Antioxidant Activity and Anticarcinogenic Effect of Extracts from Bouvardia ternifolia (Cav.) Schltdl.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extract Preparation
2.3. Antioxidant Capacity
2.4. Phytochemical Analysis
2.5. Cytotoxicity Assay
2.6. Statistical Analyses
3. Results
3.1. Antioxidant Capacity
3.2. Total Phenol, Tannin and Saponin Content
3.3. Cytotoxicity of the Extracts Obtained from the B. ternifolia Flowers, Leaves and Stems against MDA and SiHa Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, L.; Lezama-Davila, C.M.; Isaac-Marquez, A.P.; Calomeni, E.P.; Fuchs, J.R.; Satoskar, A.R.; Kinghorn, A.D. Sterols with Antileishmanial Activity Isolated from the Roots of Pentalinon Andrieuxii. Phytochemistry 2012, 82, 128–135. [Google Scholar] [CrossRef]
- Guzmán Maldonado, S.H.; Diaz Huacuz, R.S.; González Chavira, M.M. Plantas Medicinales La Realidad de Una Tradicion Ancestral. 2017. Available online: https://vun.inifap.gob.mx/VUN_MEDIA/BibliotecaWeb/_media/_folletoinformativo/1044_4729_Plantas_medicinales_la_realidad_de_una_tradici%C3%B3n_ancestral.pdf (accessed on 20 November 2023).
- Chamorro-Cevallos, G.; Mojica-Villegas, M.A.; García-Martínez, Y.; Pérez-Gutiérrez, S.; Madrigal-Santillán, E.; Vargas-Mendoza, N.; Morales-González, J.A.; Cristóbal-Luna, J.M. A Complete Review of Mexican Plants with Teratogenic Effects. Plants 2022, 11, 1675. [Google Scholar] [CrossRef]
- Fernandez, M. Familia Rubiaceae. Importancia En La Flora de Cuba. Acta Bot. Cuba 2017, 216, 62–64. [Google Scholar]
- Blackwell, W.H. Revision of Bouvardia (Rubiaceae). Ann. Mo. Bot. Gard. 1968, 55, 1–30. [Google Scholar] [CrossRef]
- García-Morales, G.; Huerta-Reyes, M.; González-Cortazar, M.; Zamilpa, A.; Jiménez-Ferrer, E.; Silva-García, R.; Román-Ramos, R.; Aguilar-Rojas, A. Anti-Inflammatory, Antioxidant and Anti-Acetylcholinesterase Activities of Bouvardia Ternifolia: Potential Implications in Alzheimer’s Disease. Arch. Pharm. Res. 2015, 38, 1369–1379. [Google Scholar] [CrossRef]
- Texas, U. of. Lady Bird Johnson Wildflower Center. Available online: https://www.wildflower.org/plants/result.php?id_plant=BOTE2 (accessed on 20 November 2023).
- Shivanand, D.J.; Hoffmann, J.J.; Torrance, S.J.; Wiedhopf, R.M.; Cole, J.R.; Arora, S.K.; Bates, R.B.; Gargiulo, R.L.; Kriek, G.R. Bouvardin and Deoxybouvardin, Antitumor Cyclic Hexapeptides from Bouvardia Ternifolia (Rubiaceae). J. Am. Chem. Soc. 1977, 99, 8040–8044. [Google Scholar] [CrossRef]
- Herrera-Ruiz, M.; García-Morales, G.; Zamilpa, A.; González-Cortazar, M.; Tortoriello, J.; Ventura-Zapata, E.; Jiménez-Ferrer, E. Inhibition of Acetylcholinesterase Activity by Hidroalcoholic Extract and Their Fractions of Bouvardia Ternifolia (Cav.) Shcltdl (Rubiaceae). Bol. Latinoam. Caribe Plantas Med. Aromat. 2012, 11, 526–541. [Google Scholar]
- Cornejo Garrido, J.; Chamorro Cevallos, G.A.; Garduño Siciliano, L.; Hernández Pando, R.; Jimenez Arellanes, M.A. Acute and Subacute Toxicity (28 Days) of a Mixture of Ursolic Acid and Oleanolic Acid Obtained from Bouvardia Ternifolia in Mice. Bol. Latinoam. Caribe Plantas Med. Aromat. 2012, 11, 91–102. [Google Scholar]
- Perez, G.R.M.; Perez, G.C.; Perez, G.S.; Zavala, S.M.A. Effect of Triterpenoids of Bouvardia Terniflora on Blood Sugar Levels of Normal and Alloxan Diabetic Mice. Phytomedicine 1998, 5, 475–478. [Google Scholar] [CrossRef]
- Zapata Lopera, Y.M.; Jiménez-Ferrer, E.; Herrera-Ruiz, M.; Zamilpa, A.; González-Cortazar, M.; Rosas-Salgado, G.; Santillán-Urquiza, M.A.; Trejo-Tapia, G.; Jiménez-Aparicio, A.R. New Chromones from Bouvardia Ternifolia (Cav.) Schltdl with Anti-Inflammatory and Immunomodulatory Activity. Plants 2023, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Tobey, R.A.; Orlicky, D.J.; Deaven, L.L.; Rail, L.B.; Kissane, R.J. Effects of Bouvardin (NSC 259968), a Cyclic Hexapeptide from Bouvardia Ternifolia, on the Progression Capacity of Cultured Chinese Hamster Cells. Cancer Res. 1978, 38, 4415–4421. [Google Scholar]
- Jiménez-Ferrer, E.; Reynosa-Zapata, I.; Pérez-Torres, Y.; Tortoriello, J. The Secretagogue Effect of the Poison from Centruroides Limpidus Limpidus on the Pancreas of Mice and the Antagonistic Action of the Bouvardia Ternifolia Extract. Phytomedicine 2005, 12, 65–71. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Schenk, G.H.; Brown, D.J. Free Radical Oxidation of Dihydric Phenols with Diphenylpicrylhydrazyl. Talanta 1967, 14, 257–261. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Price, M.L.; Van Scoyoc, S.; Butler, L.G. A Critical Evaluation of the Vanillin Reaction as an Assay for Tannin in Sorghum Grain. J. Agric. Food Chem. 1978, 26, 1214–1218. [Google Scholar] [CrossRef]
- Wrolstad, R.E.; Durst, R.W.; Lee, J. Tracking Color and Pigment Changes in Anthocyanin Products. Trends Food Sci. Technol. 2005, 16, 423–428. [Google Scholar] [CrossRef]
- Elbe, J.H.; Schwartz, S.J.; Hildenbrand, B.E. Loss and Regeneration of Betacyanin Pigments During Processing of Red Beets. J. Food Sci. 1981, 46, 1713–1715. [Google Scholar] [CrossRef]
- Hurtado, N.H.; Pérez, M. Identificación, Estabilidad y Actividad Antioxidante de Las Antocianinas Aisladas de La Cáscara Del Fruto de Capulí (Prunus serotina Spp Capuli (Cav) Mc. Vaug Cav). Inf. Tecnol. 2014, 25, 131–140. [Google Scholar] [CrossRef]
- Giron-Martinez, M.C. Determinacion Semicuantitativa de Saponinas En Muestras Vegetales Aprovechando Su Capacidad Hemolitica, Universidad Nacional Autónoma de México. 1992. Available online: https://ru.dgb.unam.mx/handle/20.500.14330/TES01000183303 (accessed on 20 November 2023).
- Valadez-Vega, C.; Alvarez-Manilla, G.; Riverón-Negrete, L.; García-Carrancá, A.; Morales-González, J.A.; Zuñiga-Pérez, C.; Madrigal-Santillán, E.; Esquivel-Soto, J.; Esquivel-Chirino, C.; Villagómez-Ibarra, R.; et al. Detection of Cytotoxic Activity of Lectin on Human Colon Adenocarcinoma (Sw480) and Epithelial Cervical Carcinoma (C33-A). Molecules 2011, 16, 2107–2118. [Google Scholar] [CrossRef] [PubMed]
- Valadez-Vega, C.; Morales-González, J.A.; Sumaya-Martínez, M.T.; Delgado-Olivares, L.; Cruz-Castañeda, A.; Bautista, M.; Sánchez-Gutiérrez, M.; Zuñiga-Pérez, C. Cytotoxic and Antiproliferative Effect of Tepary Bean Lectins on C33-A, MCF-7, SKNSH, and SW480 Cell Lines. Molecules 2014, 19, 9610–9627. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Gaviria, A.; Correa, C.E.; Mosquera, O.M.; Niño, J.; Correa, Y.M. Evaluación de Las Actividades Antioxidante y Antitopoisomerasa de Extractos de Plantas de La Ecorregión Cafetera Colombiana. Rev. Fac. Cienc. Básicas 2015, 11, 86–101. [Google Scholar] [CrossRef]
- Mosquera, O.M.; Correra, Y.M.; Niño, J. Antioxidant Activity of Plant Extracts from Colombian Flora. Rev. Bras. Farmacogn. 2009, 19, 382–387. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH Assays to Measure Antioxidant Capacity in Popular Antioxidant-Rich US Foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Kumazawa, S.; Hamasaka, T.; Nakayama, T. Antioxidant Activity of Propolis of Various Geographic Origins. Food Chem. 2004, 84, 329–339. [Google Scholar] [CrossRef]
- Ishige, K.; Schubert, D.; Sagara, Y. Flavonoids Protect Neuronal Cells from Oxidative Stress by Three Distinct Mechanisms. Free Radic. Biol. Med. 2001, 30, 433–446. [Google Scholar] [CrossRef]
- Roginsky, V.; Lissi, E.A. Review of Methods to Determine Chain-Breaking Antioxidant Activity in Food. Food Chem. 2005, 92, 235–254. [Google Scholar] [CrossRef]
- Muñoz-Jáuregui, A.; Ramos-Escudero, D.F.; Alvarado-Ortiz Ureta, C.; Castañeda-Castañeda, B. Evaluación de la capacidad antioxidante y contenido de compuestos fenólicos en recursos vegetales promisorios. Revista de La Sociedad Química Del Perú. Sociedad Química del Perú. 2007. Volume 73. Available online: http://www.scielo.org.pe/scielo.php?pid=S1810-634X2007000300003&script=sci_abstract (accessed on 20 November 2023).
- Giraldo Vásquez, L.M.; Ramírez Aristizabal, L.S. Evaluación de La Actividad Antioxidante de Extractos de Palicourea Guianensis (Rubiaceae). Rev. Cuba. Farm. 2013, 47, 483–491. [Google Scholar]
- Dudonné, S.; Vitrac, X.; Coutiére, P.; Woillez, M.; Mérillon, J.M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Koleva, I.I.; Van Beek, T.A.; Linssen, J.P.H.; De Groot, A.; Evstatieva, L.N. Screening of Plant Extracts for Antioxidant Activity: A Comparative Study on Three Testing Methods. Phytochem. Anal. 2002, 13, 8–13. [Google Scholar] [CrossRef]
- Javanmardi, J.; Stushnoff, C.; Locke, E.; Vivanco, J.M. Antioxidant Activity and Total Phenolic Content of Iranian Ocimum Access. Food Chem. 2003, 83, 547–550. [Google Scholar] [CrossRef]
- González-Gómez, J.C.; Ayala-Burgos, A.; Gutiérrez-Vázquez, E. Determinación de Fenoles Totales y Taninos Condensados En Especies Arbóreas Con Potencial Forrajero de La Región de Tierra Caliente Michoacán, México. Livest. Res. Rural Dev. 2006, 18. [Google Scholar]
- Haslam, E. Vegetable Tannins—Lessons of a Phytochemical Lifetime. Phytochemistry 2007, 68, 2713–2721. [Google Scholar] [CrossRef]
- Oda, K.; Matsuda, H.; Murakami, T.; Katayama, S.; Ohgitani, T.; Yoshikawa, M. Relationship between Adjuvant Activity and Amphipathic Structure of Soyasaponins. Vaccine 2003, 21, 2145–2151. [Google Scholar] [CrossRef] [PubMed]
- Lorent, J.H.; Quetin-Leclercq, J.; Mingeot-Leclercq, M.P. The Amphiphilic Nature of Saponins and Their Effects on Artificial and Biological Membranes and Potential Consequences for Red Blood and Cancer Cells. Org. Biomol. Chem. 2014, 12, 8803–8822. [Google Scholar] [CrossRef] [PubMed]
- Ortega, G.M.; Guerra, M. Separación, Caracterización Estructural y Cuantificación de Antocianinas Mediante Métodos Químico-Físicos. ICIDCA. Sobre Deriv. Caña Azúcar 2006, XL, 3–11. [Google Scholar]
- Rahimi, P.; Abedimanesh, S.; Mesbah-Namin, S.A.; Ostadrahimi, A. Betalains, the Nature-Inspired Pigments, in Health and Diseases. Crit. Rev. Food Sci. Nutr. 2019, 58, 2949–2978. [Google Scholar] [CrossRef]
- Rupachandra, S.; Sarada, D.V.L. Induction of Apoptotic Effects of Antiproliferative Protein from the Seeds of Borreria Hispida on Lung Cancer (A549) and Cervical Cancer (HeLa) Cell Lines. Biomed Res. Int. 2014, 2014, 179836. [Google Scholar] [CrossRef]
- Thani, W.; Vallisuta, O.; Siripong, P.; Ruangwises, N. Anti-Proliferative and Antioxidative Activities of Thai Noni/Yor (Morinda Citrifolia Linn.) Leaf Extract. Southeast Asian J. Trop. Med. Public Health 2010, 41, 482–489. [Google Scholar]
- Setzer, M.C.; Moriarity, D.M.; Lawton, R.O.; Setzer, W.N.; Gentry, G.A.; Haber, W.A. Phytomedicinal Potential of Tropical Cloudforest Plants from Monteverde, Costa Rica. Rev. Biol. Trop. 2003, 51, 647–673. [Google Scholar]
- Wu, X.D.; He, J.; Li, X.Y.; Dong, L.B.; Gong, X.; Gao, X.; Song, L.D.; Li, Y.; Peng, L.Y.; Zhao, Q.S. Triterpenoids and Steroids with Cytotoxic Activity from Emmenopterys henryi. Planta Med. 2013, 79, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Akter, R.; Uddin, S.J.; Grice, I.D.; Tiralongo, E. Cytotoxic Activity Screening of Bangladeshi Medicinal Plant Extracts. J. Nat. Med. 2014, 68, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, M.P.; Alate, A.D.; Menon, R.S. Effect of Bouvardin (NSC 259968) on the Growth Characteristics and Nucleic Acids and Protein Syntheses Profiles of P388 Leukemia Cells. Chemotherapy 1981, 27, 126–130. [Google Scholar] [CrossRef] [PubMed]
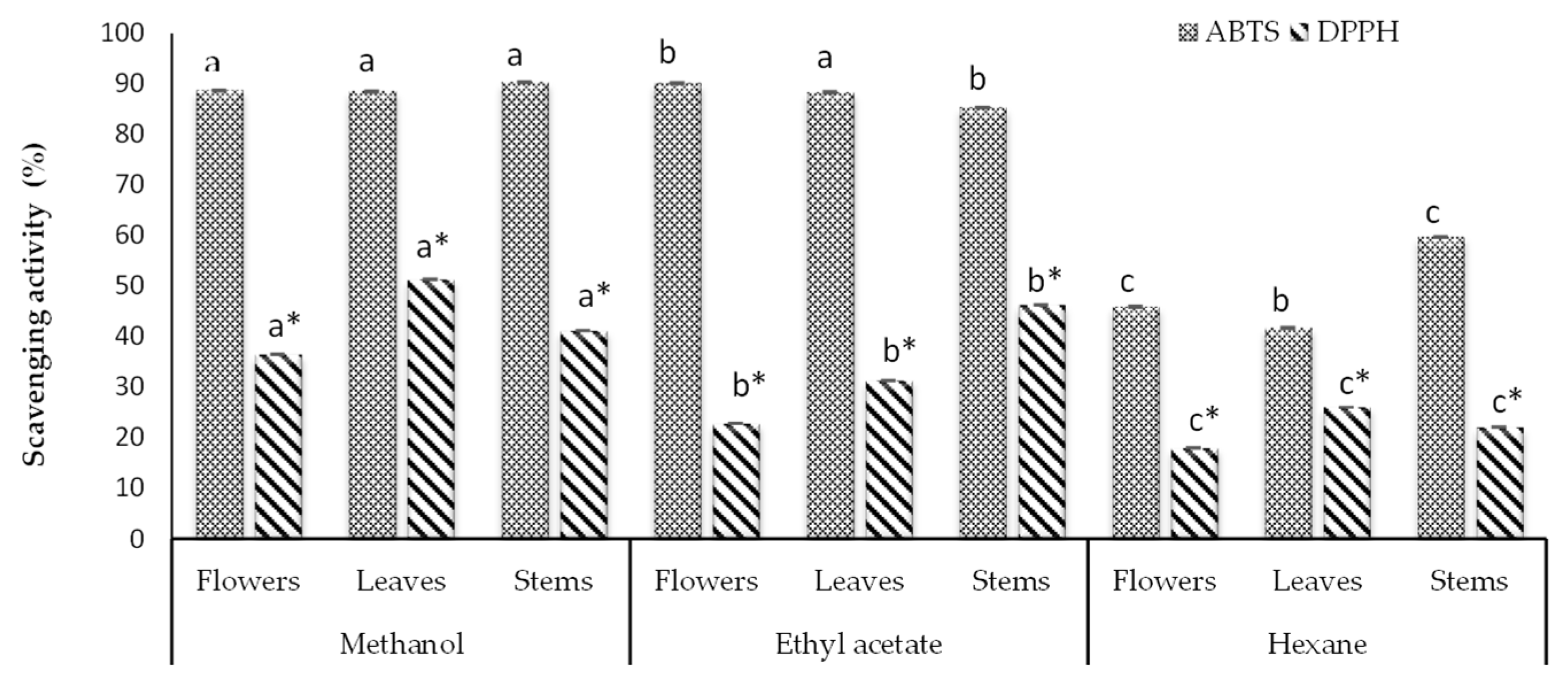
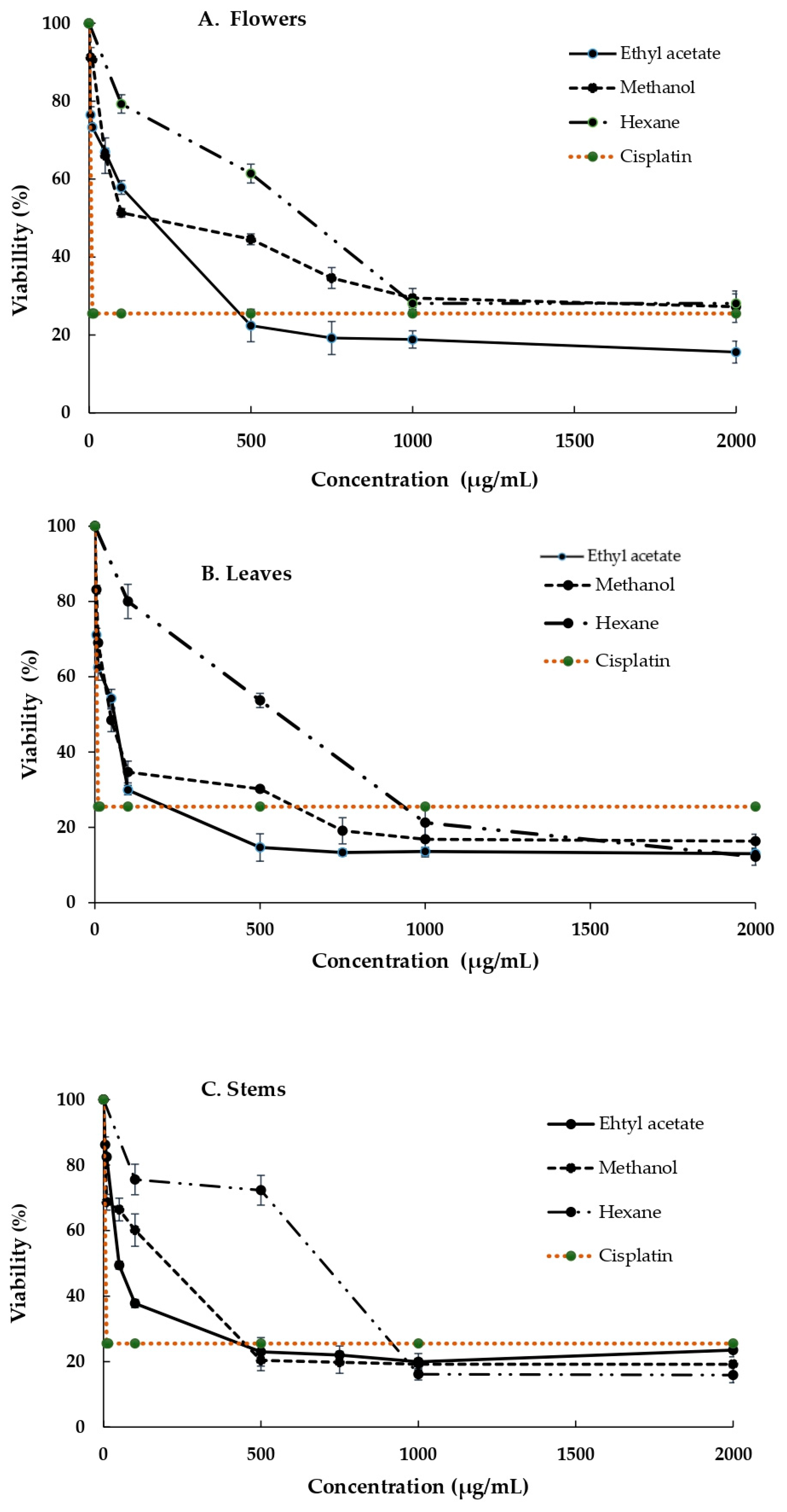
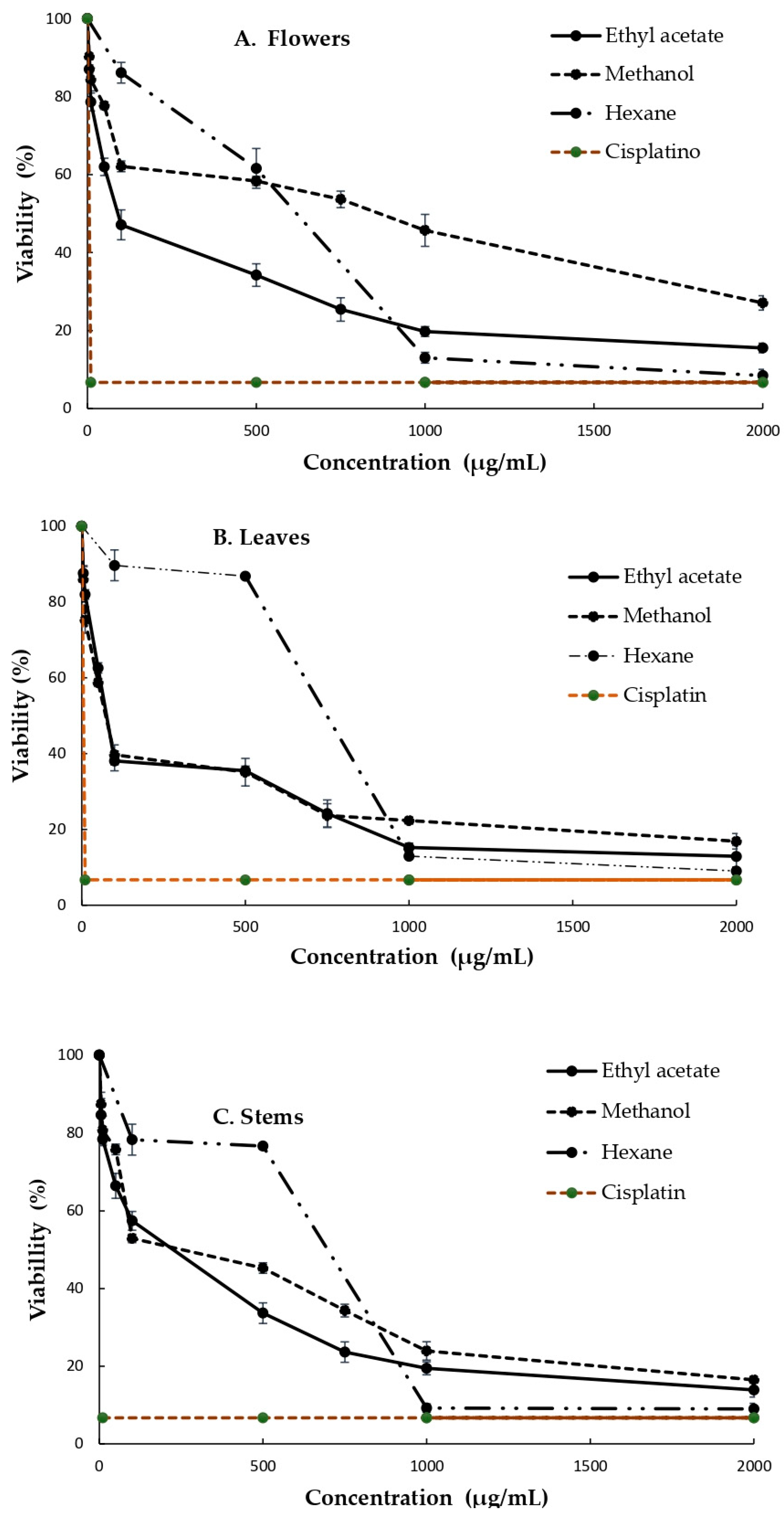
| Solvent | Parts of Plants | Antioxidant Capacity | |
|---|---|---|---|
| DPPH• | ABTS•+ | ||
| mg TEAC/g | |||
| Methanol | Flowers | 16.909 ± 0.040 a | 22.166 ± 0.043 a |
| Leaves | 23.142 ± 0.054 a | 22.123 ± 0.043 a | |
| Stems | 18.875 ± 0.054 a | 22.551 ± 0.043 a | |
| Ethyl acetate | Flowers | 11.168 ± 0.060 b | 22.509 ± 0.043 a |
| Leaves | 14.737 ± 0.040 b | 22.08 ± 0.042 a | |
| Stems | 21.013 ± 0.045 b | 22.737 ± 0.025 a | |
| Hexane | Flowers | 9.134 ± 0.040 c | 12.165 ± 0.049 b |
| Leaves | 12.522 ± 0.015 c | 11.194 ± 0.042 b | |
| Stems | 10.866 ± 0.040 c | 15.394 ± 0.042 b | |
| Parts of Plant | Solvent | Phenols (mg GAE/g) | Tannins (mg CATE/g) | Saponins (HU/mg) |
|---|---|---|---|---|
| Methanol | 78.794 ± 0.294 a | 43.721 ± 5.747 b | 20 | |
| Flowers | Ethyl acetate | 37.618 ± 0.304 b | 34.143 ± 4.619 a | ND |
| Hexane | 51.147 ± 0.284 c | 37.974 ± 1.437 ab | ND | |
| Methanol | 133.402 ± 0.34 a | 134.717 ± 6.789 a | ND | |
| Leaves | Ethyl acetate | 71.441 ± 0.294 b | 257.646 ± 27.201 b | ND |
| Hexane | 43.696 ± 0.170 c | 32.706 ± 2.991 c | ND | |
| Methanol | 117.324 ± 0.29 a | 58.089 ± 2.488 a | ND | |
| Stems | Ethyl acetate | 134.971 ± 0.89 b | 61.920 ± 3.616 a | ND |
| Hexane | 8.794 ± 0.09 c | 32.706 ± 5.982 b | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valadez-Vega, C.; Lugo-Magaña, O.; Mendoza-Guzmán, L.; Villagómez-Ibarra, J.R.; Velasco-Azorsa, R.; Bautista, M.; Betanzos-Cabrera, G.; Morales-González, J.A.; Madrigal-Santillán, E.O. Antioxidant Activity and Anticarcinogenic Effect of Extracts from Bouvardia ternifolia (Cav.) Schltdl. Life 2023, 13, 2319. https://doi.org/10.3390/life13122319
Valadez-Vega C, Lugo-Magaña O, Mendoza-Guzmán L, Villagómez-Ibarra JR, Velasco-Azorsa R, Bautista M, Betanzos-Cabrera G, Morales-González JA, Madrigal-Santillán EO. Antioxidant Activity and Anticarcinogenic Effect of Extracts from Bouvardia ternifolia (Cav.) Schltdl. Life. 2023; 13(12):2319. https://doi.org/10.3390/life13122319
Chicago/Turabian StyleValadez-Vega, Carmen, Olivia Lugo-Magaña, Lorenzo Mendoza-Guzmán, José Roberto Villagómez-Ibarra, Raul Velasco-Azorsa, Mirandeli Bautista, Gabriel Betanzos-Cabrera, José A. Morales-González, and Eduardo Osiris Madrigal-Santillán. 2023. "Antioxidant Activity and Anticarcinogenic Effect of Extracts from Bouvardia ternifolia (Cav.) Schltdl." Life 13, no. 12: 2319. https://doi.org/10.3390/life13122319
APA StyleValadez-Vega, C., Lugo-Magaña, O., Mendoza-Guzmán, L., Villagómez-Ibarra, J. R., Velasco-Azorsa, R., Bautista, M., Betanzos-Cabrera, G., Morales-González, J. A., & Madrigal-Santillán, E. O. (2023). Antioxidant Activity and Anticarcinogenic Effect of Extracts from Bouvardia ternifolia (Cav.) Schltdl. Life, 13(12), 2319. https://doi.org/10.3390/life13122319








