Dynamic MRI of the Mesenchymal Stem Cells Distribution during Intravenous Transplantation in a Rat Model of Ischemic Stroke
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cell Culture
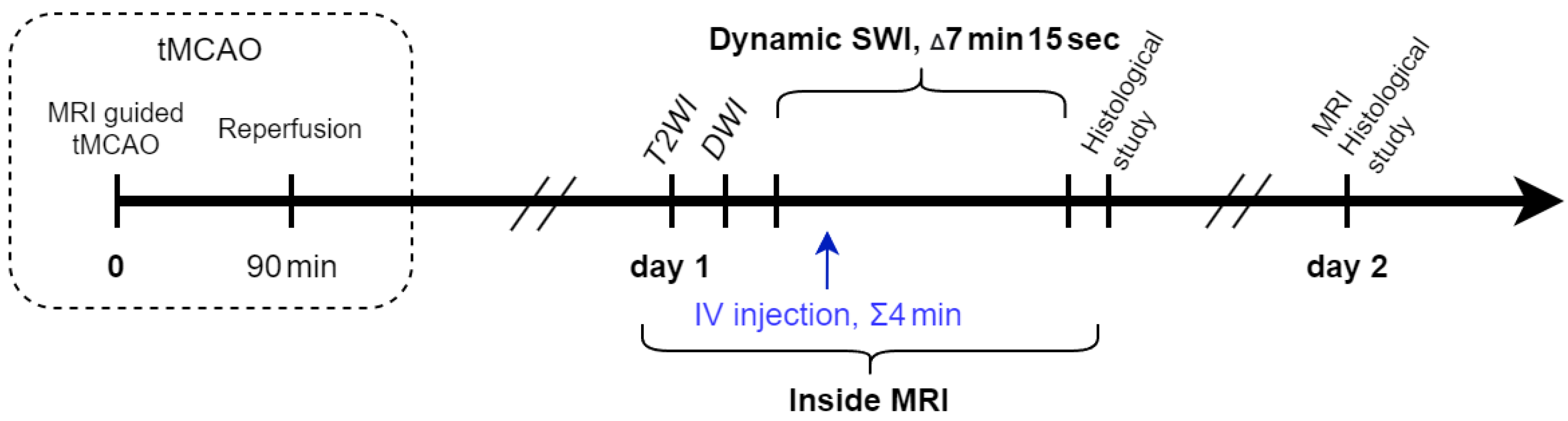
2.3. Study Design
2.4. Transient Middle Cerebral Artery Occlusion
2.5. Intravenous Transplantation
2.6. Estimation of Neurological Deficit
2.7. Magnetic Resonance Imaging
2.8. Estimation of Stroke Volume
2.9. Histology
2.10. Statistical Analysis
3. Results
3.1. Dynamic MRI Distribution of MSCs in Ischemic Rat Brain after Intravenous Transplantation
3.2. Evaluation of the Therapeutic Effects of MSCs after Intravenous Transplantation in Experimental Ischemic Stroke
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, H.; Zarriello, S.; Coats, A.; Nelson, C.; Kingsbury, C.; Gorsky, A.; Rajani, M.; Neal, E.G.; Borlongan, C.V. Stem cell therapy for neurological disorders: A focus on aging. Neurobiol. Dis. 2019, 126, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Islam, M.R.; Islam, M.T.; Harun-Or-rashid, M.; Islam, M.; Abdullah, S.; Uddin, M.B.; Das, S.; Rahaman, M.S.; Ahmed, M.; et al. Stem Cell Transplantation Therapy and Neurological Disorders: Current Status and Future Perspectives. Biology 2022, 11, 147. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Bobrovskaya, L.; Zhou, X.F. Cell therapy for neurological disorders: The perspective of promising cells. Biology 2021, 10, 1142. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Fuentes, D.E.; Fernández-Garza, L.E.; Samia-Meza, J.A.; Barrera-Barrera, S.A.; Caplan, A.I.; Barrera-Saldaña, H.A. Mesenchymal Stem Cells Current Clinical Applications: A Systematic Review. Arch. Med. Res. 2021, 52, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewska, A.; Dabrowska, S.; Lukomska, B.; Janowski, M. Mesenchymal Stem Cells for Neurological Disorders. Adv. Sci. 2021, 8, 2002944. [Google Scholar] [CrossRef]
- Li, W.; Shi, L.; Hu, B.; Hong, Y.; Zhang, H.; Li, X.; Zhang, Y. Mesenchymal Stem Cell-Based Therapy for Stroke: Current Understanding and Challenges. Front. Cell. Neurosci. 2021, 15, 1–12. [Google Scholar] [CrossRef]
- Berlet, R.; Anthony, S.; Brooks, B.; Wang, Z.J.; Sadanandan, N.; Shear, A.; Cozene, B.; Gonzales-Portillo, B.; Parsons, B.; Salazar, F.E.; et al. Combination of stem cells and rehabilitation therapies for ischemic stroke. Biomolecules 2021, 11, 1316. [Google Scholar] [CrossRef]
- Lee, S.; Kim, O.J.; Lee, K.O.; Jung, H.; Oh, S.H.; Kim, N.K. Enhancing the therapeutic potential of ccl2- overexpressing mesenchymal stem cells in acute stroke. Int. J. Mol. Sci. 2020, 21, 7795. [Google Scholar] [CrossRef]
- Shahror, R.A.; Wu, C.C.; Chiang, Y.H.; Chen, K.Y. Genetically modified mesenchymal stem cells: The next generation of stem cell-based therapy for TBI. Int. J. Mol. Sci. 2020, 21, 4051. [Google Scholar] [CrossRef]
- Yarygin, K.N.; Namestnikova, D.D.; Sukhinich, K.K.; Gubskiy, I.L.; Majouga, A.G.; Kholodenko, I. V Cell therapy of stroke: Do the intra-arterially transplanted mesenchymal stem cells cross the blood–brain barrier? Cells 2021, 10, 2997. [Google Scholar] [CrossRef]
- Borlongan, C.V. Concise Review: Stem Cell Therapy for Stroke Patients: Are We There Yet? Stem Cells Transl. Med. 2019, 8, 983–988. [Google Scholar] [CrossRef] [Green Version]
- Lalu, M.M.; Montroy, J.; Dowlatshahi, D.; Hutton, B.; Juneau, P.; Wesch, N.; Zhang, S.Y.; McGinn, R.; Corbett, D.; Stewart, D.J.; et al. From the Lab to Patients: A Systematic Review and Meta-Analysis of Mesenchymal Stem Cell Therapy for Stroke. Transl. Stroke Res. 2020, 11, 345–364. [Google Scholar] [CrossRef]
- Andrzejewska, A.; Lukomska, B.; Janowski, M. Concise Review: Mesenchymal Stem Cells: From Roots to Boost. Stem Cells 2019, 37, 855–864. [Google Scholar] [CrossRef] [Green Version]
- Babenko, V.A.; Silachev, D.N.; Popkov, V.A.; Zorova, L.D.; Pevzner, I.B.; Plotnikov, E.Y.; Sukhikh, G.T.; Zorov, D.B. Miro1 enhances mitochondria transfer from multipotent mesenchymal stem cells (MMSC) to neural cells and improves the efficacy of cell recovery. Molecules 2018, 23, 687. [Google Scholar] [CrossRef] [Green Version]
- Weiss, A.R.R.; Dahlke, M.H. Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of action of living, apoptotic, and dead MSCs. Front. Immunol. 2019, 10, 1191. [Google Scholar] [CrossRef] [Green Version]
- Song, N.; Scholtemeijer, M.; Shah, K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol. Sci. 2020, 41, 653–664. [Google Scholar] [CrossRef]
- Fan, X.-L.L.; Zhang, Y.; Li, X.; Fu, Q.-L.L. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell. Mol. Life Sci. 2020, 77, 2771–2794. [Google Scholar] [CrossRef] [Green Version]
- Mousaei Ghasroldasht, M.; Seok, J.; Park, H.S.; Liakath Ali, F.B.; Al-Hendy, A. Stem Cell Therapy: From Idea to Clinical Practice. Int. J. Mol. Sci. 2022, 23, 2850. [Google Scholar] [CrossRef]
- Sanchez-Diaz, M.; Quiñones-Vico, M.I.; de la Torre, R.S.; Montero-Vílchez, T.; Sierra-Sánchez, A.; Molina-Leyva, A.; Arias-Santiago, S. Biodistribution of mesenchymal stromal cells after administration in animal models and humans: A systematic review. J. Clin. Med. 2021, 10, 2925. [Google Scholar] [CrossRef]
- Boltze, J.; Arnold, A.; Walczak, P.; Jolkkonen, J.; Cui, L.; Wagner, D.-C. The dark side of the force—Constraints and complications of cell therapies for stroke. Front. Neurol. 2015, 6, 155. [Google Scholar] [CrossRef]
- Gubskiy, I.L.; Namestnikova, D.D.; Revkova, V.A.; Cherkashova, E.A.; Sukhinich, K.K.; Beregov, M.M.; Melnikov, P.A.; Abakumov, M.A.; Chekhonin, V.P.; Gubsky, L.V.; et al. The Impact of Cerebral Perfusion on Mesenchymal Stem Cells Distribution after Intra-Arterial Transplantation: A Quantitative MR Study. Biomedicines 2022, 10, 353. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos-dos-Santos, A.; Rosado-de-Castro, P.H.; Lopes de Souza, S.A.; Da Costa Silva, J.; Ramos, A.B.; Rodriguez de Freitas, G.; Barbosa da Fonseca, L.M.; Gutfilen, B.; Mendez-Otero, R. Intravenous and intra-arterial administration of bone marrow mononuclear cells after focal cerebral ischemia: Is there a difference in biodistribution and efficacy? Stem Cell Res. 2012, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019, 50, E344–E418. [Google Scholar] [PubMed]
- Guzman, R.; Janowski, M.; Walczak, P. Intra-arterial delivery of cell therapies for stroke. Stroke 2018, 49, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Rascón-Ramírez, F.J.; Esteban-García, N.; Barcia, J.A.; Trondin, A.; Nombela, C.; Sánchez-Sánchez-Rojas, L. Are We Ready for Cell Therapy to Treat Stroke? Front. Cell Dev. Biol. 2021, 9, 621645. [Google Scholar] [CrossRef]
- Chung, J.W.; Chang, W.H.; Bang, O.Y.; Moon, G.J.; Kim, S.J.; Kim, S.K.; Lee, J.S.; Sohn, S.I.; Kim, Y.H. Efficacy and Safety of Intravenous Mesenchymal Stem Cells for Ischemic Stroke. Neurology 2021, 96, e1012–e1023. [Google Scholar] [CrossRef]
- Gautam, J.; Alaref, A.; Hassan, A.; Sharma Kandel, R.; Mishra, R.; Jahan, N. Safety and Efficacy of Stem Cell Therapy in Patients With Ischemic Stroke. Cureus 2020, 12, e9917. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Q.; Wang, W.; Lin, F.; Wang, S.; Zhao, J. Mesenchymal stem cell therapy for ischemic stroke: A look into treatment mechanism and therapeutic potential. J. Neurol. 2021, 268, 4095–4107. [Google Scholar] [CrossRef]
- Von der Haar, K.; Lavrentieva, A.; Stahl, F.; Scheper, T.; Blume, C. Lost signature: Progress and failures in in vivo tracking of implanted stem cells. Appl. Microbiol. Biotechnol. 2015, 99, 9907–9922. [Google Scholar] [CrossRef]
- Yang, X.; Tian, D.C.; He, W.; Lv, W.; Fan, J.; Li, H.; Jin, W.N.; Meng, X. Cellular and molecular imaging for stem cell tracking in neurological diseases. Stroke Vasc. Neurol. 2021, 6, 121–127. [Google Scholar] [CrossRef]
- Bulte, J.W.M.; Daldrup-Link, H.E. Clinical tracking of cell transfer and cell transplantation: Tu. Radiology 2018, 289, 604–615. [Google Scholar] [CrossRef]
- Yahyapour, R.; Farhood, B.; Graily, G.; Rezaeyan, A.; Rezapoor, S.; Abdollahi, H.; Cheki, M.; Amini, P.; Fallah, H.; Najafi, M.; et al. Stem Cell Tracing Through MR Molecular Imaging. Tissue Eng. Regen. Med. 2018, 15, 249–261. [Google Scholar] [CrossRef]
- Geng, K.; Yang, Z.X.; Huang, D.; Yi, M.; Jia, Y.; Yan, G.; Cheng, X.; Wu, R. Tracking of mesenchymal stem cells labeled with gadolinium diethylenetriamine pentaacetic acid by 7T magnetic resonance imaging in a model of cerebral ischemia. Mol. Med. Rep. 2015, 11, 954–960. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Mu, L.; Roes, I.; Miranda-Nieves, D.; Nahrendorf, M.; Ankrum, J.A.; Zhao, W.; Karp, J.M. Nanoparticle-based monitoring of cell therapy. Nanotechnology 2011, 22, 494001. [Google Scholar] [CrossRef] [Green Version]
- Accomasso, L.; Gallina, C.; Turinetto, V.; Giachino, C. Stem cell tracking with nanoparticles for regenerative medicine purposes: An overview. Stem Cells Int. 2016, 2016, 7920358. [Google Scholar] [CrossRef] [Green Version]
- Ishibashi, H.; Hirao, K.; Yamaguchi, J.; Nabekura, J. Inhibition of chloride outward transport by gadolinium in cultured rat spinal cord neurons. Neurotoxicology 2009, 30, 155–159. [Google Scholar] [CrossRef]
- Ngen, E.J.; Wang, L.; Kato, Y.; Krishnamachary, B.; Zhu, W.; Gandhi, N.; Smith, B.; Armour, M.; Wong, J.; Gabrielson, K.; et al. Imaging transplanted stem cells in real time using an MRI dual-contrast method. Sci. Rep. 2015, 5, 13628. [Google Scholar] [CrossRef] [Green Version]
- Bull, E.; Madani, S.Y.; Sheth, R.; Seifalian, A.; Green, M.; Seifalian, A.M. Stem cell tracking using iron oxide nanoparticles. Int. J. Nanomed. 2014, 9, 1641–1653. [Google Scholar] [CrossRef] [Green Version]
- Song, B.W. In Vivo Assessment of Stem Cells for Treating Neurodegenerative Disease: Current Approaches and Future Prospects. Stem Cells Int. 2017, 2017, 9751583. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Hu, Y.; Wang, J.; Gao, X.; Qian, X.; Tang, M. Superparamagnetic iron oxide nanoparticles: Cytotoxicity, metabolism, and cellular behavior in biomedicine applications. Int. J. Nanomed. 2021, 16, 6097–6113. [Google Scholar] [CrossRef]
- Wang, Y.-X.J.; Xuan, S.; Port, M.; Idee, J.-M. Recent advances in superparamagnetic iron oxide nanoparticles for cellular imaging and targeted therapy research. Curr. Pharm. Des. 2013, 19, 6575–6593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrzejewska, A.; Jablonska, A.; Seta, M.; Dabrowska, S.; Walczak, P.; Janowski, M.; Lukomska, B. Labeling of human mesenchymal stem cells with different classes of vital stains: Robustness and toxicity. Stem Cell Res. Ther. 2019, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Haacke, E.M.; Xu, Y.; Cheng, Y.-C.N.; Reichenbach, J.R. Susceptibility weighted imaging (SWI). Magn. Reson. Med. 2004, 52, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Namestnikova, D.; Gubskiy, I.; Kholodenko, I.; Melnikov, P.; Sukhinich, K.; Gabashvili, A.; Vishnevskiy, D.; Soloveva, A.; Abakumov, M.; Vakhrushev, I.; et al. Methodological aspects of MRI of transplanted superparamagnetic iron oxide-labeled mesenchymal stem cells in live rat brain. PLoS ONE 2017, 12, e0186717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Schellenberger, A.A.; Kratz, H.; Farr, T.D.; Löwa, N.; Hauptmann, R.; Wagner, S.; Taupitz, M.; Schnorr, J.; Schellenberger, E.A. Labeling of mesenchymal stem cells for MRI with single-cell sensitivity. Int. J. Nanomed. 2016, 11, 1517–1535. [Google Scholar] [CrossRef] [Green Version]
- Dodd, S.J.; Williams, M.; Suhan, J.P.; Williams, D.S.; Koretsky, A.P.; Ho, C. Detection of single mammalian cells by high-resolution magnetic resonance imaging. Biophys. J. 1999, 76, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, E.M.; Sharer, K.; Skrtic, S.; Koretsky, A.P. In vivo detection of single cells by MRI. Magn. Reson. Med. 2006, 55, 242–249. [Google Scholar] [CrossRef]
- Heyn, C.; Ronald, J.A.; Mackenzie, L.T.; MacDonald, I.C.; Chambers, A.F.; Rutt, B.K.; Foster, P.J. In vivo magnetic resonance imaging of single cells in mouse brain with optical validation. Magn. Reson. Med. 2006, 55, 23–29. [Google Scholar] [CrossRef]
- Gorelik, M.; Orukari, I.; Wang, J.; Galpoththawela, S.; Kim, H.; Levy, M.; Gilad, A.A.; Bar-Shir, A.; Kerr, D.A.; Levchenko, A.; et al. Use of MR cell tracking to evaluate targeting of glial precursor cells to inflammatory tissue by exploiting the very late antigen-4 docking receptor. Radiology 2012, 265, 175–185. [Google Scholar] [CrossRef] [Green Version]
- Walczak, P.; Wojtkiewicz, J.; Nowakowski, A.; Habich, A.; Holak, P.; Xu, J.; Adamiak, Z.; Chehade, M.; Pearl, M.S.; Gailloud, P.; et al. Real-time MRI for precise and predictable intra-arterial stem cell delivery to the central nervous system. J. Cereb. Blood Flow Metab. 2017, 37, 2346–2358. [Google Scholar] [CrossRef]
- Namestnikova, D.D.; Gubskiy, I.L.; Revkova, V.A.; Sukhinich, K.K.; Melnikov, P.A.; Gabashvili, A.N.; Cherkashova, E.A.; Vishnevskiy, D.A.; Kurilo, V.V.; Burunova, V.V.; et al. Intra-Arterial Stem Cell Transplantation in Experimental Stroke in Rats: Real-Time MR Visualization of Transplanted Cells Starting With Their First Pass Through the Brain With Regard to the Therapeutic Action. Front. Neurosci. 2021, 15, 179. [Google Scholar] [CrossRef]
- Jolkkonen, J.; Walczak, P. Cell-Based Therapies in Stroke; Springer: Wien, Austria, 2013; ISBN 9783709111758. [Google Scholar]
- Kurtz, A. Mesenchymal stem cell delivery routes and fate. Int. J. Stem Cells 2008, 1, 1–7. [Google Scholar] [CrossRef]
- Scarfe, L.; Taylor, A.; Sharkey, J.; Harwood, R.; Barrow, M.; Comenge, J.; Beeken, L.; Astley, C.; Santeramo, I.; Hutchinson, C.; et al. Non-invasive imaging reveals conditions that impact distribution and persistence of cells after in vivo administration. Stem Cell Res. Ther. 2018, 9, 332. [Google Scholar] [CrossRef]
- Raghava, N.; Das, B.C.; Ray, S.K. Neuroprotective effects of estrogen in CNS injuries: Insights from animal models. Neurosci. Neuroeconomics 2017, 6, 15–29. [Google Scholar] [CrossRef] [Green Version]
- Burunova, V.V.; Gisina, A.M.; Kholodenko, I.V.; Lupatov, A.Y.; Shragina, O.A.; Yarygin, K.N. Standardization of biochemical profile of mesenchymal cell materials by probing the level of dehydrogenase activity. Bull. Exp. Biol. Med. 2010, 149, 497–501. [Google Scholar] [CrossRef]
- Gubskiy, I.L.; Namestnikova, D.D.; Cherkashova, E.A.; Chekhonin, V.P.; Baklaushev, V.P.; Gubsky, L.V.; Yarygin, K.N. MRI Guiding of the Middle Cerebral Artery Occlusion in Rats Aimed to Improve Stroke Modeling. Transl. Stroke Res. 2018, 9, 417–425. [Google Scholar] [CrossRef] [Green Version]
- Schaar, K.L.; Brenneman, M.M.; Savitz, S.I. Functional assessments in the rodent stroke model. Exp. Transl. Stroke Med. 2010, 2, 13. [Google Scholar] [CrossRef] [Green Version]
- Salikhova, D.; Bukharova, T.; Cherkashova, E.; Namestnikova, D.; Leonov, G.; Nikitina, M.; Gubskiy, I.; Akopyan, G.; Elchaninov, A.; Midiber, K.; et al. Therapeutic effects of hipsc-derived glial and neuronal progenitor cells-conditioned medium in experimental ischemic stroke in rats. Int. J. Mol. Sci. 2021, 22, 4694. [Google Scholar] [CrossRef]
- Skalski, K.A.; Kessler, A.T.; Bhatt, A.A. Hemorrhagic and non-hemorrhagic causes of signal loss on susceptibility-weighted imaging. Emerg. Radiol. 2018, 25, 691–701. [Google Scholar] [CrossRef]
- Bren, K.L.; Eisenberg, R.; Gray, H.B. Discovery of the magnetic behavior of hemoglobin: A beginning of bioinorganic chemistry. Proc. Natl. Acad. Sci. USA 2015, 112, 13123–13127. [Google Scholar] [CrossRef]
- Masthoff, M.; Gran, S.; Zhang, X.; Wachsmuth, L.; Bietenbeck, M.; Helfen, A.; Heindel, W.; Sorokin, L.; Roth, J.; Eisenblätter, M.; et al. Temporal window for detection of inflammatory disease using dynamic cell tracking with time-lapse MRI. Sci. Rep. 2018, 8, 9563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masthoff, M.; Freppon, F.N.; Zondler, L.; Wilken, E.; Wachsmuth, L.; Niemann, S.; Schwarz, C.; Fredrich, I.; Havlas, A.; Block, H.; et al. Resolving immune cells with patrolling behaviour by magnetic resonance time-lapse single cell tracking. eBioMedicine 2021, 73, 103670. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Wang, Z.; Gutkind, J.S.; Wang, Z.; Wang, F.; Lu, J.; Niu, G.; Teng, G.; Chen, X. Engineered mesenchymal stem cells with enhanced tropism and paracrine secretion of cytokines and growth factors to treat traumatic brain injury. Stem Cells 2015, 33, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Satake, K.; Lou, J.; Lenke, L.G. Migration of mesenchymal stem cells through cerebrospinal fluid into injured spinal cord tissue. Spine 2004, 29, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.M.; Harting, M.T.; Jimenez, F.; Monzon-Posadas, W.O.; Xue, H.; Savitz, S.I.; Laine, G.A.; Cox, C.S. Pulmonary passage is a major obstacle for intravenous stem cell delivery: The pulmonary first-pass effect. Stem Cells Dev. 2009, 18, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.M.L.; Sampasivam, Y.; Lokanathan, Y. Biodistribution of mesenchymal stem cells (MSCs) in animal models and implied role of exosomes following systemic delivery of MSCs: A systematic review. Am. J. Transl. Res. 2022, 14, 2147. [Google Scholar]
- Yang, L.; Qian, J.; Yang, B.; He, Q.; Wang, J.; Weng, Q. Challenges and Improvements of Novel Therapies for Ischemic Stroke. Front. Pharmacol. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Brooks, B.; Ebedes, D.; Usmani, A.; Gonzales-Portillo, J.V.; Gonzales-Portillo, D.; Borlongan, C.V. Mesenchymal Stromal Cells in Ischemic Brain Injury. Cells 2022, 11, 1013. [Google Scholar] [CrossRef]
- Schrepfer, S.; Deuse, T.; Reichenspurner, H.; Fischbein, M.P.; Robbins, R.C.; Pelletier, M.P. Stem Cell Transplantation: The Lung Barrier. Transplant. Proc. 2007, 39, 573–576. [Google Scholar] [CrossRef]
- Nose, N.; Nogami, S.; Koshino, K.; Chen, X.; Werner, R.A.; Kashima, S.; Rowe, S.P.; Lapa, C.; Fukuchi, K.; Higuchi, T. [18F]FDG-labelled stem cell PET imaging in different route of administrations and multiple animal species. Sci. Rep. 2021, 11, 10896. [Google Scholar] [CrossRef]
- Eggenhofer, E.; Benseler, V.; Kroemer, A.; Popp, F.C.; Geissler, E.K.; Schlitt, H.J.; Baan, C.C.; Dahlke, M.H.; Hoogduijn, M.J. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front. Immunol. 2012, 3, 297. [Google Scholar] [CrossRef]
- De Witte, S.F.H.; Luk, F.; Sierra Parraga, J.M.; Gargesha, M.; Merino, A.; Korevaar, S.S.; Shankar, A.S.; O’Flynn, L.; Elliman, S.J.; Roy, D.; et al. Immunomodulation By Therapeutic Mesenchymal Stromal Cells (MSC) Is Triggered Through Phagocytosis of MSC By Monocytic Cells. Stem Cells 2018, 36, 602–615. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Dong, N.; Hong, H.; Qi, J.; Zhang, S.; Wang, J. Mesenchymal Stem Cells: Therapeutic Mechanisms for Stroke. Int. J. Mol. Sci. 2022, 23, 2550. [Google Scholar] [CrossRef]
- Cherkashova, E.A.; Namestnikova, D.D.; Gubskiy, I.L.; Revkova, V.A.; Sukhinich, K.K.; Mel’nikov, P.A.; Chekhonin, V.P.; Gubsky, L.V.; Yarygin, K.N. Dose-Dependent Effects of Intravenous Mesenchymal Stem Cell Transplantation in Rats with Acute Focal Cerebral Ischemia. Bull. Exp. Biol. Med. 2022, 173, 514–518. [Google Scholar] [CrossRef]
- Komatsu, K.; Honmou, O.; Suzuki, J.; Houkin, K.; Hamada, H.; Kocsis, J.D. Therapeutic time window of mesenchymal stem cells derived from bone marrow after cerebral ischemia. Brain Res. 2010, 1334, 84–92. [Google Scholar] [CrossRef]
- Ma, S.; Zhong, D.; Chen, H.; Zheng, Y.; Sun, Y.; Luo, J.; Li, H.; Li, G.; Yin, Y. The immunomodulatory effect of bone marrow stromal cells (BMSCs) on interleukin (IL)-23/IL-17-mediated ischemic stroke in mice. J. Neuroimmunol. 2013, 257, 28–35. [Google Scholar] [CrossRef]
- Yavagal, D.R.; Lin, B.; Raval, A.P.; Garza, P.S.; Dong, C.; Zhao, W.; Rangel, E.B.; McNiece, I.; Rundek, T.; Sacco, R.L.; et al. Efficacy and dose-dependent safety of intra-arterial delivery of mesenchymal stem cells in a rodent stroke model. PLoS ONE 2014, 9, e93735. [Google Scholar] [CrossRef] [Green Version]
- Toyama, K.; Honmou, O.; Harada, K.; Suzuki, J.; Houkin, K.; Hamada, H.; Kocsis, J.D. Therapeutic benefits of angiogenetic gene-modified human mesenchymal stem cells after cerebral ischemia. Exp. Neurol. 2009, 216, 47–55. [Google Scholar] [CrossRef]
- Watanabe, M.; Yavagal, D. Intra-arterial delivery of mesenchymal stem cells. Brain Circ. 2016, 2, 114. [Google Scholar] [CrossRef]
- Gutiérrez-Fernández, M.; Rodríguez-Frutos, B.; Alvarez-Grech, J.; Vallejo-Cremades, M.T.; Expósito-Alcaide, M.; Merino, J.; Roda, J.M.; Díez-Tejedor, E. Functional recovery after hematic administration of allogenic mesenchymal stem cells in acute ischemic stroke in rats. Neuroscience 2011, 175, 394–405. [Google Scholar] [CrossRef]
- Zhang, H.L.; Xie, X.F.; Xiong, Y.Q.; Liu, S.M.; Hu, G.Z.; Cao, W.F.; Wu, X.M. Comparisons of the therapeutic effects of three different routes of bone marrow mesenchymal stem cell transplantation in cerebral ischemic rats. Brain Res. 2018, 1680, 143–154. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cherkashova, E.A.; Namestnikova, D.D.; Gubskiy, I.L.; Revkova, V.A.; Sukhinich, K.K.; Melnikov, P.A.; Abakumov, M.A.; Savina, G.D.; Chekhonin, V.P.; Gubsky, L.V.; et al. Dynamic MRI of the Mesenchymal Stem Cells Distribution during Intravenous Transplantation in a Rat Model of Ischemic Stroke. Life 2023, 13, 288. https://doi.org/10.3390/life13020288
Cherkashova EA, Namestnikova DD, Gubskiy IL, Revkova VA, Sukhinich KK, Melnikov PA, Abakumov MA, Savina GD, Chekhonin VP, Gubsky LV, et al. Dynamic MRI of the Mesenchymal Stem Cells Distribution during Intravenous Transplantation in a Rat Model of Ischemic Stroke. Life. 2023; 13(2):288. https://doi.org/10.3390/life13020288
Chicago/Turabian StyleCherkashova, Elvira A., Daria D. Namestnikova, Ilya L. Gubskiy, Veronica A. Revkova, Kirill K. Sukhinich, Pavel A. Melnikov, Maxim A. Abakumov, Galina D. Savina, Vladimir P. Chekhonin, Leonid V. Gubsky, and et al. 2023. "Dynamic MRI of the Mesenchymal Stem Cells Distribution during Intravenous Transplantation in a Rat Model of Ischemic Stroke" Life 13, no. 2: 288. https://doi.org/10.3390/life13020288
APA StyleCherkashova, E. A., Namestnikova, D. D., Gubskiy, I. L., Revkova, V. A., Sukhinich, K. K., Melnikov, P. A., Abakumov, M. A., Savina, G. D., Chekhonin, V. P., Gubsky, L. V., & Yarygin, K. N. (2023). Dynamic MRI of the Mesenchymal Stem Cells Distribution during Intravenous Transplantation in a Rat Model of Ischemic Stroke. Life, 13(2), 288. https://doi.org/10.3390/life13020288







