Real-World Data on Second-Line Therapy with Ramucirumab for Metastatic Gastric Cancer: A Two-Center Study on Romanian Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Treatment
2.4. Outcomes
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crew, K.D.; Neugut, A.I. Epidemiology of gastric cancer. World J. Gastroenterol. 2006, 12, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Valean, S.; Chira, R.; Dumitrascu, D. Epidemiological trends in digestive cancers in Romania, 1955–2012, compared to alcohol consumption. Correlation or coincidence? Med. Pharm. Rep. 2018, 91, 376–386. [Google Scholar] [CrossRef]
- World Health Organisation. Global Status Rapport on Alcohol and Health, 2014th ed; World Health Organisation: Geneva, Switzerland, 2014. Available online: http://www.who.int/substance_abuse/publications/global_alcohol_report/en/ (accessed on 15 October 2023).
- Corojan, A.L.; Dumitrașcu, D.; Ciobanca, P.; Leucuta, D. Prevalence of Helicobacter pylori infection among dyspeptic patients in Northwestern Romania: A decreasing epidemiological trend in the last 30 years. Exp. Ther. Med. 2020, 20, 3488–3492. [Google Scholar] [CrossRef] [PubMed]
- Turesky, R.J.; Yun, B.H.; Brennan, P.; Mates, D.; Jinga, V.; Harnden, P.; Banks, R.E.; Blanche, H.; Bihoreau, M.-T.; Chopard, P.; et al. Aristolochic acid exposure in Romania and implications for renal cell carcinoma. Br. J. Cancer 2016, 114, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Bara, T., Jr.; Gurzu, S.; Sugimura, H.; Bara, T.; Beleaua, M.A.; Jung, I. A systematic review of the possible carcinogenic role of the aristolochic acid. Rom. J. Morphol. Embryol. 2017, 58, 41–44. [Google Scholar] [PubMed]
- Machlowska, J.; Baj, J.; Sitarz, M.; Maciejewski, R.; Sitarz, R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int. J. Mol. Sci. 2020, 21, 4012. [Google Scholar] [CrossRef]
- Choi, A.H.; Kim, J.; Chao, J. Perioperative chemotherapy for resectable gastric cancer: MAGIC and beyond. World J. Gastroenterol. 2015, 21, 7343–7348. [Google Scholar] [CrossRef]
- Al-Batran, S.-E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.-G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, C.; Yao, Z.; Cui, M.; Xing, J.; Yang, H.; Zhang, N.; Liu, M.; Xu, K.; Tan, F.; et al. Adjuvant chemotherapy is an additional option for locally advanced gastric cancer after radical gastrectomy with D2 lymphadenectomy: A retrospective control study. BMC Cancer 2021, 21, 974. [Google Scholar] [CrossRef]
- Joshi, S.S.; Badgwell, B.D. Current treatment and recent progress in gastric cancer. CA Cancer J. Clin. 2021, 71, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.D.; Syn, N.L.; Moehler, M.; Grothe, W.; Yong, W.P.; Tai, B.-C.; Ho, J.; Unverzagt, S. Chemotherapy for advanced gastric cancer. Cochrane Database Syst. Rev. 2017, 2017, CD004064. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.-J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Bragagnoli, A.C.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 2021, 3, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Catalano, V.; Graziano, F.; Santini, D.; D'Emidio, S.; Baldelli, A.M.; Rossi, D.; Vincenzi, B.; Giordani, P.; Alessandroni, P.; Testa, E.; et al. Second-line chemotherapy for patients with advanced gastric cancer: Who may benefit? Br. J. Cancer 2008, 99, 1402–1407. [Google Scholar] [CrossRef] [PubMed]
- Kanagavel, D.; Fedyanin, M.; Tryakin, A.; Tjulandin, S. Second-line treatment of metastatic gastric cancer: Current options and future directions. World J. Gastroenterol. 2015, 21, 11621–11635. [Google Scholar] [CrossRef]
- Casak, S.J.; Fashoyin-Aje, I.; Lemery, S.J.; Zhang, L.; Jin, R.; Li, H.; Zhao, L.; Zhao, H.; Zhang, H.; Chen, H.; et al. FDA Approval Summary: Ramucirumab for Gastric Cancer. Clin. Cancer Res. 2015, 21, 3372–3376. [Google Scholar] [CrossRef]
- Reichert, J.M. Antibodies to watch in 2015. mAbs 2015, 7, 3372–3376. [Google Scholar] [CrossRef]
- Wilke, H.; Muro, K.; Van Cutsem, E.; Oh, S.-C.; Bodoky, G.; Shimada, Y.; Hironaka, S.; Sugimoto, N.; Lipatov, O.; Kim, T.-Y.; et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol. 2014, 15, 1224–1235. [Google Scholar] [CrossRef]
- Al-Batran, S.-E.; Van Cutsem, E.; Oh, S.C.; Bodoky, G.; Shimada, Y.; Hironaka, S.; Sugimoto, N.; Lipatov, O.N.; Kim, T.-Y.; Cunningham, D.; et al. Quality-of-life and performance status results from the phase III RAINBOW study of ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated gastric or gastroesophageal junction adenocarcinoma. Ann. Oncol. 2016, 27, 673–679. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Tomasek, J.; Yong, C.J.; Dumitru, F.; Passalacqua, R.; Goswami, C.; Safran, H.; dos Santos, L.V.; Aprile, G.; Ferry, D.R.; et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2013, 383, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Itatani, Y.; Kawada, K.; Yamamoto, T.; Sakai, Y. Resistance to Anti-Angiogenic Therapy in Cancer—Alterations to Anti-VEGF Pathway. Int. J. Mol. Sci. 2018, 19, 1232. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.S.; Tabernero, J.; Tomášek, J.; Chau, I.; Melichar, B.; Safran, H.; Tehfe, M.A.; Filip, D.; Topuzov, E.; Schlittler, L.; et al. Biomarker analyses in REGARD gastric/GEJ carcinoma patients treated with VEGFR2-targeted antibody ramucirumab. Br. J. Cancer 2016, 115, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.H.; Cecchini, M. Targeted Therapies in Advanced Gastric Cancer. Curr. Treat. Options Oncol. 2020, 21, 70. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolomeo, M.; Niger, M.; Tirino, G.; Petrillo, A.; Berenato, R.; Laterza, M.M.; Pietrantonio, F.; Morano, F.; Antista, M.; Lonardi, S.; et al. Ramucirumab as Second-Line Therapy in Metastatic Gastric Cancer: Real-World Data from the RAMoss Study. Target. Oncol. 2018, 13, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Longo, F.; Jorge, M.; Yaya, R.; Montes, A.F.; Lago, N.M.; Brozos, E.; Aparicio, J.; Quintero, G.; Ceballos, E.; Buxó, E.; et al. Real-life use of ramucirumab in gastric cancer in Spain: The RAMIS study. Futur. Oncol. 2021, 17, 1777–1791. [Google Scholar] [CrossRef] [PubMed]
- Paulson, A.S.; Hess, L.M.; Liepa, A.M.; Cui, Z.L.; Aguilar, K.M.; Clark, J.; Schelman, W. Ramucirumab for the treatment of patients with gastric or gastroesophageal junction cancer in community oncology practices. Gastric Cancer 2018, 21, 831–844. [Google Scholar] [CrossRef]
- Jung, M.; Ryu, M.-H.; Oh, D.Y.; Kang, M.; Zang, D.Y.; Hwang, I.G.; Kim, K.H.; Shim, B.Y.; Song, E.K.; Sym, S.J.; et al. Efficacy and tolerability of ramucirumab monotherapy or in combination with paclitaxel in gastric cancer patients from the Expanded Access Program Cohort by the Korean Cancer Study Group (KCSG). Gastric Cancer 2018, 21, 819–830. [Google Scholar] [CrossRef]
- Han, H.S.; Kim, B.J.; Jee, H.-J.; Ryu, M.-H.; Park, S.H.; Rha, S.Y.; Kim, J.G.; Bae, W.K.; Lee, K.-W.; Oh, D.-Y.; et al. Ramucirumab plus paclitaxel as second-line treatment in patients with advanced gastric or gastroesophageal junction adenocarcinoma: A nationwide real-world outcomes in Korea study (KCSG-ST19-16). Ther. Adv. Med Oncol. 2021, 13, 17588359211042812. [Google Scholar] [CrossRef]
- Rawla, P.; Barsouk, A. Epidemiology of gastric cancer: Global trends, risk factors and prevention. Gastroenterol. Rev. 2019, 14, 26–38. [Google Scholar] [CrossRef]
- Marupudi, N.I.; Han, J.E.; Li, K.W.; Renard, V.M.; Tyler, B.M.; Brem, H. Paclitaxel: A review of adverse toxicities and novel delivery strategies. Expert Opin. Drug Saf. 2007, 6, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.-Y.; Huang, J.-Y.; Zhao, Q.-R.; Jiang, N.; Xu, H.-M.; Wang, Z.-N.; Li, H.-Q.; Zhang, S.-B.; Sun, Z. The clinicopathological parameters and prognostic significance of HER2 expression in gastric cancer patients: A meta-analysis of literature. World J. Surg. Oncol. 2017, 15, 68. [Google Scholar] [CrossRef] [PubMed]
- Uprak, T.K.; Attaallah, W.; Celikel, C.A.; Ayranci, G.; Yegen, C. HER-2 incidence in gastric cancer, its association with prognosis and clinicopathological parameters. Turk. J. Surg. 2015, 31, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; Werner, D.; Pauligk, C.; Steinmetz, K.; Kelsen, D.P.; Jäger, E.; Altmannsberger, H.-M.; Robinson, E.; Tafe, L.J.; Tang, L.H.; et al. Prognosis of metastatic gastric and gastroesophageal junction cancer by HER2 status: A European and USA International collaborative analysis. Ann. Oncol. 2012, 23, 2656–2662. [Google Scholar] [CrossRef] [PubMed]
- Yarema, R.; Ohorchak, M.; Hyrya, P.; Kovalchuk, Y.; Safiyan, V.; Karelin, I.; Ferneza, S.; Fetsych, M.; Matusyak, M.; Oliynyk, Y.; et al. Gastric cancer with peritoneal metastases: Efficiency of standard treatment methods. World J. Gastrointest. Oncol. 2020, 12, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, J.-Q.; Liu, J.-L.; Tian, L. Issues on peritoneal metastasis of gastric cancer: An update. World J. Surg. Oncol. 2019, 17, 215. [Google Scholar] [CrossRef]
- Puliga, E.; Corso, S.; Pietrantonio, F.; Giordano, S. Microsatellite instability in Gastric Cancer: Between lights and shadows. Cancer Treat. Rev. 2021, 95, 102175. [Google Scholar] [CrossRef]
- Röcken, C. Predictive biomarkers in gastric cancer. J. Cancer Res. Clin. Oncol. 2023, 149, 467–481. [Google Scholar] [CrossRef]
- Takei, S.; Kawazoe, A.; Shitara, K. The New Era of Immunotherapy in Gastric Cancer. Cancers 2022, 14, 1054. [Google Scholar] [CrossRef]
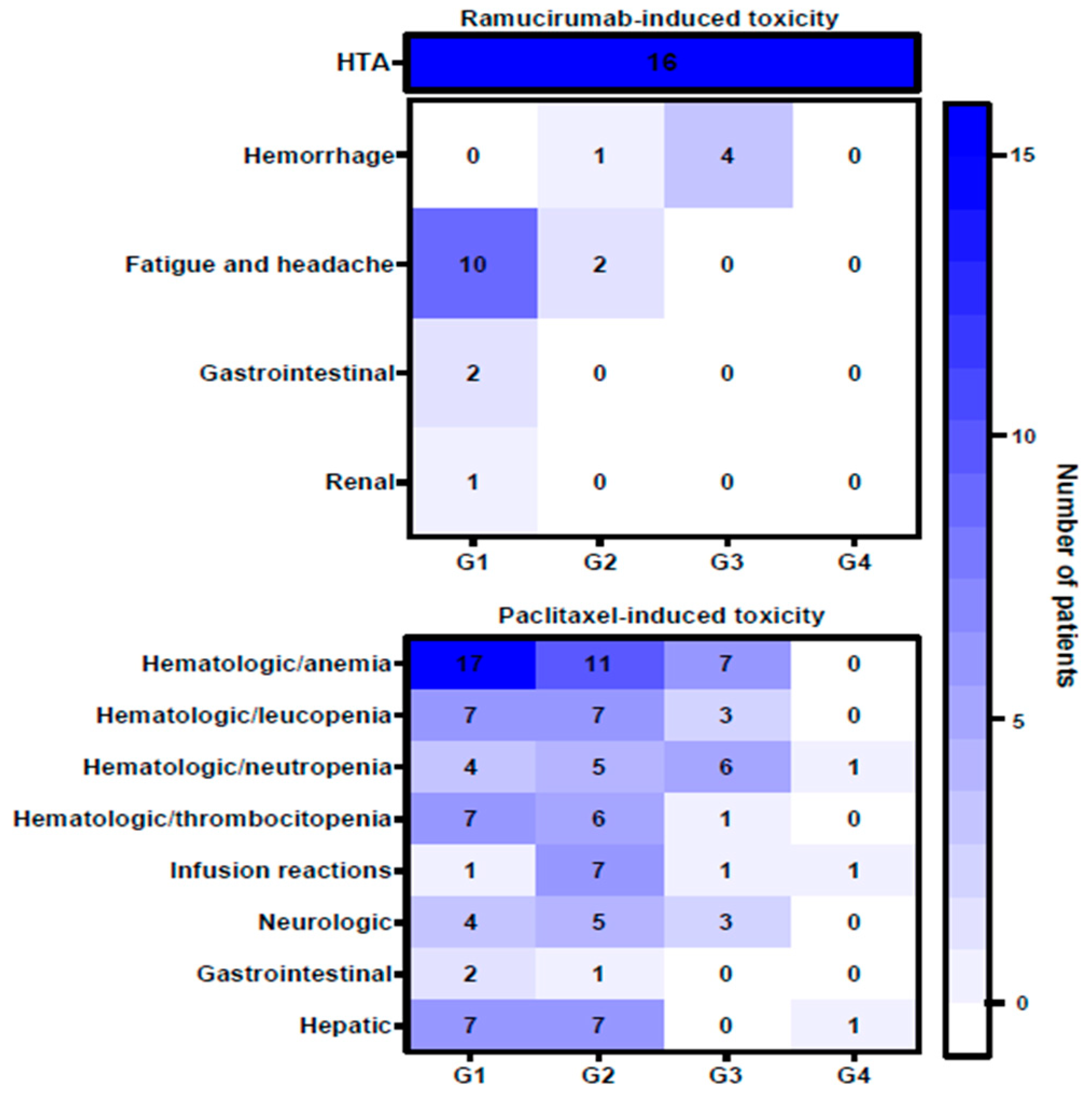







| Variable | Number of Patients (n = 69) |
|---|---|
| Age | |
| Median (range) | 62 (33–84) |
| <50 | 15 (21.7%) |
| ≥50 | 54 (78.3%) |
| Sex | |
| F | 23 (33.3%) |
| M | 46 (66.7%) |
| Tumor localization | |
| Gastric | 65 (94.2%) |
| GEJ | 4 (5.8%) |
| Histology | |
| Adenocarcinoma | 67 (97.2%) |
| Adenocarcinoma with neuroendocrine component | 1 (1.4%) |
| Squamous | 1 (1.4%) |
| Histologic subtype | |
| Mixed | 4 (5.8%) |
| Mucinous | 2 (2.9%) |
| Poorly cohesive | 18 (26.1%) |
| Tubular | 26 (37.7%) |
| NA | 19 (27.5%) |
| HER2 mutation status | |
| HER2− | 40 (58.0%) |
| HER2+ | 10 (14.5%) |
| NA | 19 (27.5%) |
| Neoadjuvant treatment | |
| Yes | 9 (13.0%) |
| No | 60 (87.0%) |
| Primary tumor surgery | |
| Yes | 17 (24.6%) |
| No | 52 (75.4%) |
| Adjuvant treatment | |
| Yes | 13 (18.8%) |
| No | 56 (81.2%) |
| Radiation therapy with curative intent | |
| Yes | 6 (8.7%) |
| No | 63 (91.3%) |
| Site of metastasis | |
| Adnexa | 2 (2.9%) |
| Bone | 4 (5.8%) |
| Liver | 19 (27.5%) |
| Lung | 2 (2.9%) |
| Lymph nodes | 5 (7.3%) |
| Peritoneum | 16 (23.3%) |
| Peritoneum + other | 9 (13.0%) |
| Skin | 1 (1.4%) |
| Multiple sites (≥2, peritoneum not involved) | 11 (15.9%) |
| Number of Patients (n = 13) | |
| Adjuvant treatment | |
| Fluoropyrimidine | 1 (7.7%) |
| Fluoropyrimidine + platinum | 7 (53.8%) |
| Fluoropyrimidine + platinum + anthracycline | 2 (15.4%) |
| Fluoropyrimidine + platinum + taxane | 3 (23.1%) |
| Time to progression on adjuvant treatment | |
| ≤6 months | 4 (30.8%) |
| >6 months | 6 (46.1%) |
| NA | 3 (23.1%) |
| Number of Patients (n = 60) | |
| First-line metastatic treatment | |
| Antimetabolite + platinum | 1 (1.7%) |
| Platinum + taxane | 1 (1.7%) |
| Fluoropyrimidine + platinum | 35 (58.2%) |
| Fluoropyrimidine + platinum + anthracycline | 6 (10.0%) |
| Fluoropyrimidine + platinum + taxane | 6 (10.0%) |
| Fluoropyrimidine + topoisomerase I inhibitor | 1 (1.7%) |
| Targeted therapy * | 10 (16.7%) * |
| * Fluoropyrimidine + platinum + antiHER2 | * 9 (15.0%) |
| * Fluoropyrimidine + platinum + anthracycline + antiHER2 | * 1 (1.7%) |
| Time to progression on first-line treatment | |
| ≤6 months | 40 (66.7%) |
| >6 months | 20 (33.3%) |
| Number of Patients (n = 69) | |
| ECOG PS at debut of R/R + P treatment | |
| <1 | 55 (79.7%) |
| ≥1 | 14 (20.3%) |
| Second-line metastatic R/R + P treatment | |
| R | 18 (26.1%) |
| R + P | 51 (73.9%) |
| Duration of second-line metastatic R/R + P treatment | |
| ≤6 months | 62 (89.9%) |
| >6 months | 7 (10.2%) |
| Reason for R/R + P treatment discontinuation | |
| Best supportive care | 8 (11.6%) |
| Decease | 18 (26.1%) |
| Disease progression | 30 (43.5%) |
| Loss of evidence | 4 (5.8%) |
| Toxicity | 5 (7.2%) |
| During treatment | 4 (5.8%) |
| Variable | Kaplan–Meier Survival Analysis | Univariate Cox Regression Analysis | Multivariate Cox Regression Analysis | |||
|---|---|---|---|---|---|---|
| Median Survival Time (Days) (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| HER2 mutation status (negative vs. positive) | 476 (405.4–546.5) 675 (504.3–845.6) | 0.053 | 2.26 (0.97–5.26) | 0.059 | 4.04 (1.43–11.38) | 0.008 |
| Second-line R/R + P treatment (R vs. R + P) | 547 (311.0–782.9) 529 (446.8–611.1) | 0.708 | 0.89 (0.46–1.67) | 0.709 | 0.82 (0.35–1.92) | 0.654 |
| Surgery (no vs. yes) | 490 (412.7–567.2) 758 (414.8–1101.1) | 0.014 | 2.57 (1.18–5.56) | 0.017 | 2.31 (0.82–6.53) | 0.114 |
| Metastasis (non-peritoneal vs. peritoneal) | 562 (477.5–646.5) 476 (377.1–574.9) | 0.911 | 1.03 (0.57–1.87) | 0.911 | 0.76 (0.37–1.57) | 0.462 |
| ECOG PS (<1 vs. >1) | 584 (505.1–662.9) 394 (140.1–647.8) | 0.084 | 0.57 (0.29–1.08) | 0.088 | 0.53 (0.22–1.24) | 0.140 |
| Number of R/R + P administrations (≤5 vs. >5) | 490 (417.0–562.9) 1263 (272.7–2253.2) | <0.0001 | 3.54 (1.67–7.49) | 0.001 | 3.40 (1.32–8.79) | 0.011 |
| Variable | Kaplan–Meier Survival Analysis | Univariate Cox Regression Analysis | Multivariate Cox Regression Analysis | |||
|---|---|---|---|---|---|---|
| Median Survival Time (Days) (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| HER2 mutation status (negative vs. positive) | 118 (94.0–141.9) 113 (56.8–169.1) | 0.717 | 1.17 (0.50–2.69) | 0.718 | 1.24 (0.51–3.00) | 0.636 |
| Second-line R/R + P treatment (R vs. R + P) | 113 (0–226.4) 141 (107.7–174.2) | 0.433 | 1.30 (0.67–2.52) | 0.437 | 0.59 (0.22–1.61) | 0.303 |
| Surgery (no vs. yes) | 119 (82.9–55.1) 175 (123.5–226.4) | 0.057 | 2.04 (0.96–4.34) | 0.063 | 1.92 (0.56–6.59) | 0.298 |
| Metastasis (non-peritoneal vs. peritoneal) | 119 (72.9–165.1) 146 (102.5–189.4) | 0.535 | 1.20 (0.67–2.17) | 0.538 | 2.28 (1.01–5.17) | 0.049 |
| ECOG PS (<1 vs. >1) | 147 (106.1–187.9) 91 (40.1–141.9) | 0.112 | 0.57 (0.28–1.15) | 0.118 | 0.31 (0.13–0.75) | 0.009 |
| Number of R/R + P administrations (≤5 vs. >5) | 92 (67.7–116.2) 210 (1.8–418.1) | <0.0001 | 8.43 (3.47–20.46) | <0.0001 | 14.65 (3.67–58.56) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galos, D.; Balacescu, L.; Vidra, R.; Sur, D. Real-World Data on Second-Line Therapy with Ramucirumab for Metastatic Gastric Cancer: A Two-Center Study on Romanian Population. Life 2023, 13, 2300. https://doi.org/10.3390/life13122300
Galos D, Balacescu L, Vidra R, Sur D. Real-World Data on Second-Line Therapy with Ramucirumab for Metastatic Gastric Cancer: A Two-Center Study on Romanian Population. Life. 2023; 13(12):2300. https://doi.org/10.3390/life13122300
Chicago/Turabian StyleGalos, Diana, Loredana Balacescu, Radu Vidra, and Daniel Sur. 2023. "Real-World Data on Second-Line Therapy with Ramucirumab for Metastatic Gastric Cancer: A Two-Center Study on Romanian Population" Life 13, no. 12: 2300. https://doi.org/10.3390/life13122300
APA StyleGalos, D., Balacescu, L., Vidra, R., & Sur, D. (2023). Real-World Data on Second-Line Therapy with Ramucirumab for Metastatic Gastric Cancer: A Two-Center Study on Romanian Population. Life, 13(12), 2300. https://doi.org/10.3390/life13122300






