A Comparative Analysis of the Protein Cargo of Extracellular Vesicles from Helminth Parasites
Abstract
1. Introduction
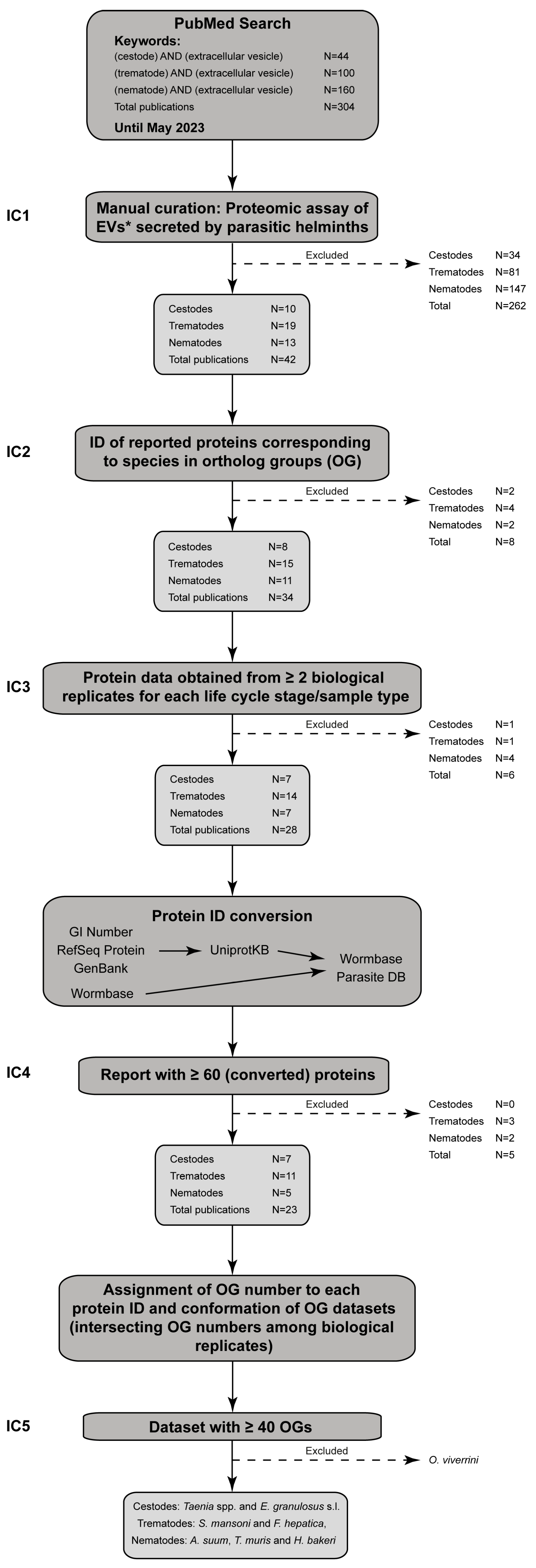
2. Materials and Methods
2.1. Orthology Analysis
2.2. Collection of Published Proteomic Data from the EVs of Helminth Parasites
2.3. Data Processing
2.4. Proteins of Unknown Function
2.5. Graphics
3. Results and Discussion
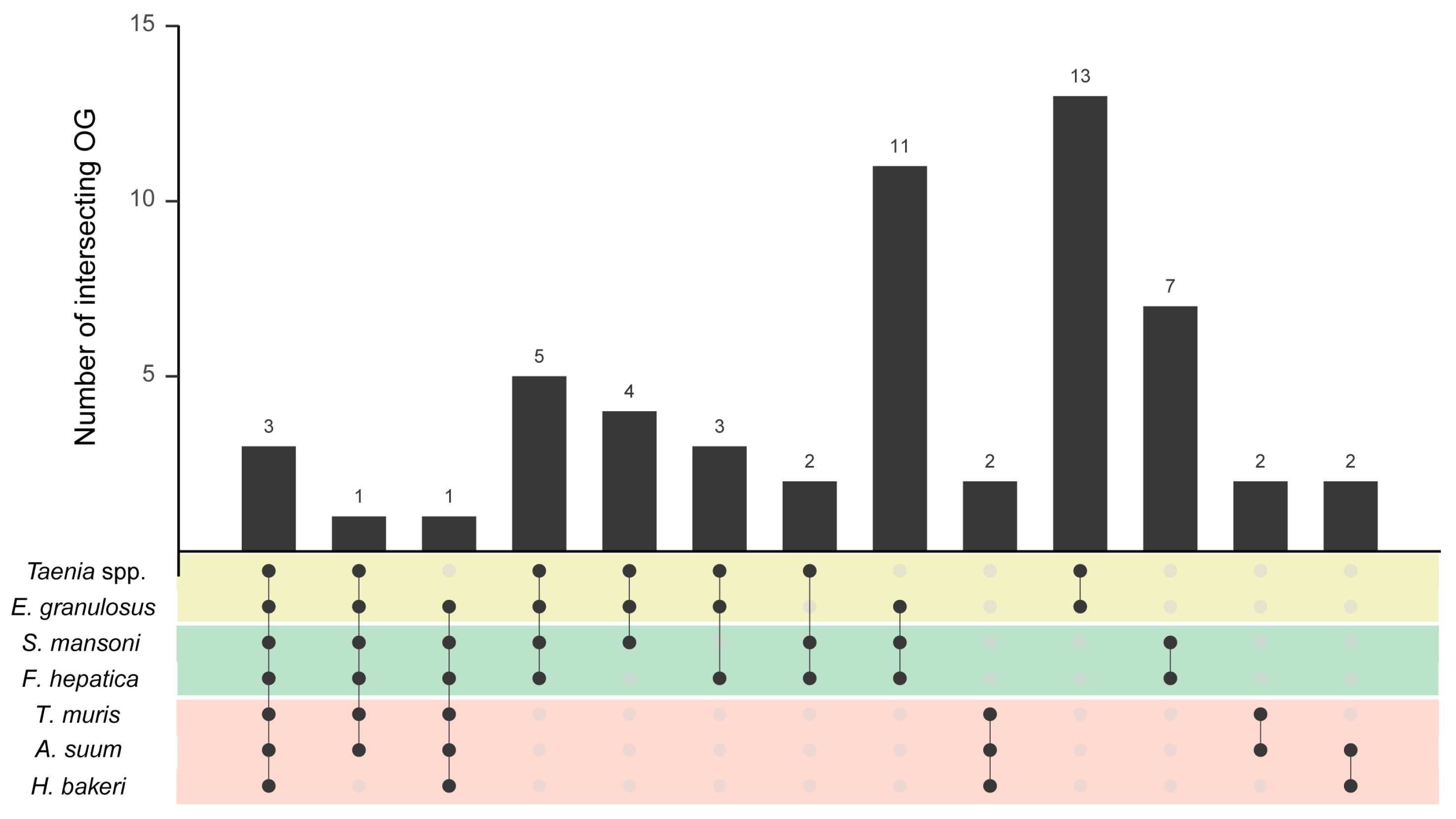
3.1. EVs from Helminth Parasites Share a Core Set of Proteins Secreted in a Taxon-Related Manner
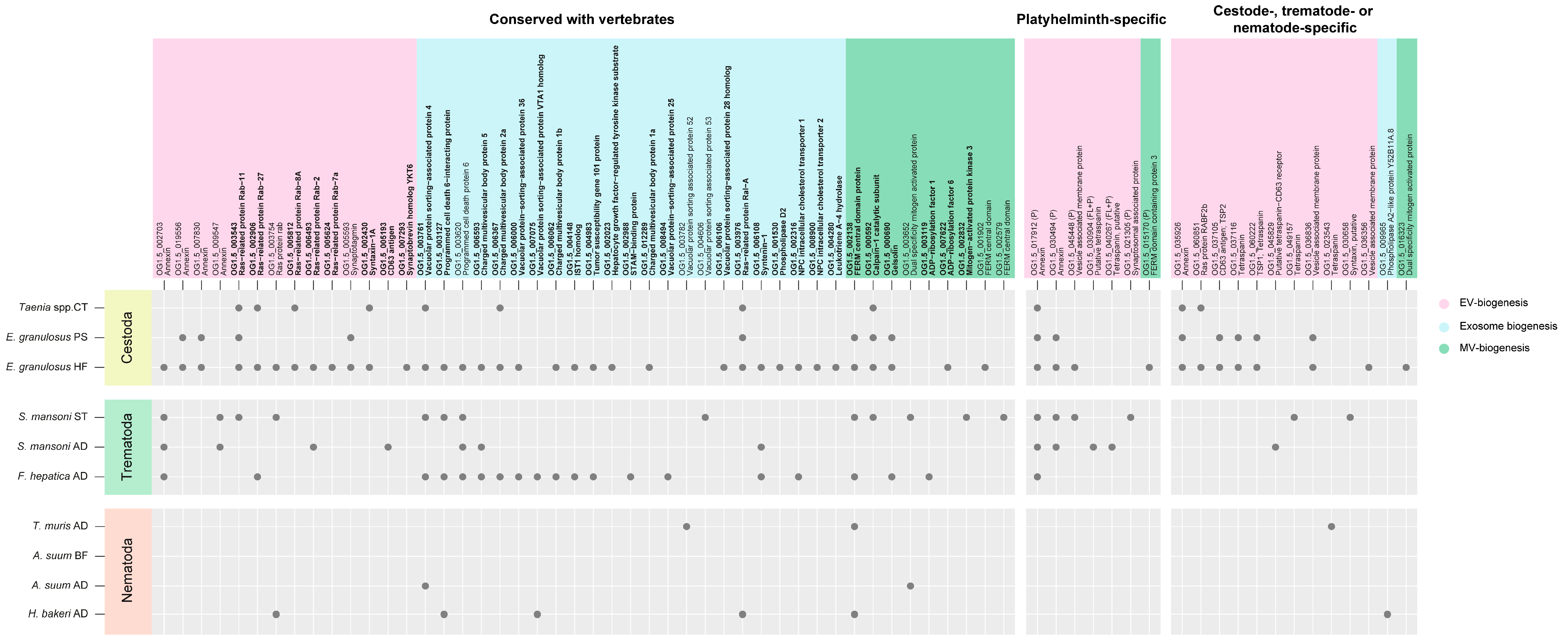
3.2. EVs from Helminth Parasites Contain Canonical and Non-Canonical Proteins Related to Exosome and Microvesicle Biogenesis
3.3. Helminth EVs Carry Taxon-Specific Proteins of Unknown Function
3.4. Helminth Parasites Secrete Moonlighting Proteins as EV-Cargo
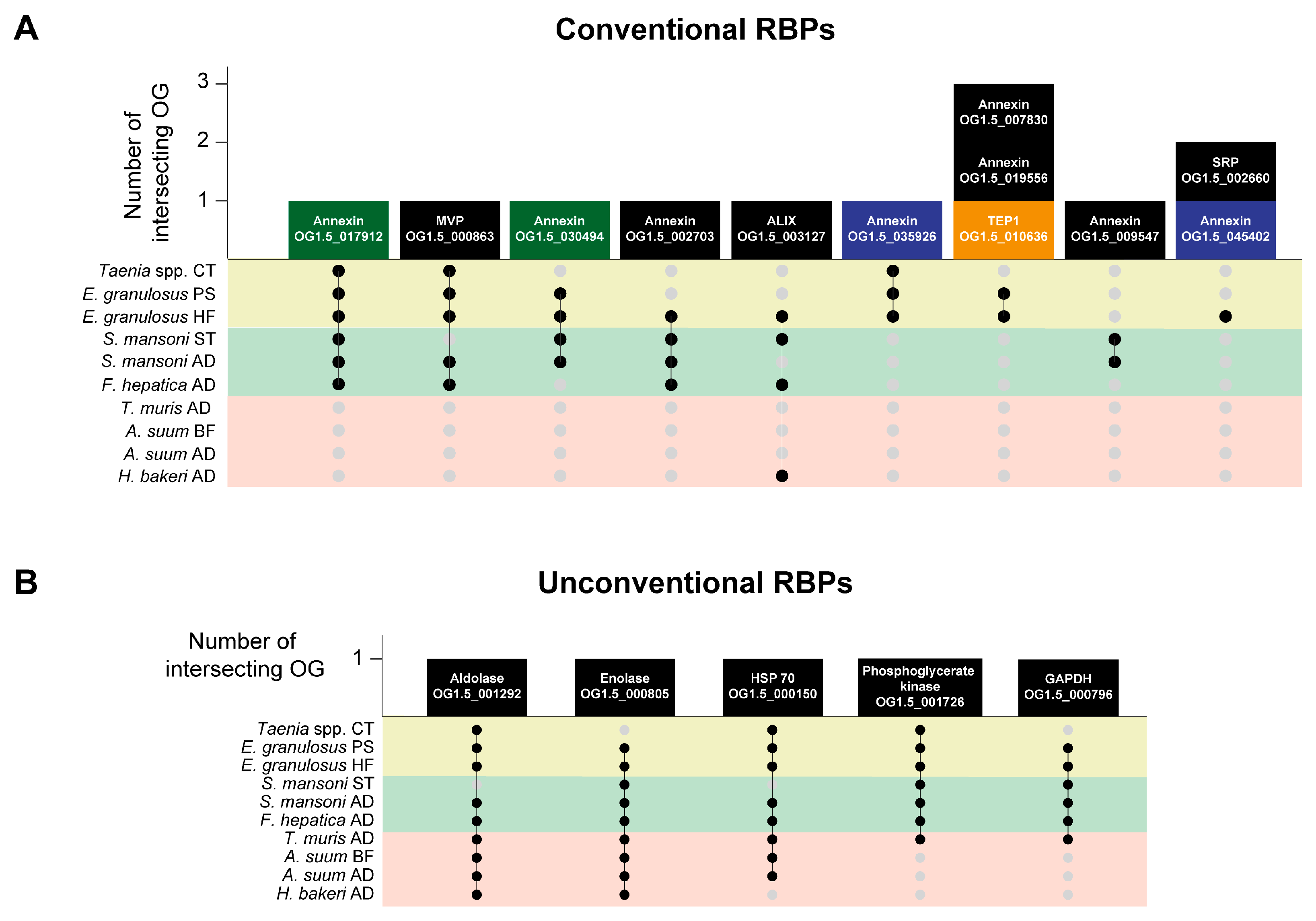
3.5. Parasitic Platyhelminths and Nematodes Secrete EVs with Different Repertoires of RNA-Binding Proteins (RBPs)
3.6. EVs as a Source of Vaccine Antigens
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030; World Health Organization: Geneva, Switzerland, 2020.
- Marcilla, A.; Trelis, M.; Cortés, A.; Sotillo, J.; Cantalapiedra, F.; Minguez, M.T.; Valero, M.L.; Sánchez del Pino, M.M.; Muñoz-Antoli, C.; Toledo, R.; et al. Extracellular Vesicles from Parasitic Helminths Contain Specific Excretory/Secretory Proteins and Are Internalized in Intestinal Host Cells. PLoS ONE 2012, 7, e45974. [Google Scholar] [CrossRef]
- Woith, E.; Fuhrmann, G.; Melzig, M.F. Extracellular Vesicles—Connecting Kingdoms. Int. J. Mol. Sci. 2019, 20, 5695. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of Secretion and Uptake of Exosomes and Other Extracellular Vesicles for Cell-to-Cell Communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.P.S.; de la Torre-Escudero, E.; Robinson, M.W. Helminth Genome Analysis Reveals Conservation of Extracellular Vesicle Biogenesis Pathways but Divergence of RNA Loading Machinery between Phyla. Int. J. Parasitol. 2020, 50, 655–661. [Google Scholar] [CrossRef]
- de la Torre-Escudero, E.; Gerlach, J.Q.; Bennett, A.P.S.; Cwiklinski, K.; Jewhurst, H.L.; Huson, K.M.; Joshi, L.; Kilcoyne, M.; O’Neill, S.; Dalton, J.P.; et al. Surface Molecules of Extracellular Vesicles Secreted by the Helminth Pathogen Fasciola Hepatica Direct Their Internalisation by Host Cells. PLoS Negl. Trop. Dis. 2019, 13, e0007087. [Google Scholar] [CrossRef]
- Cwiklinski, K.; de la Torre-Escudero, E.; Trelis, M.; Bernal, D.; Dufresne, P.J.; Brennan, G.P.; O’Neill, S.; Tort, J.; Paterson, S.; Marcilla, A.; et al. The Extracellular Vesicles of the Helminth Pathogen, Fasciola hepatica: Biogenesis Pathways and Cargo Molecules Involved in Parasite Pathogenesis. Mol. Cell. Proteom. 2015, 14, 3258–3273. [Google Scholar] [CrossRef] [PubMed]
- Chaiyadet, S.; Sotillo, J.; Smout, M.; Cantacessi, C.; Jones, M.K.; Johnson, M.S.; Turnbull, L.; Whitchurch, C.B.; Potriquet, J.; Laohaviroj, M.; et al. Carcinogenic Liver Fluke Secretes Extracellular Vesicles That Promote Cholangiocytes to Adopt a Tumorigenic Phenotype. J. Infect. Dis. 2015, 212, 1636–1645. [Google Scholar] [CrossRef]
- Chaiyadet, S.; Sotillo, J.; Krueajampa, W.; Thongsen, S.; Smout, M.; Brindley, P.J.; Laha, T.; Loukas, A. Silencing of Opisthorchis viverrini Tetraspanin Gene Expression Results in Reduced Secretion of Extracellular Vesicles. Front. Cell Infect. Microbiol. 2022, 12, 827521. [Google Scholar] [CrossRef]
- Bennett, A.P.S.; de la Torre-Escudero, E.; Oliver, N.A.M.; Huson, K.M.; Robinson, M.W. The Cellular and Molecular Origins of Extracellular Vesicles Released by the Helminth Pathogen, Fasciola hepatica. Int. J. Parasitol. 2020, 50, 671–683. [Google Scholar] [CrossRef]
- Harischandra, H.; Yuan, W.; Loghry, H.J.; Zamanian, M.; Kimber, M.J. Profiling Extracellular Vesicle Release by the Filarial Nematode Brugia malayi Reveals Sex-Specific Differences in Cargo and a Sensitivity to Ivermectin. PLoS Negl. Trop. Dis. 2018, 12, e0006438. [Google Scholar] [CrossRef]
- Sotillo, J.; Pearson, M.; Potriquet, J.; Becker, L.; Pickering, D.; Mulvenna, J.; Loukas, A. Extracellular Vesicles Secreted by Schistosoma mansoni Contain Protein Vaccine Candidates. Int. J. Parasitol. 2016, 46, 1–5. [Google Scholar] [CrossRef]
- Chaiyadet, S.; Sotillo, J.; Krueajampa, W.; Thongsen, S.; Brindley, P.J.; Sripa, B.; Loukas, A.; Laha, T. Vaccination of Hamsters with Opisthorchis viverrini Extracellular Vesicles and Vesicle-Derived Recombinant Tetraspanins Induces Antibodies That Block Vesicle Uptake by Cholangiocytes and Reduce Parasite Burden after Challenge Infection. PLoS Negl. Trop. Dis. 2019, 13, e0007450. [Google Scholar] [CrossRef]
- Sheng, Z.A.; Wu, C.L.; Wang, D.Y.; Zhong, S.H.; Yang, X.; Rao, G.S.; Peng, H.; Feng, S.W.; Li, J.; Huang, W.Y.; et al. Proteomic Analysis of Exosome-like Vesicles from Fasciola gigantica Adult Worm Provides Support for New Vaccine Targets against Fascioliasis. Parasit. Vectors 2023, 16, 62. [Google Scholar] [CrossRef] [PubMed]
- Fratini, F.; Tamarozzi, F.; Macchia, G.; Bertuccini, L.; Mariconti, M.; Birago, C.; Iriarte, A.; Brunetti, E.; Cretu, C.M.; Akhan, O.; et al. Proteomic Analysis of Plasma Exosomes from Cystic Echinococcosis Patients Provides In Vivo Support for Distinct Immune Response Profiles in Active vs. Inactive Infection and Suggests Potential Biomarkers. PLoS Negl. Trop. Dis. 2020, 14, e0008586. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhou, X.; Yang, W.; Wu, J.; Bai, M.; Zhang, Y.; Zhao, W.; Yang, H.; Nagai, A.; Yin, M.; et al. Proteomic Analysis of Plasma-Derived Extracellular Vesicles from Mice with Echinococcus granulosus at Different Infection Stages and Their Immunomodulatory Functions. Front. Cell Infect. Microbiol. 2022, 12, 805010. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.L.; Beuning, P.J.; Ondrechen, M.J. Biochemical Functional Predictions for Protein Structures of Unknown or Uncertain Function. Comput. Struct. Biotechnol. J. 2015, 13, 182–191. [Google Scholar] [CrossRef]
- Abril, J.F.; Castellano, S. Genome Annotation. Encycl. Bioinform. Comput. Biol. ABC Bioinform. 2019, 1–3, 195–209. [Google Scholar] [CrossRef]
- Sotillo, J.; Robinson, M.W.; Kimber, M.J.; Cucher, M.; Ancarola, M.E.; Nejsum, P.; Marcilla, A.; Eichenberger, R.M.; Tritten, L. The Protein and MicroRNA Cargo of Extracellular Vesicles from Parasitic Helminths—Current Status and Research Priorities. Int. J. Parasitol. 2020, 50, 635–645. [Google Scholar] [CrossRef]
- Zhou, N.; Jiang, Y.; Bergquist, T.R.; Lee, A.J.; Kacsoh, B.Z.; Crocker, A.W.; Lewis, K.A.; Georghiou, G.; Nguyen, H.N.; Hamid, M.N.; et al. The CAFA Challenge Reports Improved Protein Function Prediction and New Functional Annotations for Hundreds of Genes through Experimental Screens. Genome Biol. 2019, 20, 244. [Google Scholar] [CrossRef]
- Palevich, N.; Britton, C.; Kamenetzky, L.; Mitreva, M.; de Moraes Mourão, M.; Bennuru, S.; Quack, T.; Scholte, L.L.S.; Tyagi, R.; Slatko, B.E. Tackling Hypotheticals in Helminth Genomes. Trends Parasitol. 2018, 34, 179–183. [Google Scholar] [CrossRef]
- Nievas, Y.R.; Lizarraga, A.; Salas, N.; Cóceres, V.M.; de Miguel, N. Extracellular Vesicles Released by Anaerobic Protozoan Parasites: Current Situation. Cell Microbiol. 2020, 22, e13257. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, L.L.; Assis, J.; Araújo, F.M.G.; Salim, A.C.M.; Macchiaroli, N.; Cucher, M.; Camicia, F.; Fox, A.; Rosenzvit, M.; Oliveira, G.; et al. The Echinococcus canadensis (G7) Genome: A Key Knowledge of Parasitic Platyhelminth Human Diseases. BMC Genom. 2017, 18, 204. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A New Generation of Protein Database Search Programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Brunk, B.P.; Chen, F.; Gao, X.; Harb, O.S.; Iodice, J.B.; Shanmugam, D.; Roos, D.S.; Stoeckert, C.J. Using OrthoMCL to Assign Proteins to OrthoMCL-DB Groups or to Cluster Proteomes into New Ortholog Groups. Curr. Protoc. Bioinform. 2011, 35, 6.12.1–6.12.19. [Google Scholar] [CrossRef]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 5.0 Improves Signal Peptide Predictions Using Deep Neural Networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Krogh, A.; Larsson, B.; Von Heijne, G.; Sonnhammer, E.L.L. Predicting Transmembrane Protein Topology with a Hidden Markov Model: Application to Complete Genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef]
- Conway, J.R.; Lex, A.; Gehlenborg, N. UpSetR: An R Package for the Visualization of Intersecting Sets and Their Properties. Bioinformatics 2017, 33, 2938–2940. [Google Scholar] [CrossRef]
- Nicolao, M.C.; Rodriguez Rodrigues, C.; Cumino, A.C. Extracellular Vesicles from Echinococcus granulosus Larval Stage: Isolation, Characterization and Uptake by Dendritic Cells. PLoS Negl. Trop. Dis. 2019, 13, e0007032. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, W.; Cui, F.; Shi, C.; Ma, Y.; Yu, Y.; Zhao, W.; Zhao, J. Extracellular Vesicles Derived from Echinococcus granulosus Hydatid Cyst Fluid from Patients: Isolation, Characterization and Evaluation of Immunomodulatory Functions on T Cells. Int. J. Parasitol. 2019, 49, 1029–1037. [Google Scholar] [CrossRef]
- Yang, J.; Wu, J.; Fu, Y.; Yan, L.; Li, Y.; Guo, X.; Zhang, Y.; Wang, X.; Shen, Y.; Cho, W.C.; et al. Identification of Different Extracellular Vesicles in the Hydatid Fluid of Echinococcus granulosus and Immunomodulatory Effects of 110 K EVs on Sheep PBMCs. Front. Immunol. 2021, 12, 315. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cai, M.; Yang, J.; Li, Y.; Ding, J.; Kandil, O.M.; Kutyrev, I.; Ayaz, M.; Zheng, Y. Comparative Analysis of Different Extracellular Vesicles Secreted by Echinococcus granulosus Protoscoleces. Acta Trop. 2021, 213, 105756. [Google Scholar] [CrossRef] [PubMed]
- Siles-Lucas, M.; Sánchez-Ovejero, C.; González-Sánchez, M.; González, E.; Falcón-Pérez, J.M.; Boufana, B.; Fratini, F.; Casulli, A.; Manzano-Román, R. Isolation and Characterization of Exosomes Derived from Fertile Sheep Hydatid Cysts. Vet. Parasitol. 2017, 236, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Ancarola, M.E.; Marcilla, A.; Herz, M.; Macchiaroli, N.; Pérez, M.; Asurmendi, S.; Brehm, K.; Poncini, C.; Rosenzvit, M.; Cucher, M. Cestode Parasites Release Extracellular Vesicles with MicroRNAs and Immunodiagnostic Proteins Cargo. Int. J. Parasitol. 2017, 47, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-Q.; Liu, T.-L.; Liang, P.-H.; Zhang, S.-H.; Li, T.-S.; Li, Y.-P.; Liu, G.-X.; Mao, L.; Luo, X.-N. Characterization of Exosome-like Vesicles Derived from Taenia pisiformis Cysticercus and Their Immunoregulatory Role on Macrophages. Parasit. Vectors 2020, 13, 318. [Google Scholar] [CrossRef] [PubMed]
- Kifle, D.W.; Pearson, M.S.; Becker, L.; Pickering, D.; Loukas, A.; Sotillo, J. Proteomic Analysis of Two Populations of Schistosoma mansoni-Derived Extracellular Vesicles: 15k Pellet and 120k Pellet Vesicles. Mol. Biochem. Parasitol. 2020, 236, 111264. [Google Scholar] [CrossRef]
- Meningher, T.; Barsheshet, Y.; Ofir-Birin, Y.; Gold, D.; Brant, B.; Dekel, E.; Sidi, Y.; Schwartz, E.; Regev-Rudzki, N.; Avni, O.; et al. Schistosomal Extracellular Vesicle-enclosed MiRNAs Modulate Host T Helper Cell Differentiation. EMBO Rep. 2020, 21, e47882. [Google Scholar] [CrossRef]
- Samoil, V.; Dagenais, M.; Ganapathy, V.; Aldridge, J.; Glebov, A.; Jardim, A.; Ribeiro, P. Vesicle-Based Secretion in Schistosomes: Analysis of Protein and MicroRNA (MiRNA) Content of Exosome-like Vesicles Derived from Schistosoma mansoni. Sci. Rep. 2018, 8, 3286. [Google Scholar] [CrossRef]
- Nowacki, F.C.; Swain, M.T.; Klychnikov, O.I.; Niazi, U.; Ivens, A.; Quintana, J.F.; Hensbergen, P.J.; Hokke, C.H.; Buck, A.H.; Hoffmann, K.F. Protein and Small Non-Coding RNA-Enriched Extracellular Vesicles Are Released by the Pathogenic Blood Fluke Schistosoma mansoni. J. Extracell. Vesicles 2015, 4, 28665. [Google Scholar] [CrossRef]
- Sánchez-López, C.M.; González-Arce, A.; Soler, C.; Ramírez-Toledo, V.; Trelis, M.; Bernal, D.; Marcilla, A. Extracellular Vesicles from the Trematodes Fasciola hepatica and Dicrocoelium dendriticum Trigger Different Responses in Human THP-1 Macrophages. J. Extracell. Vesicles 2023, 12, e12317. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.; Cwiklinski, K.; Lalor, R.; O’connell, B.; Robinson, M.W.; Gerlach, J.; Joshi, L.; Kilcoyne, M.; Dalton, J.P.; O’neill, S.M. Fasciola Hepatica Extracellular Vesicles Isolated from Excretory-Secretory Products Using a Gravity Flow Method Modulate Dendritic Cell Phenotype and Activity. PLoS Negl. Trop. Dis. 2020, 14, e0008626. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.N.; Phillips, H.; Tomes, J.J.; Swain, M.T.; Wilkinson, T.J.; Brophy, P.M.; Morphew, R.M. The Importance of Extracellular Vesicle Purification for Downstream Analysis: A Comparison of Differential Centrifugation and Size Exclusion Chromatography for Helminth Pathogens. PLoS Negl. Trop. Dis. 2019, 13, e0007191. [Google Scholar] [CrossRef] [PubMed]
- Tritten, L.; Tam, M.; Vargas, M.; Jardim, A.; Stevenson, M.M.; Keiser, J.; Geary, T.G. Excretory/Secretory Products from the Gastrointestinal Nematode Trichuris muris. Exp. Parasitol. 2017, 178, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Eichenberger, R.M.; Talukder, M.H.; Field, M.A.; Wangchuk, P.; Giacomin, P.; Loukas, A.; Sotillo, J. Characterization of Trichuris muris Secreted Proteins and Extracellular Vesicles Provides New Insights into Host–Parasite Communication. J. Extracell. Vesicles 2018, 7, 1428004. [Google Scholar] [CrossRef]
- Shears, R.K.; Bancroft, A.J.; Hughes, G.W.; Grencis, R.K.; Thornton, D.J. Extracellular Vesicles Induce Protective Immunity against Trichuris muris. Parasite Immunol. 2018, 40, e12536. [Google Scholar] [CrossRef] [PubMed]
- Hansen, E.P.; Fromm, B.; Andersen, S.D.; Marcilla, A.; Andersen, K.L.; Borup, A.; Williams, A.R.; Jex, A.R.; Gasser, R.B.; Young, N.D.; et al. Exploration of Extracellular Vesicles from Ascaris Suum Provides Evidence of Parasite–Host Cross Talk. J. Extracell. Vesicles 2019, 8, 1578116. [Google Scholar] [CrossRef]
- Behnke, J.M.; Menge, D.M.; Noyes, H. Heligmosomoides Bakeri: A Model for Exploring the Biology and Genetics of Resistance to Chronic Gastrointestinal Nematode Infections. Parasitology 2009, 136, 1565–1580. [Google Scholar] [CrossRef]
- Buck, A.H.; Coakley, G.; Simbari, F.; McSorley, H.J.; Quintana, J.F.; Le Bihan, T.; Kumar, S.; Abreu-Goodger, C.; Lear, M.; Harcus, Y.; et al. Exosomes Secreted by Nematode Parasites Transfer Small RNAs to Mammalian Cells and Modulate Innate Immunity. Nat. Commun. 2014, 5, 5488. [Google Scholar] [CrossRef]
- Lim, H.J.; Yoon, H.; Kim, H.; Kang, Y.W.; Kim, J.E.; Kim, O.Y.; Lee, E.Y.; Twizere, J.C.; Rak, J.; Kim, D.K. Extracellular Vesicle Proteomes Shed Light on the Evolutionary, Interactive, and Functional Divergence of Their Biogenesis Mechanisms. Front. Cell Dev. Biol. 2021, 9, 734950. [Google Scholar] [CrossRef]
- Davis, C.N.; Winters, A.; Milic, I.; Devitt, A.; Cookson, A.; Brophy, P.M.; Morphew, R.M. Evidence of Sequestration of Triclabendazole and Associated Metabolites by Extracellular Vesicles of Fasciola hepatica. Sci. Rep. 2020, 10, 13445. [Google Scholar] [CrossRef] [PubMed]
- Chaiyadet, S.; Krueajampa, W.; Hipkaeo, W.; Plosan, Y.; Piratae, S.; Sotillo, J.; Smout, M.; Sripa, B.; Brindley, P.J.; Loukas, A.; et al. Suppression of MRNAs Encoding CD63 Family Tetraspanins from the Carcinogenic Liver Fluke Opisthorchis viverrini Results in Distinct Tegument Phenotypes. Sci. Rep. 2017, 7, 14342. [Google Scholar] [CrossRef]
- Pirovich, D.B.; Da’dara, A.A.; Skelly, P.J. Schistosoma mansoni Glyceraldehyde-3-Phosphate Dehydrogenase Enhances Formation of the Blood-Clot Lysis Protein Plasmin. Biol. Open 2020, 9, bio050385. [Google Scholar] [CrossRef]
- Mani, M.; Chen, C.; Amblee, V.; Liu, H.; Mathur, T.; Zwicke, G.; Zabad, S.; Patel, B.; Thakkar, J.; Jeffery, C.J. MoonProt: A Database for Proteins That Are Known to Moonlight. Nucleic Acids Res. 2015, 43, D277–D282. [Google Scholar] [CrossRef]
- Henderson, B.; Martin, A. Bacterial Virulence in the Moonlight: Multitasking Bacterial Moonlighting Proteins Are Virulence Determinants in Infectious Disease. Infect. Immun. 2011, 79, 3476–3491. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Arreaza, A.; Acosta, H.; Quiñones, W.; Concepción, J.L.; Michels, P.A.M.; Avilán, L. Extracellular Functions of Glycolytic Enzymes of Parasites: Unpredicted Use of Ancient Proteins. Mol. Biochem. Parasitol. 2014, 193, 75–81. [Google Scholar] [CrossRef]
- El-Dabaa, E.; Mei, H.; El-Sayed, A.; Karim, A.M.; Eldesoky, H.M.; Fahim, F.A.; LoVerde, P.T.; Saber, M.A. Cloning and Characterization of Schistosoma mansoni Fructose-1,6- Bisphosphate Aldolase Isoenzyme. J. Parasitol. 1998, 84, 954–960. [Google Scholar] [CrossRef]
- Ravida, A.; Cwiklinski, K.; Aldridge, A.M.; Clarke, P.; Thompson, R.; Gerlach, J.Q.; Kilcoyne, M.; Hokke, C.H.; Dalton, J.P.; O’Neill, S.M. Fasciola hepatica Surface Tegument: Glycoproteins at the Interface of Parasite and Host. Mol. Cell Proteom. 2016, 15, 3139. [Google Scholar] [CrossRef]
- Morales, A.; Espino, A.M. Evaluation and Characterization of Fasciola hepatica Tegument Protein Extract for Serodiagnosis of Human Fascioliasis. Clin. Vaccine Immunol. 2012, 19, 1870. [Google Scholar] [CrossRef]
- Li, S.; Bian, M.; Wang, X.; Chen, X.; Xie, Z.; Sun, H.; Jia, F.; Liang, P.; Zhou, C.; He, L.; et al. Molecular and Biochemical Characterizations of Three Fructose-1,6-Bisphosphate Aldolases from Clonorchis sinensis. Mol. Biochem. Parasitol. 2014, 194, 36–43. [Google Scholar] [CrossRef]
- Lorenzatto, K.R.; Monteiro, K.M.; Paredes, R.; Paludo, G.P.; Da Fonsêca, M.M.; Galanti, N.; Zaha, A.; Ferreira, H.B. Fructose-Bisphosphate Aldolase and Enolase from Echinococcus granulosus: Genes, Expression Patterns and Protein Interactions of Two Potential Moonlighting Proteins. Gene 2012, 506, 76–84. [Google Scholar] [CrossRef]
- McCarthy, J.S.; Wieseman, M.; Tropea, J.; Kaslow, D.; Abraham, D.; Lustigman, S.; Tuan, R.; Guderian, R.H.; Nutman, T.B. Onchocerca volvulus Glycolytic Enzyme Fructose-1,6-Bisphosphate Aldolase as a Target for a Protective Immune Response in Humans. Infect. Immun. 2002, 70, 851. [Google Scholar] [CrossRef]
- González-Miguel, J.; Morchón, R.; Carretón, E.; Montoya-Alonso, J.A.; Simón, F. Surface Associated Antigens of Dirofilaria Immitis Adult Worms Activate the Host Fibrinolytic System. Vet. Parasitol. 2013, 196, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Bai, X.; Li, C.; Tong, M.; Zhang, P.; Cai, W.; Liu, X.; Liu, M. Molecular Characterization of Fructose-1,6-Bisphosphate Aldolase from Trichinella spiralis and Its Potential in Inducing Immune Protection. Front. Cell Infect. Microbiol. 2019, 9, 454349. [Google Scholar] [CrossRef] [PubMed]
- Jolodar, A.; Fischer, P.; Bergmann, S.; Büttner, D.W.; Hammerschmidt, S.; Brattig, N.W. Molecular Cloning of an α-Enolase from the Human Filarial Parasite Onchocerca volvulus That Binds Human Plasminogen. Biochim. Biophys. Acta (BBA)—Gene Struct. Expr. 2003; 1627, 111–120. [Google Scholar] [CrossRef]
- Ayón-Núñez, D.A.; Fragoso, G.; Bobes, R.J.; Laclette, J.P. Plasminogen-Binding Proteins as an Evasion Mechanism of the Host’s Innate Immunity in Infectious Diseases. Biosci. Rep. 2018, 38, 20180705. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.N.; Gallo, M.B.C.; Silva, E.S.; Figueiredo, C.A.V.; Cooper, P.J.; Barreto, M.L.; Loureiro, S.; Pontes-de-Carvalho, L.C.; Alcantara-Neves, N.M. A Proteomic Approach to Identify Proteins from Trichuris trichiura Extract with Immunomodulatory Effects. Parasite Immunol. 2013, 35, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Pancholi, V. Multifunctional α-Enolase: Its Role in Diseases. Cell. Mol. Life Sci. 2001, 58, 902–920. [Google Scholar] [CrossRef]
- Sandini, S.; Melchionna, R.; Arancia, S.; Gomez, M.J.; Valle, R. La Generation of a Highly Immunogenic Recombinant Enolase of the Human Opportunistic Pathogen Candida albicans. Biotechnol. Appl. Biochem. 1999, 29, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, R.M.; Graven, K.K.; Tucci, M.; McDonald, R.J.; Farber, H.W. Non-Neuronal Enolase Is an Endothelial Hypoxic Stress Protein. J. Biol. Chem. 1995, 270, 27752–27757. [Google Scholar] [CrossRef]
- Singh, N.; Bhalla, N. Moonlighting Proteins. Annu. Rev. Genet. 2020, 54, 265–285. [Google Scholar] [CrossRef]
- Nakagawa, T.; Hirano, Y.; Inomata, A.; Yokota, S.; Miyachi, K.; Kaneda, M.; Umeda, M.; Furukawa, K.; Omata, S.; Horigome, T. Participation of a Fusogenic Protein, Glyceraldehyde-3-Phosphate Dehydrogenase, in Nuclear Membrane Assembly. J. Biol. Chem. 2003, 278, 20395–20404. [Google Scholar] [CrossRef] [PubMed]
- Glaser, P.E.; Gross, R.W. Rapid Plasmenylethanolamine-Selective Fusion of Membrane Bilayers Catalyzed by an Isoform of Glyceraldehyde-3-Phosphate Dehydrogenase: Discrimination between Glycolytic and Fusogenic Roles of Individual Isoforms. Biochemistry 1995, 34, 12193–12203. [Google Scholar] [CrossRef] [PubMed]
- Figuera, L.; Gómez-Arreaza, A.; Avilán, L. Parasitism in Optima Forma: Exploiting the Host Fibrinolytic System for Invasion. Acta Trop. 2013, 128, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Murugavel, S.; Devi, I.K.; Vedamurthy, G.V.; Gupta, S.C.; Singh, B.P.; Joshi, P. Glyceraldehyde-3-Phosphate Dehydrogenase of the Parasitic Nematode Haemonchus contortus Binds to Complement C3 and Inhibits Its Activity. Parasite Immunol. 2013, 35, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Franchini, G.R.; Pórfido, J.L.; Ibáñez Shimabukuro, M.; Rey Burusco, M.F.; Bélgamo, J.A.; Smith, B.O.; Kennedy, M.W.; Córsico, B. The Unusual Lipid Binding Proteins of Parasitic Helminths and Their Potential Roles in Parasitism and as Therapeutic Targets. Prostaglandins Leukot. Essent. Fat. Acids 2015, 93, 31–36. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA Delivery by Extracellular Vesicles in Mammalian Cells and Its Applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef]
- Fabbiano, F.; Corsi, J.; Gurrieri, E.; Trevisan, C.; Notarangelo, M.; D’Agostino, V.G. RNA Packaging into Extracellular Vesicles: An Orchestra of RNA-Binding Proteins? J. Extracell. Vesicles 2020, 10, e12043. [Google Scholar] [CrossRef]
- Cucher, M.A.; Ancarola, M.E.M.; Kamenetzky, L. The Challenging World of Extracellular RNAs of Helminth Parasites. Mol. Immunol. 2021, 134, 150–160. [Google Scholar] [CrossRef]
- Zhang, X.; Gong, W.; Cao, S.; Yin, J.; Zhang, J.; Cao, J.; Shen, Y. Comprehensive Analysis of Non-Coding RNA Profiles of Exosome-like Vesicles From the Protoscoleces and Hydatid Cyst Fluid of Echinococcus granulosus. Front. Cell Infect. Microbiol. 2020, 10, 316. [Google Scholar] [CrossRef]
- Minkler, S.J.; Loghry-Jansen, H.J.; Sondjaja, N.A.; Kimber, M.J. Expression and Secretion of Circular RNAs in the Parasitic Nematode, Ascaris suum. Front. Genet. 2022, 13, 884052. [Google Scholar] [CrossRef]
- Chow, F.W.N.; Koutsovoulos, G.; Ovando-Vázquez, C.; Neophytou, K.; Bermúdez-Barrientos, J.R.; Laetsch, D.R.; Robertson, E.; Kumar, S.; Claycomb, J.M.; Blaxter, M.; et al. Secretion of an Argonaute Protein by a Parasitic Nematode and the Evolution of Its SiRNA Guides. Nucleic Acids Res. 2019, 47, 3594–3606. [Google Scholar] [CrossRef]
- Castello, A.; Hentze, M.W.; Preiss, T. Metabolic Enzymes Enjoying New Partnerships as RNA-Binding Proteins. Trends Endocrinol. Metab. 2015, 26, 746–757. [Google Scholar] [CrossRef]
- Chang, C.H.; Curtis, J.D.; Maggi, L.B.; Faubert, B.; Villarino, A.V.; O’Sullivan, D.; Huang, S.C.C.; Van Der Windt, G.J.W.; Blagih, J.; Qiu, J.; et al. XPosttranscriptional Control of T Cell Effector Function by Aerobic Glycolysis. Cell 2013, 153, 1239. [Google Scholar] [CrossRef] [PubMed]
- Bouchery, T.; Kyle, R.; Ronchese, F.; Le Gros, G. The Differentiation of CD4+ T-Helper Cell Subsets in the Context of Helminth Parasite Infection. Front. Immunol. 2014, 5, 112572. [Google Scholar] [CrossRef] [PubMed]
- Wegener, M.; Dietz, K.J. The Mutual Interaction of Glycolytic Enzymes and RNA in Post-Transcriptional Regulation. RNA 2022, 28, 4446–4468. [Google Scholar] [CrossRef] [PubMed]
- Albihlal, W.S.; Gerber, A.P. Unconventional RNA-Binding Proteins: An Uncharted Zone in RNA Biology. FEBS Lett. 2018, 592, 2917–2931. [Google Scholar] [CrossRef]
- Drurey, C.; Coakley, G.; Maizels, R.M. Extracellular Vesicles: New Targets for Vaccines against Helminth Parasites. Int. J. Parasitol. 2020, 50, 623–633. [Google Scholar] [CrossRef]
- Steisslinger, V.; Korten, S.; Brattig, N.W.; Erttmann, K.D. DNA Vaccine Encoding the Moonlighting Protein Onchocerca Volvulus Glyceraldehyde-3-Phosphate Dehydrogenase (Ov-GAPDH) Leads to Partial Protection in a Mouse Model of Human Filariasis. Vaccine 2015, 33, 5861–5867. [Google Scholar] [CrossRef][Green Version]
- Molehin, A.J.; Sennoune, S.R.; Zhang, W.; Rojo, J.U.; Siddiqui, A.J.; Herrera, K.A.; Johnson, L.; Sudduth, J.; May, J.; Siddiqui, A.A. Cross-Species Prophylactic Efficacy of Sm-P80-Based Vaccine and Intracellular Localization of Sm-P80/Sm-P80 Ortholog Proteins during Development in Schistosoma mansoni, Schistosoma japonicum, and Schistosoma haematobium. Parasitol. Res. 2017, 116, 3175–3188. [Google Scholar] [CrossRef]
- Marques, H.H.; Zouain, C.S.; Torres, C.B.B.; Oliveira, J.S.; Alves, J.B.; Goes, A.M. Protective Effect and Granuloma Down-Modulation Promoted by RP44 Antigen a Fructose 1,6 Bisphosphate Aldolase of Schistosoma mansoni. Immunobiology 2008, 213, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Mahana, N.; Abd-Allah, H.A.S.; Salah, M.; Tallima, H.; El Ridi, R. Fasciola Gigantica Enolase Is a Major Component of Worm Tegumental Fraction Protective against Sheep Fasciolosis. Acta Trop. 2016, 158, 189–196. [Google Scholar] [CrossRef]
- Kalyanasundaram, A.; Jawahar, S.; Ilangopathy, M.; Palavesam, A.; Raman, M. Comparative Immunoprophylactic Efficacy of Haemonchus contortus Recombinant Enolase (RHcENO) and Con A Purified Native Glycoproteins in Sheep. Exp. Parasitol. 2015, 154, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Yuan, Z.G.; Xu, M.J.; Zhou, D.H.; Zhang, X.X.; Zhang, Y.Z.; Wang, X.W.; Yan, C.; Lin, R.Q.; Zhu, X.Q. Ascaris suum Enolase Is a Potential Vaccine Candidate against Ascariasis. Vaccine 2012, 30, 3478–3482. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Phillips, T.; Charest, H.; Podesta, R.B.; Quinlin, M.L.; Pinkston, J.R.; Lloyd, J.D.; Paz, M.; Villalovos, R.M.; Pompa, J. Induction of Protective Immunity against Schistosoma mansoni via DNA Priming and Boosting with the Large Subunit of Calpain (Sm-P80): Adjuvant Effects of Granulocyte-Macrophage Colony-Stimulating Factor and Interleukin-4. Infect. Immun. 2003, 71, 3844–3851. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.A.; Phillips, T.; Charest, H.; Podesta, R.B.; Quinlin, M.L.; Pinkston, J.R.; Lloyd, J.D.; Pompa, J.; Villalovos, R.M.; Paz, M. Enhancement of Sm-P80 (Large Subunit of Calpain) Induced Protective Immunity against Schistosoma mansoni through Co-Delivery of Interleukin-2 and Interleukin-12 in a DNA Vaccine Formulation. Vaccine 2003, 21, 2882–2889. [Google Scholar] [CrossRef]
- Leow, C.Y.; Willis, C.; Chuah, C.; Leow, C.H.; Jones, M. Immunogenicity, Antibody Responses and Vaccine Efficacy of Recombinant Annexin B30 against Schistosoma mansoni. Parasite Immunol. 2020, 42, e12693. [Google Scholar] [CrossRef]
- Newlands, G.F.J.; Skuce, P.J.; Knox, D.P.; Smith, S.K.; Smith, W.D. Cloning and Characterization of a β-Galactoside-Binding Protein (Galectin) from the Gut of the Gastrointestinal Nematode Parasite Haemonchus contortus. Parasitology 1999, 119, 483–490. [Google Scholar] [CrossRef]
- White, R.; Sotillo, J.; Ancarola, M.E.; Borup, A.; Boysen, A.T.; Brindley, P.J.; Buzás, E.I.; Cavallero, S.; Chaiyadet, S.; Chalmers, I.W.; et al. Special Considerations for Studies of Extracellular Vesicles from Parasitic Helminths: A Community-Led Roadmap to Increase Rigour and Reproducibility. J. Extracell. Vesicles 2023, 12, 12298. [Google Scholar] [CrossRef]

| Proteins Secreted in EV from | Ortholog Group | Protein Description | Orthologues | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hsa/ Mmu | Sme | Cae | Egr | Emu | Tas | Tsa | Sma | Fhe | Ovi | Gsa | Asu | Bma | Dim | Hco | Hpo | Nbr | Tci | Tmu | Tsu | |||
| Cestoda + Trematoda + Nematoda | OG1.5_000110 | Actin | ||||||||||||||||||||
| OG1.5_000150 | Heat shock protein 70 | |||||||||||||||||||||
| OG1.5_000216 | Heat shock protein 90/Endoplasmin | |||||||||||||||||||||
| OG1.5_000805 | Enolase | |||||||||||||||||||||
| OG1.5_001292 | Fructose-bisphosphate aldolase | |||||||||||||||||||||
| Cestoda + Trematoda | OG1.5_000551 | Otoferlin/Myoferlin | ||||||||||||||||||||
| OG1.5_000592 | Family C2 unassigned peptidase (C02 family)/Calpain | |||||||||||||||||||||
| OG1.5_000646 | Na+/K+-transporting ATPase subunit alpha | |||||||||||||||||||||
| OG1.5_000731 | L-lactate dehydrogenase | |||||||||||||||||||||
| OG1.5_000863 | Major vault protein | |||||||||||||||||||||
| OG1.5_001170 | Ectonucleotide pyrophosphatase/phosphodiesterase | |||||||||||||||||||||
| OG1.5_001443 | Plastin 3/Fimbrin | |||||||||||||||||||||
| OG1.5_001898 | Cathepsin B-like peptidase | |||||||||||||||||||||
| OG1.5_002072 | Receptor Mediated Endocytosis family member | |||||||||||||||||||||
| OG1.5_002703 | Annexin | |||||||||||||||||||||
| OG1.5_002906 | Ras protein Rab 27A | |||||||||||||||||||||
| OG1.5_002908 | Rab GDP dissociation inhibitor | |||||||||||||||||||||
| OG1.5_003543 | Rab | |||||||||||||||||||||
| OG1.5_003615 | BRO1 domain containing protein BROX | |||||||||||||||||||||
| OG1.5_003620 | Programmed cell death protein | |||||||||||||||||||||
| OG1.5_004233 | Nascent polypeptide associated complex subunit | |||||||||||||||||||||
| OG1.5_004775 | Ras gtpase | |||||||||||||||||||||
| OG1.5_004819 | Ras protein Rap 1b | |||||||||||||||||||||
| OG1.5_006108 | Syndecan-binding protein syntenin | |||||||||||||||||||||
| OG1.5_006367 | Charged multivesicular body protein 2a | |||||||||||||||||||||
| OG1.5_006593 | Charged multivesicular body protein 5 | |||||||||||||||||||||
| OG1.5_008557 | Alpha tocopherol transfer protein | |||||||||||||||||||||
| OG1.5_010119 | Hypothetical or predicted protein | |||||||||||||||||||||
| OG1.5_017912 | Annexin | |||||||||||||||||||||
| OG1.5_023852 | Tegumental protein | |||||||||||||||||||||
| Cestoda | OG1.5_000444 | Calcium-transporting ATPase | ||||||||||||||||||||
| OG1.5_000651 | Long chain fatty acid coenzyme A ligase 1/5 | |||||||||||||||||||||
| OG1.5_000774 | Solute carrier family 5/Na+:glucose cotransporter 2/Na+:myo inositol cotransporter | |||||||||||||||||||||
| OG1.5_000781 | V-type proton ATPase subunit a | |||||||||||||||||||||
| OG1.5_002430 | Syntaxin 1a | |||||||||||||||||||||
| OG1.5_003065 | Ras gtpase | |||||||||||||||||||||
| OG1.5_005812 | Ras protein rab 8b | |||||||||||||||||||||
| OG1.5_026801 | Major egg antigen p40 Putative hsp20 | |||||||||||||||||||||
| OG1.5_030608 | Endophilin B1 | |||||||||||||||||||||
| OG1.5_035926 | Annexin | |||||||||||||||||||||
| OG1.5_036009 | Transforming protein RhoA | |||||||||||||||||||||
| OG1.5_036631 | Expressed conserved protein | |||||||||||||||||||||
| OG1.5_060851 | Ras protein RABF2b | |||||||||||||||||||||
| Trematoda | OG1.5_000586 | Vacuolar ATP synthase subunit b | ||||||||||||||||||||
| OG1.5_004511 | Glutathione S transferase | |||||||||||||||||||||
| OG1.5_005173 | Proteasome subunit alpha type | |||||||||||||||||||||
| OG1.5_005613 | Leucyl aminopeptidase | |||||||||||||||||||||
| OG1.5_014186 | Putative lysosome-associated membrane glycoprotein | |||||||||||||||||||||
| OG1.5_015286 | Putative placenta-specific protein 8 protein (C15 protein) (Onzin) | |||||||||||||||||||||
| OG1.5_030814 | Hypothetical or predicted protein | |||||||||||||||||||||
| Nematoda | OG1.5_002305 | Small heat shock protein OV25 | ||||||||||||||||||||
| OG1.5_004286 | Peptidyl prolyl cis trans isomerase CWC27/Aldo keto reductase family 1 member B4 | |||||||||||||||||||||
| OG1.5_004557 | Vitellogenin | |||||||||||||||||||||
| OG1.5_005795 | Intestinal acid phosphatase | |||||||||||||||||||||
| OG1.5_008101 | Titin | |||||||||||||||||||||
| OG1.5_021155 | Apolipophorin | |||||||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ancarola, M.E.; Maldonado, L.L.; García, L.C.A.; Franchini, G.R.; Mourglia-Ettlin, G.; Kamenetzky, L.; Cucher, M.A. A Comparative Analysis of the Protein Cargo of Extracellular Vesicles from Helminth Parasites. Life 2023, 13, 2286. https://doi.org/10.3390/life13122286
Ancarola ME, Maldonado LL, García LCA, Franchini GR, Mourglia-Ettlin G, Kamenetzky L, Cucher MA. A Comparative Analysis of the Protein Cargo of Extracellular Vesicles from Helminth Parasites. Life. 2023; 13(12):2286. https://doi.org/10.3390/life13122286
Chicago/Turabian StyleAncarola, María Eugenia, Lucas L. Maldonado, Lucía C. A. García, Gisela R. Franchini, Gustavo Mourglia-Ettlin, Laura Kamenetzky, and Marcela A. Cucher. 2023. "A Comparative Analysis of the Protein Cargo of Extracellular Vesicles from Helminth Parasites" Life 13, no. 12: 2286. https://doi.org/10.3390/life13122286
APA StyleAncarola, M. E., Maldonado, L. L., García, L. C. A., Franchini, G. R., Mourglia-Ettlin, G., Kamenetzky, L., & Cucher, M. A. (2023). A Comparative Analysis of the Protein Cargo of Extracellular Vesicles from Helminth Parasites. Life, 13(12), 2286. https://doi.org/10.3390/life13122286







