Thymic Epithelial Tumors: An Evolving Field
Abstract
:1. Introduction
2. Histopathological Classification of Thymic Epithelial Tumors
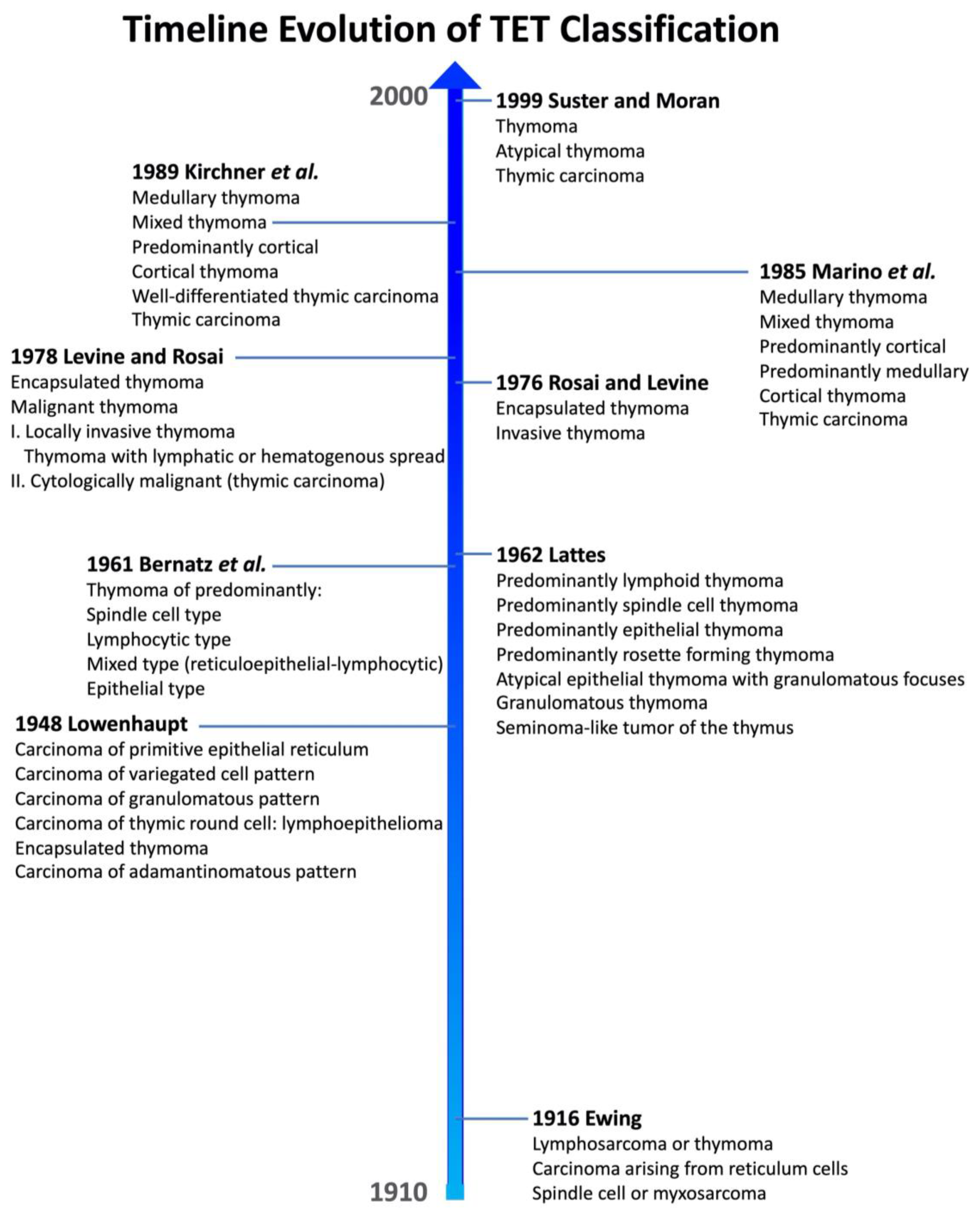
2.1. Historical Background
2.2. The WHO Classification
2.2.1. The Way to Current WHO Classification
2.2.2. Current 2021 WHO Classification
- Micronodular thymic carcinoma with lymphoid hyperplasia, the malignant counterpart of micronodular thymoma with lymphoid stroma;
- Hyalinizing clear cell carcinoma, analogous to that from the salivary glands;
- Thymic sebaceous carcinoma, similar to the homonym tumor of the skin.
3. Staging Systems
3.1. Historical Background
3.2. Main Staging Systems
3.2.1. TNM Staging
3.2.2. Masaoka–Koga Staging
3.2.3. Comparison between the TNM and Masaoka–Koga Staging Systems
3.3. Prognostic Factors
4. Molecular Pathology
TET Microenvironment
5. Diagnostic and Therapeutic Approaches
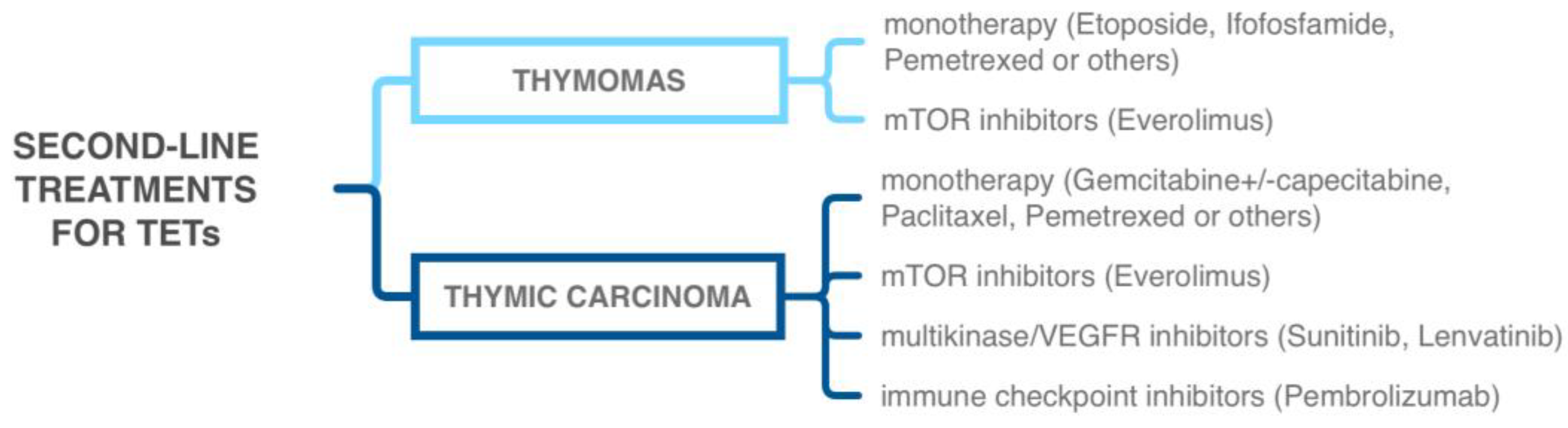
5.1. Tyrosine Kinase Inhibitors
5.2. Immune-Checkpoint Inhibitors
5.3. Other Targeted Therapies
6. Outlooks for the Future
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hsu, C.-H.; Chan, J.K.; Yin, C.-H.; Lee, C.-C.; Chern, C.-U.; Liao, C.-I. Trends in the incidence of thymoma, thymic carcinoma, and thymic neuroendocrine tumor in the United States. PLoS ONE 2019, 14, e0227197. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer; World Health Organization. Thoracic Tumours, 5th ed.; IARC Press: Lyon, France, 2021. [Google Scholar]
- Marx, A.; Willcox, N.; Leite, M.I.; Chuang, W.-Y.; Schalke, B.; Nix, W.; Ströbel, P. Thymoma and paraneoplastic myasthenia gravis. Autoimmunity 2010, 43, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Rosai, J.; Sobin, L.H. Histological Typing of Tumours of the Thymus. International Histological Classification of Tumours; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1999; 65p. [Google Scholar]
- Radovich, M.; Pickering, C.R.; Felau, I.; Ha, G.; Zhang, H.; Jo, H.; Hoadley, K.A.; Anur, P.; Zhang, J.; McLellan, M.; et al. The Integrated Genomic Landscape of Thymic Epithelial Tumors. Cancer Cell 2018, 33, 244–258.e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enkner, F.; Pichlhöfer, B.; Zaharie, A.T.; Krunic, M.; Holper, T.M.; Janik, S.; Moser, B.; Schlangen, K.; Neudert, B.; Walter, K.; et al. Molecular Profiling of Thymoma and Thymic Carcinoma: Genetic Differences and Potential Novel Therapeutic Targets. Pathol. Oncol. Res. 2017, 23, 551–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girard, N.; Basse, C.; Schrock, A.; Ramkissoon, S.; Killian, K.; Ross, J.S. Comprehensive Genomic Profiling of 274 Thymic Epithelial Tumors Unveils Oncogenic Pathways and Predictive Biomarkers. Oncologist 2022, 27, 919–929. [Google Scholar] [CrossRef]
- Tateo, V.; Manuzzi, L.; Parisi, C.; De Giglio, A.; Campana, D.; Pantaleo, M.; Lamberti, G. An Overview on Molecular Characterization of Thymic Tumors: Old and New Targets for Clinical Advances. Pharmaceuticals 2021, 14, 316. [Google Scholar] [CrossRef]
- Conforti, F.; Pala, L.; Giaccone, G.; De Pas, T. Thymic epithelial tumors: From biology to treatment. Cancer Treat. Rev. 2020, 86, 102014. [Google Scholar] [CrossRef]
- Giaccone, G.; Kim, C.; Thompson, J.; McGuire, C.; Kallakury, B.; Chahine, J.J.; Manning, M.; Mogg, R.; Blumenschein, W.M.; Tan, M.T.; et al. Pembrolizumab in patients with thymic carcinoma: A single-arm, single-centre, phase 2 study. Lancet Oncol. 2018, 19, 347–355. [Google Scholar] [CrossRef]
- Giaccone, G.; Kim, C. Durable Response in Patients With Thymic Carcinoma Treated With Pembrolizumab After Prolonged Follow-Up. J. Thorac. Oncol. 2021, 16, 483–485. [Google Scholar] [CrossRef]
- Ewing, J. The thymus and its tumors. Surg. Gynecol. Obstet. 1916, 22, 461–472. [Google Scholar]
- Rosai, J. Lowenhaupt’s embryology-based classification of thymic tumors and the concept of granulomatous thymoma. Cancer 1998, 82, 1209–1216. [Google Scholar] [CrossRef]
- Lowenhaupt, E. Tumors of the thymus in relation to the thymic epithelial anlage. Cancer 1948, 1, 547–563. [Google Scholar] [CrossRef]
- Bernatz, P.E.; Harrison, E.G.; Clagett, O.T. Thymoma: A clinicopathologic study. J. Thorac. Cardiovasc. Surg. 1961, 42, 424–444. [Google Scholar] [CrossRef] [PubMed]
- Lattes, R. Thymoma and other tumors of the thymus. An analysis of 107 cases. Cancer 1962, 15, 1224–1260. [Google Scholar] [CrossRef]
- Rosai, J.; Levine, G.D. Tumors of the Thymus: Atlas of Tumor Pathology; Second Series Fasc. 13. Armed Forces Institute of Pathology: Washington, DC, USA, 1976; 228p. [Google Scholar]
- Levine, G.D.; Rosai, J. Thymic hyperplasia and neoplasia: A review of current concepts. Hum. Pathol. 1978, 9, 495–515. [Google Scholar] [CrossRef]
- Marino, M.; Müller-Hermelink, H.K. Thymoma and thymic carcinoma. Relation of thymoma epithelial cells to the cortical and medullary differentiation of thymus. Virchows Arch. A Pathol. Anat. Histopathol. 1985, 407, 119–149. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, T.; Schalke, B.; Marx, A.; Müller-Hermelink, H.K. Evaluation of prognostic features in thymic epithelial tumors. Thymus 1989, 14, 195–203. [Google Scholar]
- Suster, S.; Moran, C.A. Thymoma, Atypical Thymoma, and Thymic Carcinoma: A Novel Conceptual Approach to the Classification of Thymic Epithelial Neoplasms. Am. J. Clin. Pathol. 1999, 111, 826–833. [Google Scholar] [CrossRef]
- Marino, M.; Roden, A.C. The evolution of the histopathologic classification of thymic epithelial tumors. Mediastinum 2018, 2, 9. [Google Scholar] [CrossRef]
- Rena, O.; Papalia, E.; Maggi, G.; Oliaro, A.; Ruffini, E.; Filosso, P.; Mancuso, M.; Novero, D.; Casadio, C. World Health Organization histologic classification: An independent prognostic factor in resected thymomas. Lung Cancer 2005, 50, 59–66. [Google Scholar] [CrossRef]
- Travis, W.D.; Brambilla, E.; Burke, A.P.; Marx, A.; Nicholson, A.G. Pathology and genetics of tumours of the lung, pleura, thymus, and heart. In World Health Organization Classification of Tumours; IARC Press: Lyon, France, 2004; 344p. [Google Scholar]
- Travis, W.; Brambilla, E.; Burke, A.P.; Marx, A.; Nicholson, A.G. WHO classification of tumours of the lung, pleura, thymus and heart. In World Health Organization Classification of Tumours, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2015; 412p. [Google Scholar]
- Di Tommaso, L.; Kuhn, E.; Kurrer, M.; Zettl, A.; Marx, A.; Müller-Hermelink, H.K.; Roncalli, M.; Rosai, J. Thymic tumor with adenoid cystic carcinomalike features: A clinicopathologic study of 4 cases. Am. J. Surg. Pathol. 2007, 31, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Filosso, P.L.; Ruffini, E.; Lausi, P.O.; Lucchi, M.; Oliaro, A.; Detterbeck, F. Historical perspectives: The evolution of the thymic epithelial tumors staging system. Lung Cancer 2014, 83, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Ruffini, E.; Rami-Porta, R.; Huang, J.; Ahmad, U.; Appel, S.; Bille, A.; Boubia, S.; Brambilla, C.; Cangir, A.K.; Cilento, V.; et al. The International Association for the Study of Lung Cancer Thymic Epithelial Tumor Staging Project: Unresolved Issues to be Addressed for the Next Ninth Edition of the TNM Classification of Malignant Tumors. J. Thorac. Oncol. 2022, 17, 838–851. [Google Scholar] [CrossRef]
- Bergh, N.P.; Gatzinsky, P.; Larsson, S.; Lundin, P.; Ridell, B. Tumors of the thymus and thymic region: I. Clinicopathological studies on thymomas. Ann. Thorac. Surg. 1978, 25, 91–98. [Google Scholar] [CrossRef]
- Masaoka, A.; Monden, Y.; Nakahara, K.; Tanioka, T. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981, 48, 2485–2492. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, Y.; Masaoka, A.; Hashimoto, T.; Niwa, H.; Mizuno, T.; Fujii, Y.; Nakahara, K. A tentative tumor-node-metastasis classification of thymoma. Cancer 1991, 68, 1984–1987. [Google Scholar] [CrossRef]
- Tsuchiya, R.; Koga, K.; Matsuno, Y.; Mukai, K.; Shimosato, Y. Thymic carcinoma: Proposal for pathological TNM and staging. Pathol. Int. 1994, 44, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Bedini, A.V.; Andreani, S.M.; Tavecchio, L.D.; Fabbri, A.; Giardini, R.; Camerini, T.; Bufalino, R.; Morabito, A.; Rosai, J. Proposal of a Novel System for the Staging of Thymic Epithelial Tumors. Ann. Thorac. Surg. 2005, 80, 1994–2000. [Google Scholar] [CrossRef]
- Ruffini, E.; Van Raemdonck, D.; Detterbeck, F.; Rocco, G.; Thomas, P.; Venuta, F. Management of Thymic Tumors: A Survey of Current Practice among Members of the European Society of Thoracic Surgeons. J. Thorac. Oncol. 2011, 6, 614–623. [Google Scholar] [CrossRef]
- Masaoka, A. Staging System of Thymoma. J. Thorac. Oncol. 2010, 5 (Suppl. 4), S304–S312. [Google Scholar] [CrossRef] [Green Version]
- Detterbeck, F.C.; Stratton, K.; Giroux, D.; Asamura, H.; Crowley, J.; Falkson, C.; Filosso, P.L.; Frazier, A.; Giaccone, G.; Huanget, J.; et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: Proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J. Thorac. Oncol. 2014, 9 (Suppl. 2), S65–S72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markowiak, T.; Hofmann, H.-S.; Ried, M. Classification and staging of thymoma. J. Thorac. Dis. 2020, 12, 7607–7612. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, A.G.; Detterbeck, F.; Marino, M.; Kim, J.; Stratton, K.; Giroux, D.; Asamura, H.; Crowley, J.; Falkson, C.; Filosso, P.L.; et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: Proposals for the T Component for the forthcoming (8th) edition of the TNM classification of malignant tumors. J. Thorac. Oncol. 2014, 9 (Suppl. 2), S73–S80. [Google Scholar] [CrossRef] [Green Version]
- Molina, T.J. Update on the TNM 8th Edition—Staging of thymic epithelial tumors, a pathologist’s perspective. Mediastinum 2022, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Asamura, H.; Nakagawa, K.; Matsuno, Y.; Suzuki, K.; Watanabe, S.-I.; Tsuchiya, R. Thymoma needs a new staging system. Interact. Cardiovasc. Thorac. Surg. 2004, 3, 163–167. [Google Scholar] [CrossRef] [Green Version]
- Blumberg, D.; Port, J.L.; Weksler, B.; Delgado, R.; Rosai, J.; Bains, M.S.; Ginsberg, R.J.; Martini, N.; McCormack, P.M.; Rusch, V.; et al. Thymoma: A multivariate analysis of factors predicting survival. Ann. Thorac. Surg. 1995, 60, 908–914. [Google Scholar] [CrossRef]
- Lewis, J.E.; Wick, M.R.; Scheithauer, B.W.; Bernatz, P.E.; Taylor, W.F. Thymoma. A clinicopathologic review. Cancer 1987, 60, 2727–2743. [Google Scholar] [CrossRef]
- Ruffini, E.; Detterbeck, F.; Van Raemdonck, D.; Rocco, G.; Thomas, P.; Weder, W.; Brunelli, A.; Evangelista, A.; Venuta, F.; Khaled, A.; et al. Tumours of the thymus: A cohort study of prognostic factors from the European Society of Thoracic Surgeons database. Eur. J. Cardio-Thorac. Surg. 2014, 46, 361–368. [Google Scholar] [CrossRef] [Green Version]
- Bian, D.; Zhou, F.; Yang, W.; Zhang, K.; Chen, L.; Jiang, G.; Zhang, P.; Wu, C.; Fei, K.; Zhang, L. Thymoma size significantly affects the survival, metastasis and effectiveness of adjuvant therapies: A population based study. Oncotarget 2018, 9, 12273–12283. [Google Scholar] [CrossRef] [Green Version]
- Okumura, M.; Yoshino, I.; Yano, M.; Watanabe, S.-I.; Tsuboi, M.; Yoshida, K.; Date, H.; Yokoi, K.; Nakajima, J.; Toyooka, S.-I.; et al. Tumour size determines both recurrence-free survival and disease-specific survival after surgical treatment for thymoma. Eur. J. Cardio-Thorac. Surg. 2019, 56, 174–181. [Google Scholar] [CrossRef]
- Cangir, A.K.; Yenigün, B.M.; Direk, T.; Kocaman, G.; Yücemen, U.; Kahya, Y.; Sak, S.D.; Enön, S. Different View on Tumor Size Dilemma in Tumor-Node-Metastasis Staging System for Thymoma. Thorac. Cardiovasc. Surg. 2021, 69, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Koga, K.; Matsuno, Y.; Noguchi, M.; Mukai, K.; Asamura, H.; Goya, T.; Shimosato, Y. A review of 79 thymomas: Modification of staging system and reappraisal of conventional division into invasive and non-invasive thymoma. Pathol. Int. 1994, 44, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Roden, A.C. Evolution of Classification of Thymic Epithelial Tumors in the Era of Dr Thomas V. Colby. Arch. Pathol. Lab. Med. 2017, 141, 232–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondo, K.; Monden, Y. Therapy for thymic epithelial tumors: A clinical study of 1320 patients from Japan. Ann. Thorac. Surg. 2003, 76, 878–884. [Google Scholar] [CrossRef]
- Detterbeck, F.C.; Nicholson, A.G.; Kondo, K.; Van Schil, P.; Moran, C. The Masaoka-Koga Stage Classification for Thymic Malignancies: Clarification and Definition of Terms. J. Thorac. Oncol. 2011, 6 (Suppl. 3), S1710–S1716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weis, C.-A.; Yao, X.; Deng, Y.; Detterbeck, F.C.; Marino, M.; Nicholson, A.G.; Huang, J.; Ströbel, P.; Antonicelli, A.; Marx, A. The Impact of Thymoma Histotype on Prognosis in a Worldwide Database. J. Thorac. Oncol. 2015, 10, 367–372. [Google Scholar] [CrossRef] [Green Version]
- Safieddine, N.; Liu, G.; Cuningham, K.; Ming, T.; Hwang, D.; Brade, A.; Bezjak, A.; Fischer, S.; Xu, W.; Azad, S.; et al. Prognostic Factors for Cure, Recurrence and Long-Term Survival After Surgical Resection of Thymoma. J. Thorac. Oncol. 2014, 9, 1018–1022. [Google Scholar] [CrossRef] [Green Version]
- Tseng, Y.C.; Tseng, Y.-H.; Kao, H.-L.; Hsieh, C.-C.; Chou, T.-Y.; Goan, Y.-G.; Hsu, W.-H.; Hsu, H.-S. Long term oncological outcome of thymoma and thymic carcinoma—An analysis of 235 cases from a single institution. PLoS ONE 2017, 12, e0179527. [Google Scholar]
- Valdivia, D.; Cheufou, D.; Fels, B.; Puhlvers, S.; Mardanzai, K.; Zaatar, M.; Weinreich, G.; Taube, C.; Theegarten, D.; Stuschke, M.; et al. Potential Prognostic Value of Preoperative Leukocyte Count, Lactate Dehydrogenase and C-Reactive Protein in Thymic Epithelial Tumors. Pathol. Oncol. Res. 2021, 27, 629993. [Google Scholar] [CrossRef]
- Muriana, P.; Carretta, A.; Ciriaco, P.; Bandiera, A.; Negri, G. Assessment of the prognostic role of neutrophil-to-lymphocyte ratio following complete resection of thymoma. J. Cardiothorac. Surg. 2018, 13, 119. [Google Scholar] [CrossRef]
- Comin, C.E.; Messerini, L.; Novelli, L.; Boddi, V.; Dini, S. KI-67 antigen expression predicts survival and correlates with histologic subtype in the WHO classification of thymic epithelial tumors. Int. J. Surg. Pathol. 2004, 12, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-F.; Yan, J.-J.; Jin, Y.-T.; Su, I.-J. Detection of bcl-2 and p53 in thymoma: Expression of bcl-2 as a reliable marker of tumor aggressiveness. Hum. Pathol. 1996, 27, 1089–1092. [Google Scholar] [CrossRef] [PubMed]
- Sogawa, K.-I.; Kondo, K.; Fujino, H.; Takahashi, Y.; Miyoshi, T.; Sakiyama, S.; Mukai, K.; Monden, Y. Increased expression of matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 is correlated with poor prognostic variables in patients with thymic epithelial tumors. Cancer 2003, 98, 1822–1829. [Google Scholar] [CrossRef] [PubMed]
- Mimori, T.; Shukuya, T.; Ko, R.; Okuma, Y.; Koizumi, T.; Imai, H.; Takiguchi, Y.; Miyauchi, E.; Kagamu, H.; Sugiyama, T.; et al. Clinical Significance of Tumor Markers for Advanced Thymic Carcinoma: A Retrospective Analysis from the NEJ023 Study. Cancers 2022, 14, 331. [Google Scholar] [CrossRef] [PubMed]
- Leisibach, P.; Schneiter, D.; Soltermann, A.; Yamada, Y.; Weder, W.; Jungraithmayr, W. Prognostic value of immunohistochemical markers in malignant thymic epithelial tumors. J. Thorac. Dis. 2016, 8, 2580–2591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhn, E.; Wistuba, I.I. Molecular Pathology of Thymic Epithelial Neoplasms. Hematol. Clin. N. Am. 2008, 22, 443–455. [Google Scholar] [CrossRef]
- Rajan, A.; Girard, N.; Marx, A. State of the Art of Genetic Alterations in Thymic Epithelial Tumors. J. Thorac. Oncol. 2014, 9 (Suppl. 2), S131–S136. [Google Scholar] [CrossRef] [Green Version]
- Petrini, I.; Meltzer, P.S.; Kim, I.-K.; Lucchi, M.; Park, K.-S.; Fontanini, G.; Gao, J.; Zucali, P.A.; Calabrese, F.; Favaretto, A.; et al. A specific missense mutation in GTF2I occurs at high frequency in thymic epithelial tumors. Nat. Genet. 2014, 46, 844–849. [Google Scholar] [CrossRef] [Green Version]
- Petrini, I.; Meltzer, P.S.; Zucali, P.A.; Luo, J.; Lee, C.; Santoro, A.; Lee, H.S.; Killian, K.J.; Wang, Y.; Tsokos, M.; et al. Copy number aberrations of BCL2 and CDKN2A/B identified by array-CGH in thymic epithelial tumors. Cell Death Dis. 2012, 3, e351. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Yin, N.; Yin, B.; Lu, Q. DNA methylation in thoracic neoplasms. Cancer Lett. 2011, 301, 7–16. [Google Scholar] [CrossRef]
- Mokhtar, M.; Kondo, K.; Namura, T.; Ali, A.H.; Fujita, Y.; Takai, C.; Takizawa, H.; Nakagawa, Y.; Toba, H.; Kajiura, K.; et al. Methylation and expression profiles of MGMT gene in thymic epithelial tumors. Lung Cancer 2014, 83, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; Jang, H.-J.; Shah, R.; Yoon, D.; Hamaji, M.; Wald, O.; Lee, J.-S.; Sugarbaker, D.J.; Burt, B.M. Genomic Analysis of Thymic Epithelial Tumors Identifies Novel Subtypes Associated with Distinct Clinical Features. Clin. Cancer Res. 2017, 23, 4855–4864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, A.L. Role of the multifunctional transcription factor TFII-I in DNA damage repair. DNA Repair 2021, 106, 103175. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yuan, Y.; Xiao, H.; Dai, J.; Ye, Y.; Zhang, Q.; Zhang, Z.; Jiang, Y.; Luo, J.; Hu, J.; et al. Discovery and validation of DNA methylation markers for overall survival prognosis in patients with thymic epithelial tumors. Clin. Epigenet. 2019, 11, 38. [Google Scholar] [CrossRef] [Green Version]
- Bi, Y.; Meng, Y.; Niu, Y.; Li, S.; Liu, H.; He, J.; Zhang, Y.; Liang, N.; Liu, L.; Mao, X.; et al. Genome-wide DNA methylation profile of thymomas and potential epigenetic regulation of thymoma subtypes. Oncol. Rep. 2019, 41, 2762–2774. [Google Scholar] [CrossRef]
- Hirabayashi, H.; Fujii, Y.; Sakaguchi, M.; Tanaka, H.; Yoon, H.E.; Komoto, Y.; Inoue, M.; Miyoshi, S.; Matsuda, H. p16INK4, pRB, p53 and cyclin D1 expression and hypermethylation of CDKN2 gene in thymoma and thymic carcinoma. Int. J. Cancer 1997, 73, 639–644. [Google Scholar] [CrossRef]
- Lopomo, A.; Ricciardi, R.; Maestri, M.; De Rosa, A.; Melfi, F.; Lucchi, M.; Mussi, A.; Coppedè, F.; Migliore, L. Gene-Specific Methylation Analysis in Thymomas of Patients with Myasthenia Gravis. Int. J. Mol. Sci. 2016, 17, 2121. [Google Scholar] [CrossRef] [Green Version]
- Hirose, Y.; Kondo, K.; Takizawa, H.; Nagao, T.; Nakagawa, Y.; Fujino, H.; Toba, H.; Kenzaki, K.; Sakiyama, S.; Tangoku, A. Aberrant methylation of tumour-related genes in thymic epithelial tumours. Lung Cancer 2009, 64, 155–159. [Google Scholar] [CrossRef]
- Psilopatis, I.; Pergaris, A.; Vrettou, K.; Theocharis, S.; Troungos, C. Thymic Epithelial Neoplasms: Focusing on the Epigenetic Alterations. Int. J. Mol. Sci. 2022, 23, 4045. [Google Scholar] [CrossRef]
- Ganci, F.; Vico, C.; Korita, E.; Sacconi, A.; Gallo, E.; Mori, F.; Cambria, A.; Russo, E.; Anile, M.; Vitolo, D.; et al. MicroRNA expression profiling of thymic epithelial tumors. Lung Cancer 2014, 85, 197–204. [Google Scholar] [CrossRef]
- Radovich, M.; Solzak, J.P.; Hancock, B.A.; Conces, M.L.; Atale, R.; Porter, R.F.; Zhu, J.; Glasscock, J.; Kesler, K.A.; Badve, S.S.; et al. A large microRNA cluster on chromosome 19 is a transcriptional hallmark of WHO type A and AB thymomas. Br. J. Cancer 2016, 114, 477–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Chen, Y.; Xu, S.; Yang, Y.; Wei, D.; Wang, W.; Huang, X. Aberrant decrease of microRNA19b regulates TSLP expression and contributes to Th17 cells development in myasthenia gravis related thymomas. J. Neuroimmunol. 2015, 288, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Ren, R.; Fang, X. Identification and Characterization of Non-Coding RNAs in Thymoma. Experiment 2021, 27, e929727. [Google Scholar] [CrossRef]
- Roden, A.C.; Erickson-Johnson, M.R.; Yi, E.S.; García, J.J. Analysis of MAML2 rearrangement in mucoepidermoid carcinoma of the thymus. Hum. Pathol. 2013, 44, 2799–2805. [Google Scholar] [CrossRef]
- Lee, J.-K.; Louzada, S.; An, Y.; Kim, S.; Youk, J.; Park, S.; Koo, S.; Keam, B.; Jeon, Y.; Ku, J.-L.; et al. Complex chromosomal rearrangements by single catastrophic pathogenesis in NUT midline carcinoma. Ann. Oncol. 2017, 28, 890–897. [Google Scholar] [CrossRef]
- Porubsky, S.; Rudolph, B.; Rückert, J.; Küffer, S.; Ströbel, P.; Roden, A.C.; Jain, D.; Tousseyn, T.; Van Veer, H.; Huang, J.; et al. EWSR1 translocation in primary hyalinising clear cell carcinoma of the thymus. Histopathology 2019, 75, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Massoth, L.R.; Hung, Y.P.; Dias-Santagata, D.; Onozato, M.; Shah, N.; Severson, E.; Duncan, D.; Gillespie, B.J.; Williams, N.F.; Ross, J.S.; et al. Pan-Cancer Landscape Analysis Reveals Recurrent KMT2A-MAML2 Gene Fusion in Aggressive Histologic Subtypes of Thymoma. JCO Precis. Oncol. 2020, 4, 109–115. [Google Scholar] [CrossRef]
- O’Neill, I.D. Gefitinib as targeted therapy for mucoepidermoid carcinoma of the lung: Possible significance of CRTC1–MAML2 oncogene. Lung Cancer 2009, 64, 129–130. [Google Scholar] [CrossRef]
- Vivero, M.; Davineni, P.; Nardi, V.; Chan, J.K.C.; Sholl, L.M. Metaplastic thymoma: A distinctive thymic neoplasm characterized by YAP1-MAML2 gene fusions. Mod. Pathol. 2020, 33, 560–565. [Google Scholar] [CrossRef]
- Yu, L.; Ke, J.; Du, X.; Yu, Z.; Di Gao, D. Genetic characterization of thymoma. Sci. Rep. 2019, 9, 2369. [Google Scholar] [CrossRef] [Green Version]
- Manca, N.; Perandin, F.; De Simone, N.; Giannini, F.; Bonifati, D.; Angelini, C. Detection of HTLV-I tax-rex and pol gene sequences of thymus gland in a large group of patients with myasthenia gravis. J. Acquir. Immune Defic. Syndr. 2002, 29, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chen, S.; Cheng, X.; Xu, B.; Zeng, H.; Zou, J.; Su, C.; Chen, Z. Characteristics of genomic mutations and signaling pathway alterations in thymic epithelial tumors. Ann. Transl. Med. 2021, 9, 1659. [Google Scholar] [CrossRef]
- Wang, Y.; Thomas, A.; Lau, C.; Rajan, A.; Zhu, Y.; Killian, J.K.; Petrini, I.; Pham, T.; Morrow, B.; Zhong, X.; et al. Mutations of epigenetic regulatory genes are common in thymic carcinomas. Sci. Rep. 2014, 4, 7336. [Google Scholar] [CrossRef] [PubMed]
- Thomas de Montpréville, V.; Ghigna, M.-R.; Lacroix, L.; Besse, B.; Broet, P.; Dartevelle, P.; Fadel, E.; Dorfmuller, P. Thymic carcinomas: Clinicopathologic study of 37 cases from a single institution. Virchows Arch. 2013, 462, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Fujiwara, Y.; Asao, T.; Honda, T.; Shimada, Y.; Kanai, Y.; Tsuta, K.; Kono, K.; Watanabe, S.; Ohe, Y.; et al. The genomic and epigenomic landscape in thymic carcinoma. Carcinogenesis 2017, 38, 1084–1091. [Google Scholar] [CrossRef]
- Umemura, S.; Zhu, J.; Chahine, J.J.; Kallakury, B.; Chen, V.; Kim, I.-K.; Zhang, Y.-W.; Goto, K.; He, Y.; Giaccone, G. Downregulation of CYLD promotes IFN-γ mediated PD-L1 expression in thymic epithelial tumors. Lung Cancer 2020, 147, 221–228. [Google Scholar] [CrossRef]
- Moreira, A.L.; Won, H.H.; McMillan, R.; Huang, J.; Riely, G.J.; Ladanyi, M.; Berger, M.F. Massively Parallel Sequencing Identifies Recurrent Mutations in TP53 in Thymic Carcinoma Associated with Poor Prognosis. J. Thorac. Oncol. 2015, 10, 373–380. [Google Scholar] [CrossRef] [Green Version]
- Masaoutis, C.; Palamaris, K.; Kokkali, S.; Levidou, G.; Theocharis, S. Unraveling the Immune Microenvironment of Thymic Epithelial Tumors: Implications for Autoimmunity and Treatment. Int. J. Mol. Sci. 2022, 23, 7864. [Google Scholar] [CrossRef]
- Hou, X.; Lin, S.; Liu, Y.; Wang, K.; Yu, Z.; Jia, J.; Yu, J.; Zheng, W.; Bai, J.; Chang, L.; et al. Analysis of the tumor microenvironment and mutation burden identifies prognostic features in thymic epithelial tumors. Am. J. Cancer Res. 2022, 12, 2387–2396. [Google Scholar]
- Kang, R.; Chen, R.; Zhang, Q.; Hou, W.; Wu, S.; Cao, L.; Huang, J.; Yu, Y.; Fan, X.-G.; Yan, Z.; et al. HMGB1 in health and disease. Mol. Asp. Med. 2014, 40, 1–116. [Google Scholar]
- Fend, F.; Kirchner, T.; Marx, A.; Müller-Hermelink, H.-K. B-cells in thymic epithelial tumours. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1993, 63, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Sato, J.; Fujiwara, M.; Kawakami, T.; Sumiishi, A.; Sakata, S.; Sakamoto, A.; Kurata, A. Fascin expression in dendritic cells and tumor epithelium in thymoma and thymic carcinoma. Oncol. Lett. 2011, 2, 1025–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omatsu, M.; Kunimura, T.; Mikogami, T.; Shiokawa, A.; Nagai, T.; Masunaga, A.; Kitami, A.; Suzuki, T.; Kadokura, M. Difference in distribution profiles between CD163+ tumor-associated macrophages and S100+ dendritic cells in thymic epithelial tumors. Diagn. Pathol. 2014, 9, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Xu, Q.; Kaul, D.; Ismail, M.; Badakhshi, H. Significance of tumor mutation burden and immune infiltration in thymic epithelial tumors. Thorac. Cancer 2021, 12, 1995–2006. [Google Scholar] [CrossRef] [PubMed]
- Agrafiotis, A.C.; Siozopoulou, V.; Hendriks, J.M.H.; Pauwels, P.; Koljenovic, S.; Van Schil, P.E. Tumor Microenvironment in Thymic Epithelial Tumors: A Narrative Review. Cancers 2022, 14, 6082. [Google Scholar] [CrossRef]
- Janik, S.; Schiefer, A.I.; Bekos, C.; Hacker, P.; Haider, T.; Moser, J.; Klepetko, W.; Müllauer, L.; Ankersmit, H.J.; Moser, B. HSP27 and 70 expression in thymic epithelial tumors and benign thymic alterations: Diagnostic, prognostic and physiologic implications. Sci. Rep. 2016, 6, 24267. [Google Scholar] [CrossRef] [Green Version]
- Rick, J.W.; Chandra, A.; Ore, C.D.; Nguyen, A.T.; Yagnik, G.; Aghi, M.K. Fibronectin in malignancy: Cancer-specific alterations, protumoral effects, and therapeutic implications. Semin. Oncol. 2019, 46, 284–290. [Google Scholar] [CrossRef]
- Chiappetta, M.; Mendogni, P.; Cattaneo, M.; Evangelista, J.; Farina, P.; Pizzuto, D.A.; Annunziata, S.; Castello, A.; Congedo, M.T.; Tabacco, D.; et al. Is PET/CT Able to Predict Histology in Thymic Epithelial Tumours? A Narrative Review. Diagnostics 2022, 13, 98. [Google Scholar] [CrossRef]
- Mendogni, P.; Toker, A.; Moser, B.; Trancho, F.H.; Aigner, C.; Bravio, I.G.; Novoa, N.M.; Molins, L.; Costardi, L.; Voltolini, L.; et al. Surgical resection of Masaoka stage III thymic epithelial tumours with great vessels involvement: A retrospective multicentric analysis from the European Society of Thoracic Surgeons thymic database. Eur. J. Cardio-Thorac. Surg. 2022, 62, ezac02. [Google Scholar] [CrossRef]
- Tosi, D.; Damarco, F.; Franzi, S.; Mohamed, S.; Palleschi, A.; Mendogni, P. Outcomes of extended surgical resections for locally advanced thymic malignancies: A narrative review. Gland. Surg. 2022, 11, 611–621. [Google Scholar] [CrossRef]
- Bruni, A.; Stefani, A.; Perna, M.; Borghetti, P.; Levra, N.G.; D’Angelo, E.; D’Onofrio, A.; Rubino, L.; Frassinelli, L.; Salvestrini, V.; et al. The role of postoperative radiotherapy for thymomas: A multicentric retrospective evaluation from three Italian centers and review of the literature. J. Thorac. Dis. 2020, 12, 7518–7530. [Google Scholar] [CrossRef] [PubMed]
- Hamaji, M.; Shah, R.M.; Ali, S.O.; Bettenhausen, A.; Lee, H.-S.; Burt, B.M. A Meta-Analysis of Postoperative Radiotherapy for Thymic Carcinoma. Ann. Thorac. Surg. 2017, 103, 1668–1675. [Google Scholar] [CrossRef] [Green Version]
- Giaccone, G.; Lischalk, J.W. Best practices and guidelines for the management of thymic epithelial tumors. Mediastinum 2019, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Petrini, I.; Zucali, P.A.; Lee, H.S.; Pineda, M.A.; Meltzer, P.S.; Walter-Rodriguez, B.; Roncalli, M.; Santoro, A.; Wang, Y.; Giaccone, G. Expression and Mutational Status of c-kit in Thymic Epithelial Tumors. J. Thorac. Oncol. 2010, 5, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Yoh, K.; Nishiwaki, Y.; Ishii, G.; Goto, K.; Kubota, K.; Ohmatsu, H.; Niho, S.; Nagai, K.; Saijo, N. Mutational status of EGFR and KIT in thymoma and thymic carcinoma. Lung Cancer 2008, 62, 316–320. [Google Scholar] [CrossRef]
- Tsuchida, M.; Umezu, H.; Hashimoto, T.; Shinohara, H.; Koike, T.; Hosaka, Y.; Eimoto, T.; Hayashi, J.-I. Absence of gene mutations in KIT-positive thymic epithelial tumors. Lung Cancer 2008, 62, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Schirosi, L.; Nannini, N.; Nicoli, D.; Cavazza, A.; Valli, R.; Buti, S.; Garagnani, L.; Sartori, G.; Calabrese, F.; Marchetti, A.; et al. Activating c-KIT mutations in a subset of thymic carcinoma and response to different c-KIT inhibitors. Ann. Oncol. 2012, 23, 2409–2414. [Google Scholar] [CrossRef]
- Palmieri, G.; Marino, M.; Buonerba, C.; Federico, P.; Conti, S.; Milella, M.; Petillo, L.; Evoli, A.; Lalle, M.; Ceribelli, A.; et al. Imatinib mesylate in thymic epithelial malignancies. Cancer Chemother. Pharmacol. 2011, 69, 309–315. [Google Scholar] [CrossRef]
- Salter, J.T.; Lewis, D.; Yiannoutsos, C.; Loehrer, P.J.; Risley, L.; Chiorean, E.G. Imatinib for the treatment of thymic carcinoma. J. Clin. Oncol. 2008, 26 (Suppl. 15), 8116. [Google Scholar] [CrossRef]
- Giaccone, G.; Rajan, A.; Ruijter, R.; Smit, E.; van Groeningen, C.; Hogendoorn, P. Imatinib Mesylate in Patients with WHO B3 Thymomas and Thymic Carcinomas. J. Thorac. Oncol. 2009, 4, 1270–1273. [Google Scholar] [CrossRef] [Green Version]
- Ströbel, P.; Hartmann, M.; Jakob, A.; Mikesch, K.; Brink, I.; Dirnhofer, S.; Marx, A. Thymic Carcinoma with Overexpression of Mutated KIT and the Response to Imatinib. N. Engl. J. Med. 2004, 350, 2625–2626. [Google Scholar] [CrossRef] [PubMed]
- Rossi, V.; Donini, M.; Sergio, P.; Passalacqua, R.; Rossi, G.; Buti, S. When a thymic carcinoma “becomes” a GIST. Lung Cancer 2013, 80, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Hirai, F.; Edagawa, M.; Shimamatsu, S.; Toyozawa, R.; Toyokawa, G.; Nosaki, K.; Yamaguchi, M.; Seto, T.; Twakenoyama, M.; Ichinose, Y. c-kit mutation-positive advanced thymic carcinoma successfully treated as a mediastinal gastrointestinal stromal tumor: A case report. Mol. Clin. Oncol. 2016, 4, 527–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmieri, G. Cetuximab is an active treatment of metastatic and chemorefractory thymoma. Front. Biosci. 2007, 12, 757–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farina, G.; Garassino, M.C.; Gambacorta, M.; La Verde, N.; Gherardi, G.; Scanni, A. Response of thymoma to cetuximab. Lancet Oncol. 2007, 8, 449–450. [Google Scholar] [CrossRef] [PubMed]
- Weissferdt, A.; Lin, H.; Woods, D.; Tang, X.; Fujimoto, J.; Wistuba, I.I.; Moran, C.A. HER family receptor and ligand status in thymic carcinoma. Lung Cancer 2012, 77, 515–521. [Google Scholar] [CrossRef]
- Pan, C.C.; Chen, P.C.-H.; Wang, L.-S.; Lee, J.-Y.; Chiang, H. Expression of apoptosis-related markers and HER-2/neu in thymic epithelial tumours. Histopathology 2003, 43, 165–172. [Google Scholar] [CrossRef]
- Thomas, A.; Rajan, A.; Berman, A.; Tomita, Y.; Brzezniak, C.; Lee, M.-J.; Lee, S.; Ling, A.; Spittler, A.J.; Carter, C.A.; et al. Sunitinib in patients with chemotherapy-refractory thymoma and thymic carcinoma: An open-label phase 2 trial. Lancet Oncol. 2015, 16, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Tomita, M.; Matsuzaki, Y.; Edagawa, M.; Maeda, M.; Shimizu, T.; Hara, M.; Onitsuka, T. Correlation between tumor angiogenesis and invasiveness in thymic epithelial tumors. J. Thorac. Cardiovasc. Surg. 2002, 124, 493–498. [Google Scholar] [CrossRef] [Green Version]
- Antonarelli, G.; Corti, C.; Zucali, P.A.; Perrino, M.; Manglaviti, S.; Russo, G.L.; Varano, G.M.; Salvini, P.; Curigliano, G.; Catania, C.; et al. Continuous sunitinib schedule in advanced platinum refractory thymic epithelial neoplasms: A retrospective analysis from the ThYmic MalignanciEs (TYME) Italian collaborative group. Eur. J. Cancer 2022, 174, 31–36. [Google Scholar] [CrossRef]
- Remon, J.; Girard, N.; Mazieres, J.; Dansin, E.; Pichon, E.; Greillier, L.; Dubos, C.; Lindsay, C.R.; Besse, B. Sunitinib in patients with advanced thymic malignancies: Cohort from the French RYTHMIC network. Lung Cancer 2016, 97, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Sato, J.; Satouchi, M.; Itoh, S.; Okuma, Y.; Niho, S.; Mizugaki, H.; Murakami, H.; Fujisaka, Y.; Kozuki, T.; Nakamura, K.; et al. Lenvatinib in patients with advanced or metastatic thymic carcinoma (REMORA): A multicentre, phase 2 trial. Lancet Oncol. 2020, 21, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Perrino, M.; De Pas, T.; Bozzarelli, S.; Giordano, L.; De Vincenzo, F.; Conforti, F.; Digiacomo, N.; Cordua, N.; D’Antonio, F.; Borea, F.; et al. Resound Trial: A phase 2 study of regorafenib in patients with thymoma (type B2-B3) and thymic carcinoma previously treated with chemotherapy. Cancer 2022, 128, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Alberobello, A.T.; Wang, Y.; Beerkens, F.J.; Conforti, F.; McCutcheon, J.N.; Rao, G.; Raffeld, M.; Liu, J.; Rahhal, R.; Zhang, Y.-W.; et al. PI3K as a Potential Therapeutic Target in Thymic Epithelial Tumors. J. Thorac. Oncol. 2016, 11, 1345–1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmieri, G.; Buonerba, C.; Federico, P.; Formisano, L.; Nappi, L.; Di Lorenzo, G.; Marino, M.; Damiano, V.; Federico, P. Everolimus plus long-acting somatostatin analogs in thymic epithelial malignancies. World J. Clin. Oncol. 2012, 3, 111–115. [Google Scholar] [CrossRef]
- Zucali, P.A.; De Pas, T.; Palmieri, G.; Favaretto, A.; Chella, A.; Tiseo, M.; Caruso, M.; Simonelli, M.; Perrino, M.; De Vincenzo, F.; et al. Phase II Study of Everolimus in Patients With Thymoma and Thymic Carcinoma Previously Treated With Cisplatin-Based Chemotherapy. J. Clin. Oncol. 2017, 36, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Abu Zaid, M.I.; Radovich, M.; Althouse, S.; Liu, H.; Spittler, A.J.; Solzak, J.; Badve, S.; Loehrer, P.J.S. A phase II study of buparlisib in relapsed or refractory thymomas. Front. Oncol. 2022, 12, 891383. [Google Scholar] [CrossRef]
- Padda, S.K.; Riess, J.W.; Schwartz, E.J.; Tian, L.; Kohrt, H.E.; Neal, J.W.; West, R.B.; Wakelee, H.A. Diffuse High Intensity PD–L1 Staining in Thymic Epithelial Tumors. J. Thorac. Oncol. 2015, 10, 500–508. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Ramesh, A.; Gusev, Y.; Bhuvaneshwar, K.; Giaccone, G. Molecular predictors of response to pembrolizumab in thymic carcinoma. Cell Rep. Med. 2021, 2, 100392. [Google Scholar] [CrossRef]
- Remon, J.; Girard, N.; Novello, S.; de Castro, J.; Bigay-Game, L.; Bernabé, R.; Greillier, L.; Mosquera, J.; Cousin, S.; Juan, O.; et al. PECATI: A Multicentric, Open-Label, Single-Arm Phase II Study to Evaluate the Efficacy and Safety of Pembrolizumab and Lenvatinib in Pretreated B3-Thymoma and Thymic Carcinoma Patients. Clin. Lung Cancer 2022, 23, e243–e246. [Google Scholar] [CrossRef]
- Conforti, F.; Zucali, P.A.; Pala, L.; Catania, C.; Bagnardi, V.; Sala, I.; Della Vigna, P.; Perrino, M.; Zagami, P.; Corti, C.; et al. Avelumab plus axitinib in unresectable or metastatic type B3 thymomas and thymic carcinomas (CAVEATT): A single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022, 23, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Nokihara, H.; Yamada, Y.; Shibata, T.; Tamura, Y.; Seki, Y.; Honda, K.; Tanabe, Y.; Wakui, H.; Tamura, T. Phase I study of Nivolumab, an anti-PD-1 antibody, in patients with malignant solid tumors. Investig. New Drugs 2017, 35, 207–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.; Rajan, A. Immune checkpoint inhibitors for treatment of thymic epithelial tumors: How to maximize benefit and optimize risk? Mediastinum 2019, 3, 35. [Google Scholar] [CrossRef] [PubMed]
- Konstantina, T.; Konstantinos, R.; Anastasios, K.; Anastasia, M.; Eleni, L.; Ioannis, S.; Sofia, A.; Dimitris, M. Fatal adverse events in two thymoma patients treated with anti-PD-1 immune check point inhibitor and literature review. Lung Cancer 2019, 135, 29–32. [Google Scholar] [CrossRef]
- Cho, J.; Ahn, M.-J.; Yoo, K.H.; Lee, H.; Kim, H.K.; Heo, M.H.; Hong, J.H.; Sun, J.-M.; Lee, S.-H.; Ahn, J.S.; et al. A phase II study of pembrolizumab for patients with previously treated advanced thymic epithelial tumor. J. Clin. Oncol. 2017, 35, 8521. [Google Scholar] [CrossRef]
- Cho, J.; Kim, H.S.; Ku, B.M.; Choi, Y.-L.; Cristescu, R.; Han, J.; Sun, J.-M.; Lee, S.-H.; Ahn, J.S.; Park, K.; et al. Pembrolizumab for Patients With Refractory or Relapsed Thymic Epithelial Tumor: An Open-Label Phase II Trial. J. Clin. Oncol. 2019, 37, 2162–2170. [Google Scholar] [CrossRef]
- Rajan, A.; Heery, C.R.; Thomas, A.; Mammen, A.L.; Perry, S.; Coyne, G.O.; Guha, U.; Berman, A.; Szabo, E.; Madan, R.A.; et al. Efficacy and tolerability of anti-programmed death-ligand 1 (PD-L1) antibody (Avelumab) treatment in advanced thymoma. J. Immunother. Cancer 2019, 7, 269. [Google Scholar] [CrossRef]
- Zhai, L.; Bell, A.; Ladomersky, E.; Lauing, K.L.; Bollu, L.; Sosman, J.A.; Zhang, B.; Wu, J.D.; Miller, S.D.; Meeks, J.J.; et al. Immunosuppressive IDO in Cancer: Mechanisms of Action, Animal Models, and Targeting Strategies. Front. Immunol. 2020, 11, 1185. [Google Scholar] [CrossRef]
- Katsuya, Y.; Horinouchi, H.; Seto, T.; Umemura, S.; Hosomi, Y.; Satouchi, M.; Nishio, M.; Kozuki, T.; Hida, T.; Sukigara, T.; et al. Single-arm, multicentre, phase II trial of nivolumab for unresectable or recurrent thymic carcinoma: PRIMER study. Eur. J. Cancer 2019, 113, 78–86. [Google Scholar] [CrossRef] [Green Version]
- Girard, N.; Aix, S.P.; Cedres, S.; Berghmans, T.; Burgers, S.; Toffart, A.; Popat, S.; Janssens, A.; Gervais, R.; Hochstenbag, M.; et al. LBA66 Efficacy and safety of nivolumab for patients with pre-treated type B3 thymoma and thymic carcinoma: Results from the EORTC-ETOP NIVOTHYM phase II trial. Ann. Oncol. 2021, 32, S1342. [Google Scholar] [CrossRef]
- Besse, B.; Garassino, M.C.; Rajan, A.; Novello, S.; Mazieres, J.; Weiss, G.J.; Kocs, D.M.; Barnett, J.M.; Davite, C.; Crivori, P.; et al. Efficacy of milciclib (PHA-848125AC), a pan-cyclin d-dependent kinase inhibitor, in two phase II studies with thymic carcinoma (TC) and B3 thymoma (B3T) patients. J. Clin. Oncol. 2018, 36, 8519. [Google Scholar] [CrossRef]
- Jung, H.A.; Kim, M.; Kim, H.S.; Kim, J.-H.; Choi, Y.H.; Cho, J.; Park, J.H.; Park, K.U.; Ku, B.M.; Park, S.; et al. A Phase 2 Study of Palbociclib for Recurrent or Refractory Advanced Thymic Epithelial Tumors (KCSG LU17-21). J. Thorac. Oncol. 2022, in press. [Google Scholar] [CrossRef]
- Principe, D.R.; Kamath, S.D.; Munshi, H.G.; Mohindra, N.A. Metastatic Thymoma Harboring a Deleterious BRCA2 Mutation Derives Durable Clinical Benefit from Olaparib. Oncologist 2020, 25, 301–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giaccone, G.; Rajan, A.; Berman, A.; Kelly, R.J.; Szabo, E.; Lopez-Chavez, A.; Trepel, J.; Lee, M.-J.; Cao, L.; Espinoza-Delgado, I.; et al. Phase II Study of Belinostat in Patients With Recurrent or Refractory Advanced Thymic Epithelial Tumors. J. Clin. Oncol. 2011, 29, 2052–2059. [Google Scholar] [CrossRef] [Green Version]
- Thomas, A.; Rajan, A.; Szabo, E.; Tomita, Y.; Carter, C.A.; Scepura, B.; Lopez-Chavez, A.; Lee, M.-J.; Redon, C.E.; Frosch, A.; et al. A Phase I/II Trial of Belinostat in Combination with Cisplatin, Doxorubicin, and Cyclophosphamide in Thymic Epithelial Tumors: A Clinical and Translational Study. Clin. Cancer Res. 2014, 20, 5392–5402. [Google Scholar] [CrossRef] [PubMed]
- Petrini, I.; Sollini, M.; Bartoli, F.; Barachini, S.; Montali, M.; Pardini, E.; Burzi, I.S.; Erba, P.A. ED-B-Containing Isoform of Fibronectin in Tumor Microenvironment of Thymomas: A Target for a Theragnostic Approach. Cancers 2022, 14, 2592. [Google Scholar] [CrossRef]

| 1999 WHO Classification (2nd Edition) | 2004 WHO Classification (3rd Edition) | 2015 WHO Classification (4th Edition) | 2021 WHO Classification (5th Edition) |
|---|---|---|---|
| Thymomas | |||
| Thymoma, type A Thymoma, type AB Thymoma, type B1 Thymoma, type B2 Thymoma, type B3 | Thymoma, NOS Thymoma, type A Thymoma, type AB Thymoma, type B1 Thymoma, type B2 Thymoma, type B3 Micronodular thymoma Metaplastic thymoma Lipofibroadenoma Microscopic thymoma Sclerosing thymomas | Thymoma, NOS Thymoma, type A * Thymoma, type AB Thymoma, type B1 Thymoma, type B2 Thymoma, type B3 Micronodular thymoma with lymphoid stroma Metaplastic thymoma Lipofibroadenoma Microscopic thymoma Sclerosing thymomas | Thymoma, NOS Thymoma, type A * Thymoma, type AB Thymoma, type B1 Thymoma, type B2 Thymoma, type B3 Micronodular thymoma with lymphoid stroma Metaplastic thymoma Lipofibroadenoma |
| Carcinomas | |||
| Thymoma, type C Epidermoid keratinizing (squamous cell) Epidermoid non-keratinizing Lymphoepithelioma-like Sarcomatoid (carcinosarcoma) Clear cell Basaloid Mucoepidermoid Papillary Undifferentiated | Thymic carcinoma Squamous cell Basaloid Mucoepidermoid Lymphoepithelioma-like Sarcomatoid (carcinosarcoma) Clear cell Adenocarcinoma Papillary adenocarcinoma Carcinoma with t(15;19) Undifferentiated | Thymic carcinoma Squamous cell Basaloid Mucoepidermoid Lymphoepithelioma-like Clear cell Sarcomatoid Adenocarcinoma Papillary adenocarcinoma Thymic carcinoma with ACC-like features Mucinous adenocarcinoma Adenocarcinoma NOS NUT carcinoma Undifferentiated carcinoma Combined thymic carcinomas Others | Squamous carcinomas Squamous cell Basaloid Lymphoepithelioma Adenocarcinomas Adenocarcinoma, NOS Low-grade papillary adenocarcinoma Thymic carcinoma with ACC-like features Adenocarcinoma, enteric-type Adenosquamous carcinoma NUT carcinoma Salivary gland-like carcinoma Mucoepidermoid Clear cell Sarcomatoid Carcinosarcoma Undifferentiated carcinomas Thymic carcinoma, NOS |
| Thymic neuroendocrine neoplasms | |||
| Carcinoid tumors (well-differentiated NECs) Classic Spindle cell Pigmented With amyloid (extrathyroidal medullary carcinoma) Atypical LCNEC SCC Mixed small cell-epidermoid keratinizing carcinoma | Carcinoid tumors Typical carcinoid Atypical carcinoid LCNEC SCC Combined TETs, including NEC | Carcinoid tumors Typical carcinoid Atypical carcinoid LCNEC Combined LCNEC SCC Combined SCC | Carcinoid tumor, NOS/NET, NOS Typical carcinoid/NET, grade 1 Atypical carcinoid/NET, grade 2 LLCNEC SCC Combined SCC |
| TNM Staging of TETs, 8th Edition (IASLC/ITMIG) | |
|---|---|
| Category | Definition |
| T descriptor | |
| T1a | Encapsulated or unencapsulated, with or without extension into mediastinal fat |
| T1b | Extension into mediastinal pleura |
| T2 | Direct invasion of the pericardium (partial or full thickness) |
| T3 | Direct invasion of lung, brachiocephalic vein, superior vena cava, chest wall, phrenic nerve, hilar (extrapericardial) pulmonary vessels |
| T4 | Direct invasion of aorta, arch vessels, main pulmonary artery, myocardium, trachea, or esophagus |
| N descriptor | |
| N0 | No nodal involvement |
| N1 | Anterior perithymic nodes |
| N2 | Deep intrathoracic or cervical nodes |
| M descriptor | |
| M0 | No metastatic pleural, pericardial, or distant sites |
| M1a | Separate pleural or pericardial nodule(s) |
| M1b | Pulmonary intraparenchymal or distant organ metastasis |
| Masaoka–Koga staging | |
| Stage I | Grossly and microscopically encapsulated tumor |
| Stage II a | Microscopic transcapsular invasion |
| Stage II b | Macroscopic invasion into thymic or surrounding fatty tissue, or grossly adherent but not breaking through mediastinal pleura or pericardium |
| Stage III | Macroscopic invasion into neighboring organs (i.e., pericardium, great vessels, or lung) |
| Stage IV a | Pleural or pericardial dissemination |
| Stage IV b | Lymphatic or hematogenous metastasis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuhn, E.; Pescia, C.; Mendogni, P.; Nosotti, M.; Ferrero, S. Thymic Epithelial Tumors: An Evolving Field. Life 2023, 13, 314. https://doi.org/10.3390/life13020314
Kuhn E, Pescia C, Mendogni P, Nosotti M, Ferrero S. Thymic Epithelial Tumors: An Evolving Field. Life. 2023; 13(2):314. https://doi.org/10.3390/life13020314
Chicago/Turabian StyleKuhn, Elisabetta, Carlo Pescia, Paolo Mendogni, Mario Nosotti, and Stefano Ferrero. 2023. "Thymic Epithelial Tumors: An Evolving Field" Life 13, no. 2: 314. https://doi.org/10.3390/life13020314
APA StyleKuhn, E., Pescia, C., Mendogni, P., Nosotti, M., & Ferrero, S. (2023). Thymic Epithelial Tumors: An Evolving Field. Life, 13(2), 314. https://doi.org/10.3390/life13020314







