Difficulties in Adaptation of the Mother and Newborn via Cesarean Section versus Natural Birth—A Narrative Review
Abstract
:1. Introduction
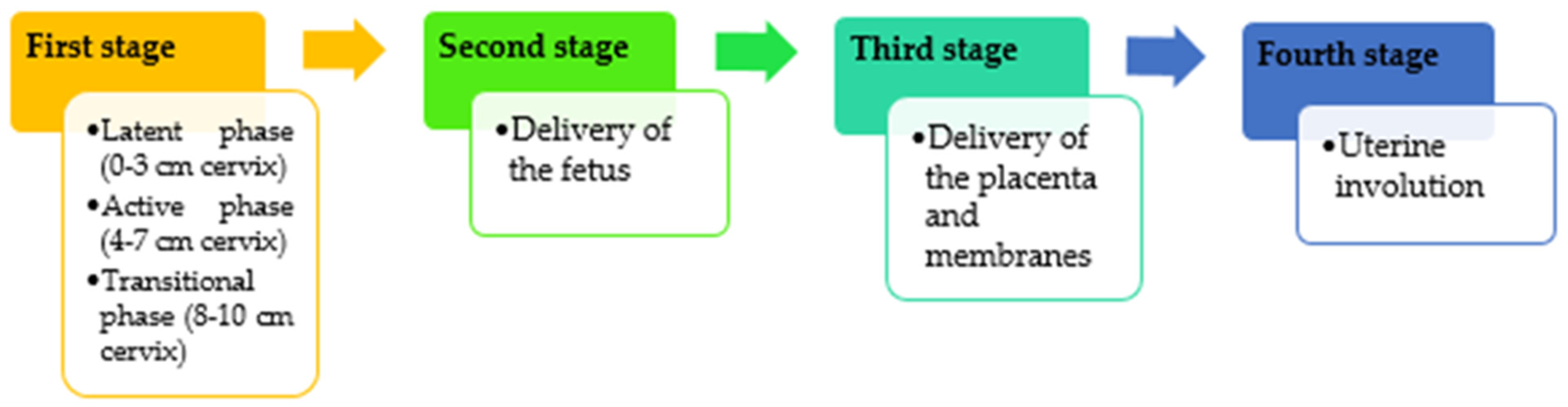
2. Impact of Vaginal Birth on the Mother
3. The Impact of Vaginal Birth on the Newborn
4. Cesarean Section as a Medical Solution
5. The Morphological Implications of Cesarean Section on the Parturient
6. The Impact of Cesarean on the Fetus
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aburel, E. Obsterică și Ginecologie; Didactică și Pedagogică: București, Romania, 1971. [Google Scholar]
- Lupu, V. Știința Creșterii și Dezvoltării Copilului; Cutia Pandorei: București, Romania, 1996; pp. 32–45. [Google Scholar]
- Technical Working Group, World Health Organization. Care in normal birth: A practical guide. Birth 1997, 24, 121–123. [Google Scholar] [CrossRef]
- Negrini, R.; da Silva Ferreira, R.D.; Guimarães, D.Z. Value-based care in obstetrics: Comparison between vaginal birth and caesarean section. BMC Pregnancy Childbirth 2021, 21, 333. [Google Scholar] [CrossRef] [PubMed]
- Bakke, E.; Øseth, E.H.; Fofanah, T.; Sesay, I.; van Duinen, A.; Bolkan, H.A.; Westendorp, J.; Lonnee-Hoffmann, R. Vacuum births and barriers to its use: An observational study in governmental hospitals in Sierra Leone. BMJ. Open 2022, 12, e060773. [Google Scholar] [CrossRef] [PubMed]
- Stelzer, I.A.; Ghaemi, M.S.; Han, X.; Ando, K.; Hédou, J.J.; Feyaerts, D.; Peterson, L.S.; Rumer, K.K.; Tsai, E.S.; Ganio, E.A.; et al. Integrated trajectories of the maternal metabolome, proteome, and immunome predict labor onset. Sci. Transl. Med. 2021, 13, eabd9898. [Google Scholar] [CrossRef]
- Vannuccini, S.; Bocchi, C.; Severi, F.M.; Challis, J.R.; Petraglia, F. Endocrinology of human parturition. Ann Endocrinol. 2016, 77, 105–113. [Google Scholar]
- Trifan, N.N. Pediatrie Preventive; Medicală: București, Romania, 1982; pp. 95–103. [Google Scholar]
- Constantinescu, C.; Petrescu, C. Puericultură și Pediatrie; Medicală: București, Romania, 1968. [Google Scholar]
- Shynlova, O.; Lee, Y.H.; Srikhajon, K.; Lye, S.J. Physiologic uterine inflammation and labor onset: Integration of endocrine and mechanical signals. Reprod. Sci. 2013, 20, 154–167. [Google Scholar] [PubMed]
- Abalos, E.; Oladapo, O.T.; Chamillard, M.; Díaz, V.; Pasquale, J.; Bonet, M.; Souza, J.P.; Gülmezoglu, A.M. Duration of spontaneous labour in ‘low-risk’ women with ‘normal’ perinatal outcomes: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 223, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Caughey, A.B. Is Zhang the new Friedman: How should we evaluate the first stage of labor? Semin. Perinatol. 2020, 44, 151215. [Google Scholar] [CrossRef]
- Management of the Third Stage of Labor. Available online: https://emedicine.medscape.com/article/275304-overview (accessed on 14 December 2022).
- Normal Labour and Delivery. Available online: https://emedicine.medscape.com/article/260036-overview#a3 (accessed on 14 December 2022).
- Ouzounian, J.G.; Elkayam, U. Physiologic changes during normal pregnancy and delivery. Cardiol. Clin. 2012, 30, 317–329. [Google Scholar]
- Hesson, K.; Hill, T.; Bakal, D. Variability in breathing patterns during latent labor. A pilot study. J. Nurse Midwifery 1997, 42, 99–103. [Google Scholar] [CrossRef]
- Labor, S.; Maguire, S. The Pain of Labour. Rev Pain 2008, 2, 15–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanderlaan, J.; Sadler, C.; Kjerulff, K. Association of Delivery Outcomes with the Number of Childbirth Education Sessions. J. Perinat Neonatal Nurs. 2021, 35, 228–236. [Google Scholar] [CrossRef]
- Uvnäs-Moberg, K.; Ekström-Bergström, A.; Berg, M.; Buckley, S.; Pajalic, Z.; Hadjigeorgiou, E.; Kotłowska, A.; Lengler, L.; Kielbratowska, B.; Leon-Larios, F.; et al. Maternal plasma levels of oxytocin during physiological childbirth—A systematic review with implications for uterine contractions and central actions of oxytocin. BMC Pregnancy Childbirth 2019, 19, 285. [Google Scholar]
- Olza, I.; Uvnas-Moberg, K.; Ekström-Bergström, A.; Leahy-Warren, P.; Karlsdottir, S.I.; Nieuwenhuijze, M.; Villarmea, S.; Hadjigeorgiou, E.; Kazmierczak, M.; Spyridou, A.; et al. Birth as a neuro-psycho-social event: An integrative model of maternal experiences and their relation to neurohormonal events during childbirth. PLoS ONE 2020, 15, e0230992. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.H.; Abele, H.; Plappert, C.F. The Role of Oxytocin and the Effect of Stress During Childbirth: Neurobiological Basics and Implications for Mother and Child. Front. Endocrinol. 2021, 12, 742236. [Google Scholar] [CrossRef]
- Altınayak, S.Ö.; Özkan, H. The effects of conventional, warm and cold acupressure on the pain perceptions and beta-endorphin plasma levels of primiparous women in labor: A randomized controlled trial. Explore 2022, 18, 545–550. [Google Scholar] [CrossRef]
- Wisner, K.L.; Sit, D.K.Y.; McShea, M.C.; Rizzo, D.M.; Zoretich, R.A.; Hughes, C.L.; Eng, H.F.; Luther, J.F.; Wisniewski, S.; Costantino, M.L.; et al. Onset Timing, Thoughts of Self-harm, and Diagnoses in Postpartum Women With Screen-Positive Depression Findings. JAMA Psychiatry 2013, 13, 490–498. [Google Scholar] [CrossRef]
- Ferrari, B.; Mesiano, L.; Benacchio, L.; Ciulli, B.; Donolato, A.; Riolo, R. Prevalence and risk factors of postpartum depression and adjustment disorder during puerperium—A retrospective research. J. Reprod. Infant Psychol. 2021, 39, 486–498. [Google Scholar] [CrossRef]
- Qi, W.; Zhao, F.; Liu, Y.; Li, Q.; Hu, J. Psychosocial risk factors for postpartum depression in Chinese women: A meta-analysis. BMC Pregnancy Childbirth 2021, 21, 174. [Google Scholar]
- Waller, R.; Kornfield, S.L.; White, L.K.; Chaiyachati, B.H.; Barzilay, R.; Njoroge, W.; Parish-Morris, J.; Duncan, A.; Himes, M.M.; Rodriguez, Y.; et al. Clinician-reported childbirth outcomes, patient-reported childbirth trauma, and risk for postpartum depression. Arch. Womens Ment. Health 2022, 25, 985–993. [Google Scholar]
- Khsim, I.E.F.; Rodríguez, M.M.; Riquelme Gallego, B.; Caparros-Gonzalez, R.A.; Amezcua-Prieto, C. Risk Factors for Post-Traumatic Stress Disorder after Childbirth: A Systematic Review. Diagnostics 2022, 12, 2598. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Isumi, A.; Fujiwara, T. Association between Lack of Social Support from Partner or Others and Postpartum Depression among Japanese Mothers: A Population-Based Cross-Sectional Study. Int. J. Environ. Res. Public Health 2020, 17, 4270. [Google Scholar] [CrossRef] [PubMed]
- Berman, Z.; Thiel, F.; Dishy, G.A.; Chan, S.J.; Dekel, S. Maternal psychological growth following childbirth. Arch. Womens Ment. Health 2021, 24, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Tasuji, T.; Reese, E.; van Mulukom, V.; Whitehouse, H. Band of mothers: Childbirth as a female bonding experience. PLoS ONE 2020, 15, e0240175. [Google Scholar] [CrossRef] [PubMed]
- Hulsbosch, L.P.; Boekhorst, M.G.B.M.; Potharst, E.S.; Pop, V.J.M.; Nyklíček, I. Trait mindfulness during pregnancy and perception of childbirth. Arch. Womens Ment. Health 2021, 24, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Lin, Y.; Yang, L.; Liang, Q.; Fu, B.; Li, H.; Zhang, H.; Liu, Y. A comparison of maternal fear of childbirth, labor pain intensity and intrapartum analgesic consumption between primiparas and multiparas: A cross-sectional study. Int. J. Nurs. Sci. 2021, 8, 380–387. [Google Scholar] [CrossRef]
- Zeleke, T.A.; Getinet, W.; Tadesse Tessema, Z.; Gebeyehu, K. Prevalence and associated factors of post-partum depression in Ethiopia. A systematic review and meta-analysis. PLoS ONE 2021, 16, e0247005. [Google Scholar] [CrossRef]
- Shakarami, A.; Mirghafourvand, M.; Abdolalipour, S.; Jafarabadi, M.A.; Iravani, M. Comparison of fear, anxiety and self-efficacy of childbirth among primiparous and multiparous women. BMC Pregnancy Childbirth 2021, 21, 642. [Google Scholar] [CrossRef]
- Munk-Olsen, T.; Laursen, T.M.; Pedersen, C.B.; Mors, O.; Mortensen, P.B. New Parents and Mental Disorders: A Population-Based Register Study. JAMA 2006, 296, 2582–2589. [Google Scholar] [CrossRef] [Green Version]
- Harlow, B.L.; Vitonis, A.F.; Sparén, P.; Cnattingius, S.; Joffe, H.; Hultman, C.M. Incidence of hospitalization for postpartum psychotic and bipolar episodes in women with and without prior prepregnancy or prenatal psychiatric hospitalizations. Arch. Gen Psychiatry 2007, 64, 42–48. [Google Scholar] [CrossRef] [Green Version]
- Schuller, C.; Känel, N.; Müller, O.; Kind, A.B.; Tinner, E.M.; Hösli, I.; Zimmermann, R.; Surbek, D. Stress and pain response of neonates after spontaneous birth and vacuum-assisted and cesarean delivery. Am. J. Obst. Gynecol. 2012, 207, 416.e1–416.e6. [Google Scholar] [CrossRef]
- Iftimovici, R. Istoria universală a medicinei și farmaciei; Editura Academiei Române: București, Romania, 2015. [Google Scholar]
- Bellieni, C.V.; Buonocore, G. Is fetal pain a real evidence? J. Matern.-Fetal Neonatal Med. 2012, 25, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Pierucci, R. Fetal Pain: The Science Behind Why It Is the Medical Standard of Care. Linacre Q. 2020, 87, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.R.; Hall, S. L Trauma-informed Care in the Newborn Intensive Care Unit: Promoting Safety, Security and Connectedness. J. Perinatol. 2017, 38, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellieni Carlo, V.; Vannuccini Silvia, V.; Petraglia Felice, V. Is Fetal Analgesia Necessary during Prenatal Surgery? J. Matern -Fetal Neonatal Med. 2018, 31, 1241–1245. [Google Scholar] [CrossRef]
- Zwergel, C.; Kaisenberg, C. Maternal and Fetal Risks in Higher Multiple Cesarean Deliveries. In Recent Advances in Cesarean Delivery; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Dumpa, V.; Kamity, R. Birth Trauma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Słabuszewska-Jóźwiak, A.; Szymański, J.K.; Ciebiera, M.; Sarecka-Hujar, B.; Jakiel, G. Pediatrics Consequences of Caesarean Section-A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 8031. [Google Scholar] [CrossRef] [PubMed]
- LoMauro, A.; Aliverti, A. Physiology masterclass: Extremes of age: Newborn and infancy. Breathe 2016, 12, 65–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, A.H.; Fahley, J.T. The transition from fetal to neonatal circulation: Normal responses and implications for infants with heart disease. Semin. Perinatol. 1993, 17, 106–121. [Google Scholar] [PubMed]
- Wigert, H.; Nilsson, C.; Dencker, A.; Begley, C.; Jangsten, E.; Sparud-Lundin, C.; Mollberg, M.; Patel, H. Women’s experiences of fear of childbirth: A metasynthesis of qualitative studies. Int. J. Qual.Stud. Health Well-Being 2020, 15, 1704484. [Google Scholar] [CrossRef] [Green Version]
- Miller, Y.D.; Danoy-Monet, M. Reproducing fear: The effect of birth stories on nulligravid women’s birth preferences. BMC Pregnancy Childbirth 2021, 21, 451. [Google Scholar] [CrossRef]
- Coates, D.; Thirukumar, P.; Spear, V.; Brown, G.; Henry, A. What are women’s mode of birth preferences and why? A systematic scoping review. Women Birth 2020, 33, 323–333. [Google Scholar] [CrossRef]
- Fineschi, V.; Arcangeli, M.; Di Fazio, N.; Del Fante, Z.; Fineschi, B.; Santoro, P.; Frati, P. Defensive Medicine in the Management of Cesarean Delivery: A Survey among Italian Physicians. Healthcare 2021, 9, 1097. [Google Scholar] [CrossRef] [PubMed]
- Appendix: Creative Etymology: “Caesarean Section” from Pliny to Rousset. Available online: https://www.jstor.org/stable/10.7591/j.ctvn1tb31.9 (accessed on 14 December 2022).
- Boley, J.P. The history of caesarean section. Can. Med. Assoc. J. 1935, 32, 557–559. [Google Scholar]
- National Institute for Health and Clinical Excellence Cesarean Section. Available online: https://www.nice.org.uk/guidance/cg132/documents/cesarean-section-update-full-guideline2 (accessed on 14 December 2022).
- World Health Organization. Appropriate technology for birth. Lancet 1985, 2, 436–437. [Google Scholar]
- Thomas, J.; Paranjothy, S. Royal College of Obstetricians and Gynaecologists Clinical Effectiveness Support Unit; The National Sentinel Caesarean Section Audit Report; RCOG Press: London, UK, 2001. [Google Scholar]
- National Collaborating Centre for Women’s and Children’s Health. Caesarean Section: Clinical Guideline; RCOG Press: London, UK, 2004. [Google Scholar]
- Timor-Tritsch, I.E.; Monteagudo, A. Unforeseen consequences of the increasing rate of cesarean deliveries: Early placenta accreta and cesarean scar pregnancy. A review. Am. J. Obstet. Gynecol. 2012, 207, 14–29. [Google Scholar] [CrossRef]
- Gregory, K.D.; Jackson, S.; Korst, L.; Fridman, M. Cesarean versus vaginal delivery: Whose risks? Whose benefits? Am. J. Perinatol. 2012, 29, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Opiyo, N.; Kingdon, C.; Oladapo, O.T.; Souza, J.P.; Vogel, J.P.; Bonet, M.; Bucagu, M.; Portela, A.; McConville, F.; Downe, S.; et al. Non-clinical interventions to reduce unnecessary caesarean sections: WHO recommendations. Bull. World Health Organ. 2020, 98, 66–68. [Google Scholar] [CrossRef]
- Zwecker, P.; Azoulay, L.; Abenhaim, H.A. Effect of fear of litigation on obstetric care: A nationwide analysis on obstetric practice. Am. J. Perinatol. 2011, 28, 277–284. [Google Scholar] [CrossRef]
- Mi, J.; Liu, F. Rate of caesarean section is alarming in China. Lancet 2014, 383, 1463–1464. [Google Scholar] [CrossRef]
- Gibbons, L.; Belizan, J.M.; Lauer, J.A.; Betran, A.P.; Merialdi, M.; Althabe, F. Inequities in the use of cesarean section deliveries in the world. Am. J. Obstet. Gynecol. 2012, 206, 331.e1–331.e19. [Google Scholar] [CrossRef]
- Ronsmans, C.; Holtz, S.; Stanton, C. Socioeconomic differentials in caesarean rates in developing countries: A retrospective analysis. Lancet 2006, 368, 1516–1523. [Google Scholar] [CrossRef] [PubMed]
- Truven Health Analytics. The Cost of Having a Baby in the United States. 2013. Available online: http://transform.childbirthconnection.org/wp-content/uploads/2013/01/Cost-of-Having-aBaby-Executive-Summary.pdf (accessed on 14 December 2022).
- MacLellan, A.N.; Woolcott, C.G.; Brown, M.M.; Dodds, L.; McDonald, S.D.; Kuhle, S. Cesarean Delivery and Healthcare Utilization and Costs in the Offspring: A Retrospective Cohort Study. J. Pediatr. 2019, 209, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Etringer, A.P.; Pinto, M.F.T.; Gomes, M.A.S.M. Análise de custos da atenção hospitalar ao parto vaginal e à cesariana eletiva para gestantes de risco habitual no Sistema Único de Saúde. Cien Saude Colet 2019, 24, 1527–1536. [Google Scholar] [CrossRef] [Green Version]
- Blumenfeld, Y.J.; El-Sayed, Y.Y.; Lyell, D.J.; Nelson, L.M.; Butwick, A.J. Risk Factors for Prolonged Postpartum Length of Stay Following Cesarean Delivery. Am. J. Perinatol. 2015, 32, 825–832. [Google Scholar] [PubMed] [Green Version]
- Sobhy, S.; Arroyo-Manzano, D.; Murugesu, N.; Karthikeyan, G.; Kumar, V.; Kaur, I.; Fernandez, E.; Gundabattula, S.R.; Betran, A.P.; Khan, K.; et al. Maternal and perinatal mortality and complications associated with caesarean section in low-income and middle-income countries: A systematic review and meta-analysis. Lancet 2019, 393, 1973–1982. [Google Scholar] [CrossRef]
- Teigen, N.C.; Sahasrabudhe, N.; Doulaveris, G.; Xie, X.; Negassa, A.; Bernstein, J.; Bernstein, P.S. Enhanced recovery after surgery at cesarean delivery to reduce postoperative length of stay: A randomized controlled trial. Am. J. Obstet. Gynecol. 2020, 222, 372.e1–372.e10. [Google Scholar] [CrossRef] [PubMed]
- Gabbai, D.; Attali, E.; Ram, S.; Amikam, U.; Ashwal, E.; Hiersch, L.; Gamzu, R.; Yogev, Y. Prediction model for prolonged hospitalization following cesarean delivery. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 274, 23–27. [Google Scholar] [CrossRef]
- Vogt, S.E.; Silva, K.S.; Dias, M.A.B. Comparison of childbirth care models in publicmhospitals, Brazil. Rev. Saude Publica 2014, 48, 304–313. [Google Scholar] [CrossRef] [Green Version]
- Betran, A.P.; Ye, J.; Moller, A.B.; Souza, J.P.; Zhang, J. Trends and projections of caesarean section rates: Global and regional estimates. BMJ. Glob. Health 2021, 6, e005671. [Google Scholar] [CrossRef]
- Caesarean Section Rates Continue to Rise, Amid Growing Inequalities in Access. Available online: https://www.who.int/news/item/16-06-2021-caesarean-section-rates-continue-to-rise-amid-growing-inequalities-in-access (accessed on 14 December 2022).
- Sandall, J.; Tribe, R.M.; Avery, L.; Mola, G.; Visser, G.H.; Homer, C.S.; Gibbons, D.; Kelly, N.M.; Kennedy, H.P.; Kidanto, H.; et al. Short-term and long-term effects of caesarean section on the health of women and children. Lancet 2018, 392, 1349–1357. [Google Scholar] [CrossRef]
- Breastfeeding. Available online: https://www.who.int/news-room/facts-in-pictures/detail/breastfeeding (accessed on 14 December 2022).
- WHO Global Strategy for Infant and Young Child Feeding. Available online: http://apps.who.int/iris/bitstream/10665/42590/1/9241562218.pdf?ua=1&ua=1 (accessed on 20 October 2022).
- UNICEF; WHO. Capture the Moment: Early Initiation of Breastfeeding: The Best Start for Every Newborn; UNICEF: New York, NY, USA; World Health Organization: Geneva, Switzerland, 2018; Available online: https://www.unicef.org/reports/capture-moment (accessed on 20 October 2022).
- Karaahmet, A.Y.; Bilgiç, F.Ş. Breastfeeding success in the first 6 months of online breastfeeding counseling after cesarean delivery and its effect on anthropometric measurements of the baby: A randomized controlled study. Rev. Assoc. Med. Bras. 2022, 68, 1434–1440. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, A.J.; Mannion, C.A.; McDonald, S.W.; Brockway, M.; Tough, S.C. The impact of caesarean section on breastfeeding initiation, duration and difficulties in the first four months postpartum. BMC Pregnancy Childbirth 2016, 16, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juan, J.; Zhang, X.; Wang, X.; Liu, J.; Cao, Y.; Tan, L.; Gao, Y.; Qiu, Y.; Yang, H. Association between Skin-to-Skin Contact Duration after Caesarean Section and Breastfeeding Outcomes. Children 2022, 9, 1742. [Google Scholar] [CrossRef] [PubMed]
- Biler, A.; Ekin, A.; Ozcan, A.; Inan, A.H.; Vural, T.; Toz, E. Is it safe to have multiple repeat cesarean sections? A high volume tertiary care center experience. Pak. J. Med. Sci. 2017, 33, 1074–1079. [Google Scholar] [CrossRef]
- Narava, S.; Pokhriyal, S.C.; Singh, S.B.; Barpanda, S.; Bricker, L. Outcome of multiple cesarean sections in a tertiary maternity hospital in the United Arab Emirates: A retrospective analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 247, 143–148. [Google Scholar] [CrossRef]
- Keag, O.E.; Norman, J.E.; Stock, S.J. Long-term risks and benefits associated with cesarean delivery for mother, baby, and subsequent pregnancies: Systematic review and meta-analysis. PLoS Med. 2018, 15, e1002494. [Google Scholar] [CrossRef]
- Antoine, C.; Young, B.K. Cesarean section one hundred years 1920–2020: The Good, the Bad and the Ugly. J. Perinat. Med. 2020, 49, 5–16. [Google Scholar] [CrossRef]
- Gu, L.; Zhang, W.; Yang, W.; Liu, H. Systematic review and meta-analysis of whether cesarean section contributes to the incidence of allergic diseases in children: A protocol for systematic review and meta-analysis. Medicine 2019, 98, e18394. [Google Scholar] [CrossRef]
- Hoang, D.M.; Levy, E.I.; Vandenplas, Y. The impact of Caesarean section on the infant gut microbiome. Acta Paediatr. 2021, 110, 60–67. [Google Scholar] [CrossRef]
- Nobuta, Y.; Tsuji, S.; Kitazawa, J.; Hanada, T.; Nakamura, A.; Zen, R.; Amano, T.; Murakami, T. Decreased Fertility in Women with Cesarean Scar Syndrome Is Associated with Chronic Inflammation in the Uterine Cavity. Tohoku J. Exp Med. 2022, 258, 237–242. [Google Scholar] [CrossRef]
- Kamlin, C.O.; O’Donnell, C.P.; Davis, P.G.; Morley, C.J. Oxygen saturation in healthy infants immediately after birth. J. Pediatr. 2006, 148, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Iddrisu, M.; Khan, Z.H. Anesthesia for cesarean delivery: General or regional anesthesia—A systematic review. Ain-Shams J. Anesthesiol. 2021, 13, 1. [Google Scholar] [CrossRef]
- Bao, Y.; Zhang, T.; Li, L.; Zhou, C.; Liang, M.; Zhou, J.; Wang, C. A retrospective analysis of maternal complications and newborn outcomes of general anesthesia for cesarean delivery in a single tertiary hospital in China. BMC Anesthesiol. 2022, 22, 208. [Google Scholar] [CrossRef] [PubMed]
- Metogo, J.A.M.; Nana, T.N.; Ngongheh, B.A.; Nyuydzefon, E.B.; Adjahoung, C.A.; Tochie, J.N.; Minkande, J.Z. General versus regional anaesthesia for caesarean section indicated for acute foetal distress: A retrospective cohort study. BMC Anesthesiol. 2021, 21, 68. [Google Scholar] [CrossRef] [PubMed]
- Sung, T.Y.; Jee, Y.S.; You, H.J.; Cho, C.K. Comparison of the effect of general and spinal anesthesia for elective cesarean section on maternal and fetal outcomes: A retrospective cohort study. Anesth. Pain Med. 2021, 16, 49–55. [Google Scholar] [CrossRef]
- Knigin, D.; Avidan, A.; Weiniger, C.F. The effect of spinal hypotension and anesthesia-to-delivery time interval on neonatal outcomes in planned cesarean delivery. Am. J. Obstet. Gynecol. 2020, 223, 747.e1–747.e13. [Google Scholar] [CrossRef]
- Willfurth, I.; Baik-Schneditz, N.; Schwaberger, B.; Mileder, L.; Schober, L.; Urlesberger, B.; Pichler, G. Cerebral Oxygenation in Neonates Immediately after Cesarean Section and Mode of Maternal Anesthesia. Neonatology 2019, 116, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Ozgen, Z.S.U.; Toraman, F.; Erkek, E.; Sungur, T.; Guclu, P.; Durmaz, S.; Bilgili, C.O. Cesarean under general or epidural anesthesia: Does it differ in terms of regional cerebral oxygenation? Acta Anaesthesiol. Taiwan 2014, 52, 159–162. [Google Scholar] [CrossRef]
- Urlesberger, B.; Kratky, E.; Rehak, T.; Pocivalnik, M.; Avian, A.; Czihak, J.; Müller, W.; Pichler, G. Regional oxygen saturation of the brain during birth transition of term infants: Comparison between elective cesarean and vaginal deliveries. J. Pediatr. 2011, 159, 404–408. [Google Scholar] [CrossRef]
- Symonds, I.; Arulkumaran, S. Essential Obstetrics Gynaecology, 5th ed.; Livingstone, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; 173p. [Google Scholar]
- Hajjo, R.; Sabbah, D.A.; Al Bawab, A.Q. Unlocking the Potential of the Human Microbiome for Identifying Disease Diagnostic Biomarkers. Diagnostics 2022, 12, 1742. [Google Scholar] [CrossRef]
- Bozomitu, L.; Miron, I.; Adam Raileanu, A.; Lupu, A.; Paduraru, G.; Marcu, F.M.; Buga, A.M.L.; Rusu, D.C.; Dragan, F.; Lupu, V.V. The Gut Microbiome and Its Implication in the Mucosal Digestive Disorders. Biomedicines 2022, 10, 3117. [Google Scholar] [CrossRef] [PubMed]
- Darabi, B.; Rahmati, S.; HafeziAhmadi, M.R.; Badfar, G.; Azami, M. The association between caesarean section and childhood asthma: An updated systematic review and meta-analysis. Allergy Asthma Clin. Immunol. 2019, 15, 62. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.H. Revisiting the hygiene hypothesis for allergy and asthma. J. Allergy Clin. Immunol. 2015, 136, 860–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gürdeniz, G.; Ernst, M.; Rago, D.; Kim, M.; Courraud, J.; Stokholm, J.; Bønnelykke, K.; Björkbom, A.; Trivedi, U.; Sørensen, S.J.; et al. Neonatal metabolome of caesarean section and risk of childhood asthma. Eur. Resp. J. 2022, 59, 2102406. [Google Scholar] [CrossRef]
- Chu, S.; Zhang, Y.; Jiang, Y.; Sun, W.; Zhu, Q.; Wang, B.; Jiang, F.; Zhang, J. Cesarean section without medical indication and risks of childhood allergic disorder, attenuated by breastfeeding. Sci. Rep. 2017, 7, 9762. [Google Scholar] [CrossRef] [Green Version]
- Lavin, T.; Preen, D.B. Investigating Caesarean Section Birth as a Risk Factor for Childhood Overweight. Child Obes. 2018, 14, 131–138. [Google Scholar] [CrossRef]
- Zhang, S.; Qin, X.; Li, P.; Huang, K. Effect of Elective Cesarean Section on Children’s Obesity From Birth to Adolescence: A Systematic Review and Meta-Analysis. Front. Pediatr. 2022, 9, 793400. [Google Scholar] [CrossRef]
- Ficara, M.; Pietrella, E.; Spada, C.; Muttini, E.D.C.; Lucaccioni, L.; Iughetti, L.; Berardi, A. Changes of intestinal microbiota in early life. J. Matern.-Fetal Neonatal Med. 2020, 33, 1036–1043. [Google Scholar] [CrossRef]
- Zhang, T.; Sidorchuk, A.; Sevilla-Cermeño, L.; Vilaplana-Pérez, A.; Chang, Z.; Larsson, H.; Mataix-Cols, D.; de la Cruz, L.F. Association of Cesarean Delivery With Risk of Neurodevelopmental and Psychiatric Disorders in the Offspring: A Systematic Review and Meta-analysis. JAMA Netw. Open 2019, 2, e1910236. [Google Scholar] [CrossRef]
- Zhang, T.; Brander, G.; Mantel, Ä.; Kuja-Halkola, R.; Stephansson, O.; Chang, Z.; Larsson, H.; Mataix-Cols, D.; de la Cruz, L.F. Assessment of Cesarean Delivery and Neurodevelopmental and Psychiatric Disorders in the Children of a Population-Based Swedish Birth Cohort. JAMA Netw. Open 2021, 4, e210837. [Google Scholar] [CrossRef]
- Blazkova, B.; Pastorkova, A.; Solansky, I.; Veleminsky, M.; Veleminsky, M.; Rossnerova, A.; Honkova, K.; Rossner, P.; Sram, R. The Impact of Cesarean and Vaginal Delivery on Results of Psychological Cognitive Test in 5 Year Old Children. Medicina 2020, 56, 554. [Google Scholar] [CrossRef] [PubMed]
- Khadem, N.; Khadivzadeh, T. The intelligence quotient of school aged children delivered by cesarean section and vaginal delivery. Iran. J. Nurs. Midwifery Res. 2010, 15, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, Y.; Cai, R.; Li, Y.; Gu, B. A narrative review of relationship between gut microbiota and neuropsychiatric disorders: Mechanisms and clinical application of probiotics and prebiotics. Ann. Palliat. Med. 2021, 10, 2304–2313. [Google Scholar] [CrossRef] [PubMed]
| Associated Risk | Reference | ||
|---|---|---|---|
| Mother | Intraoperative | Anesthesia-associated risks | [84] |
| Infections | [69,75] | ||
| Organ injury (bladder, bowel, ureter, etc.) | [75] | ||
| Need for blood transfusion or hysterectomy | [84] | ||
| Postoperative | Thromboembolic complications (embolism, thrombosis) | [84] | |
| Pelvic adhesions | [85] | ||
| Persistent pain | [84] | ||
| Delayed initiation of breastfeeding | [80] | ||
| Newborn | Difficult adaptation to extrauterine life | [69,75] | |
| Altered immune system development | [75] | ||
| Increased risk of developing asthma and allergies | [86] | ||
| Reduced gut microbiome diversity | [87] | ||
| Future pregnancy | Intrauterine growth restriction | [69] | |
| Ectopic pregnancy | [75] | ||
| Preterm birth | [75] | ||
| Stillbirth | [75] | ||
| Spontaneous abortion | [84] | ||
| Uterine rupture | [85] | ||
| Abnormal placentation (placenta praevia, increta, or accreta) with possbile bleeding and need for blood transfusion and hysterectomy | [85] | ||
| Infertility | [84,87,88] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lupu, V.V.; Miron, I.C.; Raileanu, A.A.; Starcea, I.M.; Lupu, A.; Tarca, E.; Mocanu, A.; Buga, A.M.L.; Lupu, V.; Fotea, S. Difficulties in Adaptation of the Mother and Newborn via Cesarean Section versus Natural Birth—A Narrative Review. Life 2023, 13, 300. https://doi.org/10.3390/life13020300
Lupu VV, Miron IC, Raileanu AA, Starcea IM, Lupu A, Tarca E, Mocanu A, Buga AML, Lupu V, Fotea S. Difficulties in Adaptation of the Mother and Newborn via Cesarean Section versus Natural Birth—A Narrative Review. Life. 2023; 13(2):300. https://doi.org/10.3390/life13020300
Chicago/Turabian StyleLupu, Vasile Valeriu, Ingrith Crenguta Miron, Anca Adam Raileanu, Iuliana Magdalena Starcea, Ancuta Lupu, Elena Tarca, Adriana Mocanu, Ana Maria Laura Buga, Valeriu Lupu, and Silvia Fotea. 2023. "Difficulties in Adaptation of the Mother and Newborn via Cesarean Section versus Natural Birth—A Narrative Review" Life 13, no. 2: 300. https://doi.org/10.3390/life13020300
APA StyleLupu, V. V., Miron, I. C., Raileanu, A. A., Starcea, I. M., Lupu, A., Tarca, E., Mocanu, A., Buga, A. M. L., Lupu, V., & Fotea, S. (2023). Difficulties in Adaptation of the Mother and Newborn via Cesarean Section versus Natural Birth—A Narrative Review. Life, 13(2), 300. https://doi.org/10.3390/life13020300








