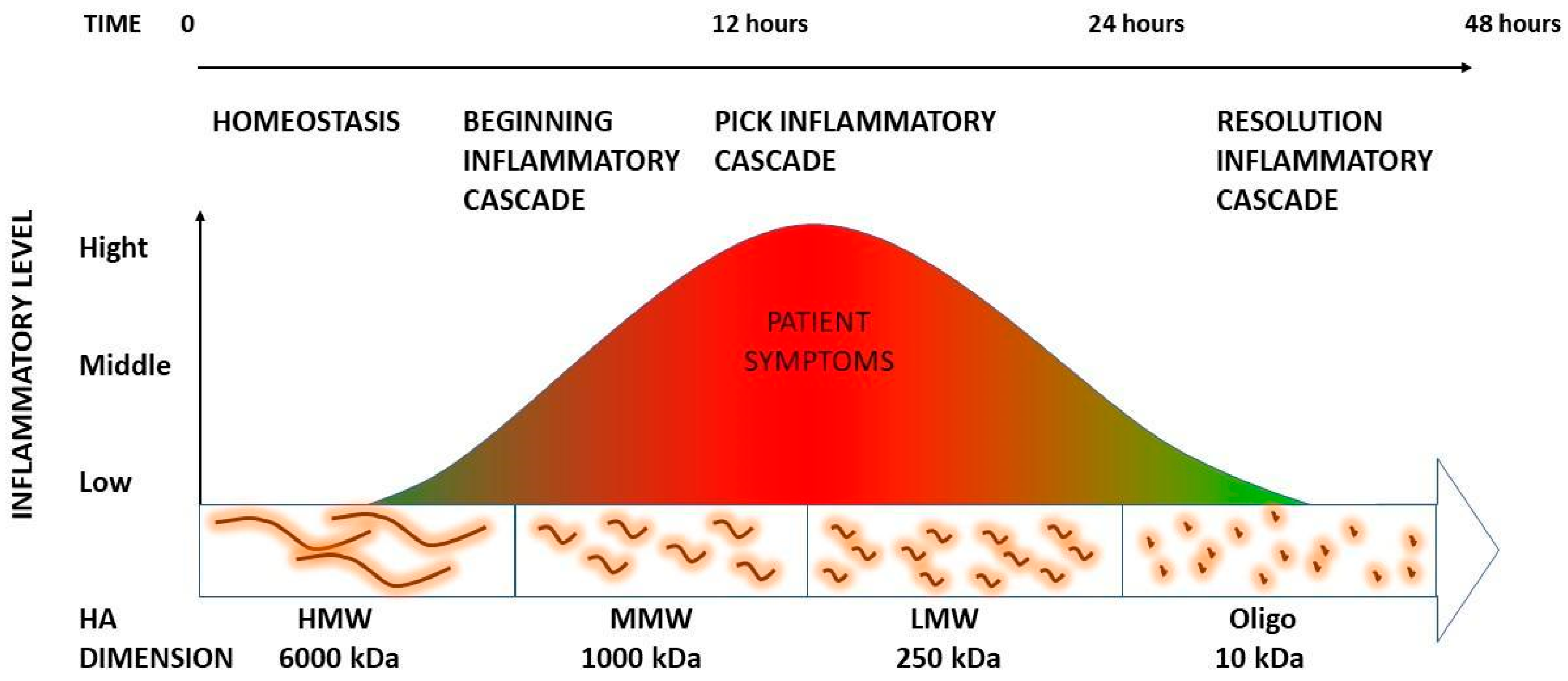
3.1. The Role of HA Weight: A Decremental Cascade during Inflammation
3.2. The Inflammation Cascade: Influencing Factors
3.3. HA Polymer Fragments: Diverse Biological Activities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Paanalahti, K.; Holm, L.W.; Nordin, M.; Asker, M.; Lyander, J.; Skillgate, E. Adverse events after manual therapy among patients seeking care for neck and/or back pain: A randomized controlled trial. BMC Musculoskelet. Disord. 2014, 15, 77. [Google Scholar] [CrossRef]
- Fidut-Wrońska, J.; Chołuj, K.; Chmiel, J.; Pikto-Pitkiewicz, K.; Majcher, P. Observation using thermography of post-operative reaction after fascial manipulation®. Ann. Agric. Environ. Med. 2019, 26, 468–471. [Google Scholar] [CrossRef]
- Stecco, C.; Day, J.A. The fascial manipulation technique and its biomechanical model: A guide to the human fascial system. Int. J. Ther. Massage Bodyw. 2010, 3, 38–40. [Google Scholar]
- Day, J.A.; Copetti, L.; Rucli, G. From clinical experience to a model for the human fascial system. J. Bodyw. Mov. Ther. 2012, 16, 372–380. [Google Scholar] [CrossRef]
- Pintucci, M.; Simis, M.; Imamura, M.; Pratelli, E.; Stecco, A.; Ozcakar, L.; Battistella, L.R. Successful treatment of rotator cuff tear using Fascial Manipulation® in a stroke patient. J. Bodyw. Mov. Ther. 2017, 21, 653–657. [Google Scholar] [CrossRef]
- Branchini, M.; Lopopolo, F.; Andreoli, E.; Loreti, I.; Marchand, A.M.; Stecco, A. Fascial Manipulation® for chronic aspecific low back pain: A single blinded randomized controlled trial. F1000Res 2015, 4, 1208. [Google Scholar] [CrossRef]
- Cowman, M.K.; Schmidt, T.A.; Raghavan, P.; Stecco, A. Viscoelastic Properties of Hyaluronan in Physiological Conditions. F1000Res 2015, 4, 622. [Google Scholar] [CrossRef]
- Lee, J.Y.; Spicer, A.P. Hyaluronan: A multifunctional, megaDalton, stealth molecule. Curr. Opin. Cell Biol. 2000, 12, 581–586. [Google Scholar] [CrossRef]
- Abatangelo, G.; Vindigni, V.; Avruscio, G.; Pandis, L.; Brun, P. Hyaluronic Acid: Redefining Its Role. Cells 2020, 9, 1743. [Google Scholar] [CrossRef]
- Anderegg, U.; Simon, J.C.; Averbeck, M. More than just a filler—The role of hyaluronan for skin homeostasis. Exp. Dermatol. 2014, 23, 295–303. [Google Scholar] [CrossRef]
- Fraser, J.R.; Laurent, T.C.; Laurent, U.B. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Oh, J.H.; Chung, J.H. Glycosaminoglycan and proteoglycan in skin aging. J. Dermatol. Sci. 2016, 83, 174–181. [Google Scholar] [CrossRef]
- Tømmeraas, K.; Melander, C. Kinetics of hyaluronan hydrolysis in acidic solution at various pH values. Biomacromolecules 2008, 9, 1535–1540. [Google Scholar] [CrossRef]
- Menon, R.G.; Oswald, S.F.; Raghavan, P.; Regatte, R.R.; Stecco, A. T1ρ-Mapping for Musculoskeletal Pain Diagnosis: Case Series of Variation of Water Bound Glycosaminoglycans Quantification before and after Fascial Manipulation® in Subjects with Elbow Pain. Int. J. Environ. Res. Public Health 2020, 17, 708. [Google Scholar] [CrossRef]
- Menon, R.G.; Raghavan, P.; Regatte, R.R. Quantifying muscle glycosaminoglycan levels in patients with post-stroke muscle stiffness using T1ρ MRI. Sci. Rep. 2019, 9, 14513. [Google Scholar] [CrossRef]
- Han, W.; Lv, Y.; Sun, Y.; Wang, Y.; Zhao, Z.; Shi, C.; Chen, X.; Wang, L.; Zhang, M.; Wei, B.; et al. The anti-inflammatory activity of specific-sized hyaluronic acid oligosaccharides. Carbohydr. Polym. 2022, 276, 118699, Erratum in Carbohydr. Polym. 2022, 282, 119101. [Google Scholar] [CrossRef]
- Bohaumilitzky, L.; Huber, A.K.; Stork, E.M.; Wengert, S.; Woelfl, F.; Boehm, H. A Trickster in Disguise: Hyaluronan’s Ambivalent Roles in the Matrix. Front. Oncol. 2017, 7, 242. [Google Scholar] [CrossRef]
- Laurent, T.C.; Fraser, J.R. Hyaluronan. FASEB J. 1992, 6, 2397–2404. [Google Scholar] [CrossRef]
- Cowman, M.K. Hyaluronan and Hyaluronan Fragments. Adv. Carbohydr. Chem. Biochem. 2017, 74, 1–59. [Google Scholar]
- Balazs, E.A. Viscoelastic properties of hyaluronic acid and biological lubrication. Univ. Mich. Med. Cent. J. 1968, 1, 255–259. [Google Scholar]
- Anderegg, U.; Halfter, N.; Schnabelrauch, M.; Hintze, V. Collagen/glycosaminoglycan-based matrices for controlling skin cell responses. Biol. Chem. 2021, 402, 1325–1335. [Google Scholar] [CrossRef] [PubMed]
- Spicer, A.P.; Tien, J.Y. Hyaluronan and morphogenesis. Birth Defects Res. C Embryo Today 2004, 72, 89–108. [Google Scholar] [CrossRef] [PubMed]
- Garg, H.; Hales, C. Chemistry and Biology of Hyaluronan, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Yu, L.; Quinn, D.A.; Garg, H.G.; Hales, C.A. Cyclin-dependent kinase inhibitor p27Kip1, but not p21WAF1/Cip1, is required for inhibition of hypoxia-induced pulmonary hypertension and remodeling by heparin in mice. Circ. Res. 2005, 97, 937–945. [Google Scholar] [CrossRef]
- Heldin, P. Chemistry and Biology of Hyaluronan. Edited by Hari G. Garg and Charles A. Hales. ChemBioChem 2005, 6, 1288–1289. [Google Scholar] [CrossRef]
- Tian, X.; Azpurua, J.; Hine, C.; Vaidya, A.; Myakishev-Rempel, M.; Ablaeva, J.; Mao, Z.; Nevo, E.; Gorbunova, V.; Seluanov, A. High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature 2013, 499, 346–349. [Google Scholar] [CrossRef]
- Itano, N.; Sawai, T.; Yoshida, M.; Lenas, P.; Yamada, Y.; Imagawa, M.; Shinomura, T.; Hamaguchi, M.; Yoshida, Y.; Ohnuki, Y.; et al. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J. Biol. Chem. 1999, 274, 25085–25092. [Google Scholar] [CrossRef]
- Huin-Amargier, C.; Marchal, P.; Payan, E.; Netter, P.; Dellacherie, E. New Physically and Chemically Crosslinked Hyaluronate (HA)-based Hydrogels for Cartilage Repair. J. Biomed. Mater. 2005, 76, 416–424. [Google Scholar] [CrossRef]
- Capila, I.; Sasisekharan, R. Chapter 2—Methods for Analysis of Hyaluronan and Its Fragments. In Chemistry and Biology of Hyaluronan; Hari, G.G., Charles, A.H., Eds.; Elsevier Press: Amsterdam, The Netherlands, 2004; pp. 21–40. [Google Scholar]
- Nazet, U.; Feulner, L.; Muschter, D.; Neubert, P.; Schatz, V.; Grässel, S.; Jantsch, J.; Proff, P.; Schröder, A.; Kirschneck, C. Mechanical Stress Induce PG-E2 in Murine Synovial Fibroblasts Originating from the Temporomandibular Joint. Cells 2021, 10, 298. [Google Scholar] [CrossRef]
- Parsons, B.J.; Spickett, C.M. Special issue on “Analytical methods for the detection of oxidized biomolecules and antioxidants”. Free Radic. Res. 2015, 49, 473–476. [Google Scholar] [CrossRef]
- Kasai, S.; Furuichi, Y.; Ando, N.; Kagami, K.; Abe, M.; Nakane, T.; Goi, K.; Inukai, T.; Saitoh, S.; Ohno, S.; et al. Inflammatory mediator ultra-low-molecular-weight hyaluronan triggers necrosis of B-precursor leukemia cells with high surface CD44 expression. Cell Death Dis. 2017, 8, e2857. [Google Scholar] [CrossRef]
- Stern, R. Hyaluronan catabolism: A new metabolic pathway. Eur. J. Cell Biol. 2004, 83, 317–325. [Google Scholar] [CrossRef]
- Avenoso, A.; Bruschetta, G.; D’Ascola, A.; Scuruchi, M.; Mandraffino, G.; Saitta, A.; Campo, S.; Campo, G.M. Hyaluronan Fragmentation During Inflammatory Pathologies: A Signal that Empowers Tissue Damage. Mini Rev. Med. Chem. 2020, 20, 54–65. [Google Scholar] [CrossRef]
- Horton, M.R.; Shapiro, S.; Bao, C.; Lowenstein, C.J.; Noble, P.W. Induction and regulation of macrophage metalloelastase by hyaluronan fragments in mouse macrophages. J. Immunol. 1999, 162, 4171–4176. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Hascall, V.C.; Markwald, R.R.; Ghatak, S. Interactions between hyaluronan and its receptors (CD44, RHAMM) regulate the activities of inflammation and cancer. Front. Immunol. 2015, 6, 201. [Google Scholar] [CrossRef]
- Yamamoto, H.; Tobisawa, Y.; Inubushi, T.; Irie, F.; Ohyama, C.; Yamaguchi, Y. A mammalian homolog of the zebrafish transmembrane protein 2 (TMEM2) is the long-sought-after cell-surface hyaluronidase. J. Biol. Chem. 2017, 292, 7304–7313. [Google Scholar] [CrossRef] [PubMed]
- Tavianatou, A.G.; Caon, I.; Franchi, M.; Piperigkou, Z.; Galesso, D.; Karamanos, N.K. Hyaluronan: Molecular size-dependent signaling and biological functions in inflammation and cancer. FEBS J. 2019, 286, 2883–2908. [Google Scholar] [CrossRef]
- Yamasaki, K.; Muto, J.; Taylor, K.R.; Cogen, A.L.; Audish, D.; Bertin, J.; Grant, E.P.; Coyle, A.J.; Misaghi, A.; Hoffman, H.M.; et al. NLRP3/cryopyrin is necessary for interleukin-1beta (IL-1beta) release in response to hyaluronan, an endogenous trigger of inflammation in response to injury. J. Biol. Chem. 2009, 284, 12762–12771. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Takahashi, M. CD44-dependent intracellular and extracellular catabolism of hyaluronic acid by hyaluronidase-1 and -2. J. Biol. Chem. 2007, 282, 5597–5607. [Google Scholar] [CrossRef] [PubMed]
- Donelan, W.; Dominguez-Gutierrez, P.R.; Kusmartsev, S. Deregulated hyaluronan metabolism in the tumor microenvironment drives cancer inflammation and tumor-associated immune suppression. Front. Immunol. 2022, 13, 971278. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.D.; Horton, M.R. Threat matrix: Low-molecular-weight hyaluronan (HA) as a danger signal. Immunol. Res. 2005, 31, 207–218. [Google Scholar] [CrossRef]
- Tolg, C.; McCarthy, J.B.; Yazdani, A.; Turley, E.A. Hyaluronan and RHAMM in wound repair and the “cancerization” of stromal tissues. Biomed. Res. Int. 2014, 2014, 103923. [Google Scholar] [CrossRef] [PubMed]
- Slevin, M.; Krupinski, J.; Gaffney, J.; Matou, S.; West, D.; Delisser, H.; Savani, R.C.; Kumar, S. Hyaluronan-mediated angiogenesis in vascular disease: Uncovering RHAMM and CD44 receptor signaling pathways. Matrix Biol. 2007, 26, 58–68. [Google Scholar] [CrossRef]
- Wolf, D.; Schümann, J.; Koerber, K.; Kiemer, A.K.; Vollmar, A.M.; Sass, G.; Papadopoulos, T.; Bang, R.; Klein, S.D.; Brüne, B.; et al. Low-molecular-weight hyaluronic acid induces nuclear factor-kappaB-dependent resistance against tumor necrosis factor alpha-mediated liver injury in mice. Hepatology 2001, 34, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.F.; Qiao, S.P.; Shi, S.L.; Yao, L.F.; Hou, X.L.; Li, C.F.; Lin, F.H.; Guo, K.; Acharya, A.; Chen, X.B.; et al. Modulating Three-Dimensional Microenvironment with Hyaluronan of Different Molecular Weights Alters Breast Cancer Cell Invasion Behavior. ACS Appl. Mater. Interfaces 2017, 9, 9327–9338. [Google Scholar] [CrossRef]
- Misra, S.; Toole, B.P.; Ghatak, S. Hyaluronan constitutively regulates activation of multiple receptor tyrosine kinases in epithelial and carcinoma cells. J. Biol. Chem. 2006, 281, 34936–34941. [Google Scholar] [CrossRef] [PubMed]
- Kolar, S.L.; Kyme, P.; Tseng, C.W.; Soliman, A.; Kaplan, A.; Liang, J.; Nizet, V.; Jiang, D.; Murali, R.; Arditi, M.; et al. Group B Streptococcus Evades Host Immunity by Degrading Hyaluronan. Cell Host Microbe 2015, 18, 694–704. [Google Scholar] [CrossRef]
- Xu, H.; Ito, T.; Tawada, A.; Maeda, H.; Yamanokuchi, H.; Isahara, K.; Yoshida, K.; Uchiyama, Y.; Asari, A. Effect of hyaluronan oligosaccharides on the expression of heat shock protein 72. J. Biol. Chem. 2002, 277, 17308–17314. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, C.; Chen, Y.; Cowell, J.A.; Wei, G.; Kultti, A.; Huang, L.; Thompson, C.B.; Rosengren, S.; Frost, G.I.; et al. Recombinant human hyaluronidase PH20 does not stimulate an acute inflammatory response and inhibits lipopolysaccharide-induced neutrophil recruitment in the air pouch model of inflammation. J. Immunol. 2014, 192, 5285–5295. [Google Scholar] [CrossRef]
- Tammi, M.I.; Oikari, S.; Pasonen-Seppänen, S.; Rilla, K.; Auvinen, P.; Tammi, R.H. Activated hyaluronan metabolism in the tumor matrix—Causes and consequences. Matrix Biol. 2019, 78–79, 147–164. [Google Scholar] [CrossRef]
- McDevitt, A.W.; Cooper, C.G.; Friedrich, J.M.; Anderson, D.J.M.; Arnold, E.A.; Clewley, D.J. Effect of Physical Therapy Timing on Patient Reported Outcomes for Individuals with Acute Low Back Pain: A Systematic Review with Meta Analysis of Randomized Controlled Trials. PM&R 2023, 15, 1466–1477. [Google Scholar]
- Daecke, W.; Kusnierczak, D.; Loew, M. Long-term effects of extracorporeal shockwave therapy in chronic calcific tendinitis of the shoulder. J. Shoulder Elbow Surg. 2002, 11, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Matteini, P.; Dei, L.; Carretti, E.; Volpi, N.; Goti, A.; Pini, R. Structural behavior of highly concentrated hyaluronan. Biomacromolecules 2009, 10, 1516–1522. [Google Scholar] [CrossRef] [PubMed]
- Stecco, C.; Fede, C.; Macchi, V.; Porzionato, A.; Petrelli, L.; Biz, C.; Stern, R.; De Caro, R. The fasciacytes: A new cell devoted to fascial gliding regulation. Clin. Anat. 2018, 31, 667–676. [Google Scholar] [CrossRef]
- Nagy, N.; Kuipers, H.F.; Marshall, P.L.; Wang, E.; Kaber, G.; Bollyky, P.L. Hyaluronan in immune dysregulation and autoimmune diseases. Matrix Biol. 2019, 78–79, 292–313. [Google Scholar] [CrossRef]
- Stern, R.; Asari, A.A.; Sugahara, K.N. Hyaluronan fragments: An information-rich system. Eur. J. Cell Biol. 2006, 85, 699–715. [Google Scholar] [CrossRef] [PubMed]
- Sekito, F.; Pintucci, M.; Pirri, C.; Ribeiro de Moraes Rego, M.; Cardoso, M.; Soares Paixão, K.; Ribeiro da Silva, V.; Stecco, A. Facial Pain: RCT between Conventional Treatment and Fascial Manipulation® for Temporomandibular Disorders. Bioengineering 2022, 9, 279. [Google Scholar] [CrossRef]
- Brandolini, S.; Lugaresi, G.; Santagata, A.; Ermolao, A.; Zaccaria, M.; Marchand, A.M.; Stecco, A. Sport injury prevention in individuals with chronic ankle instability: Fascial Manipulation® versus control group: A randomized controlled trial. J. Bodyw. Mov. Ther. 2019, 23, 316–323. [Google Scholar] [CrossRef]
- Hinghofer-Szalkay, H.G.; Mekonen, W.; Rössler, A.; Schwaberger, G.; Lamprecht, M.; Hofmann, P. Post-exercise decrease of plasma hyaluronan: Increased clearance or diminished production? Physiol. Res. 2002, 51, 139–144. [Google Scholar] [CrossRef]
- Borgini, E.; Antonio, S.; Julie Ann, D.; Stecco, C. How much time is required to modify a fascial fibrosis? J. Bodyw. Mov. Ther. 2010, 14, 318–325. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stecco, A.; Bonaldi, L.; Fontanella, C.G.; Stecco, C.; Pirri, C. The Effect of Mechanical Stress on Hyaluronan Fragments’ Inflammatory Cascade: Clinical Implications. Life 2023, 13, 2277. https://doi.org/10.3390/life13122277
Stecco A, Bonaldi L, Fontanella CG, Stecco C, Pirri C. The Effect of Mechanical Stress on Hyaluronan Fragments’ Inflammatory Cascade: Clinical Implications. Life. 2023; 13(12):2277. https://doi.org/10.3390/life13122277
Chicago/Turabian StyleStecco, Antonio, Lorenza Bonaldi, Chiara Giulia Fontanella, Carla Stecco, and Carmelo Pirri. 2023. "The Effect of Mechanical Stress on Hyaluronan Fragments’ Inflammatory Cascade: Clinical Implications" Life 13, no. 12: 2277. https://doi.org/10.3390/life13122277







