Age-Related Changes in the Clustering of Blood Populations in Cynomolgus Monkeys Depend on Sex and Immune Status
Abstract
:1. Introduction
2. Methods
2.1. Husbandry of Animals and Sampling
2.2. Blood Sampling and Analysis
2.3. Statistical Analysis
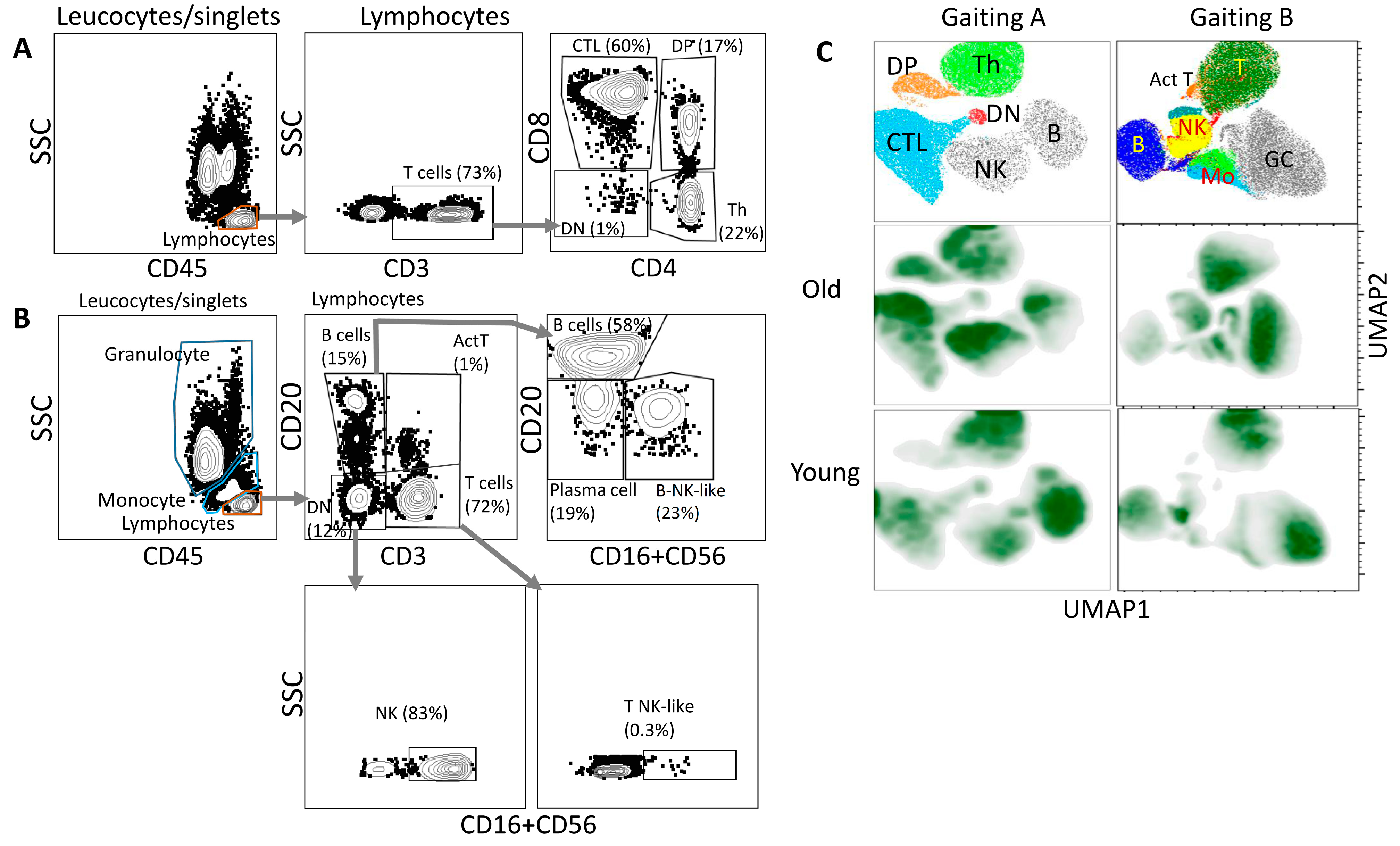
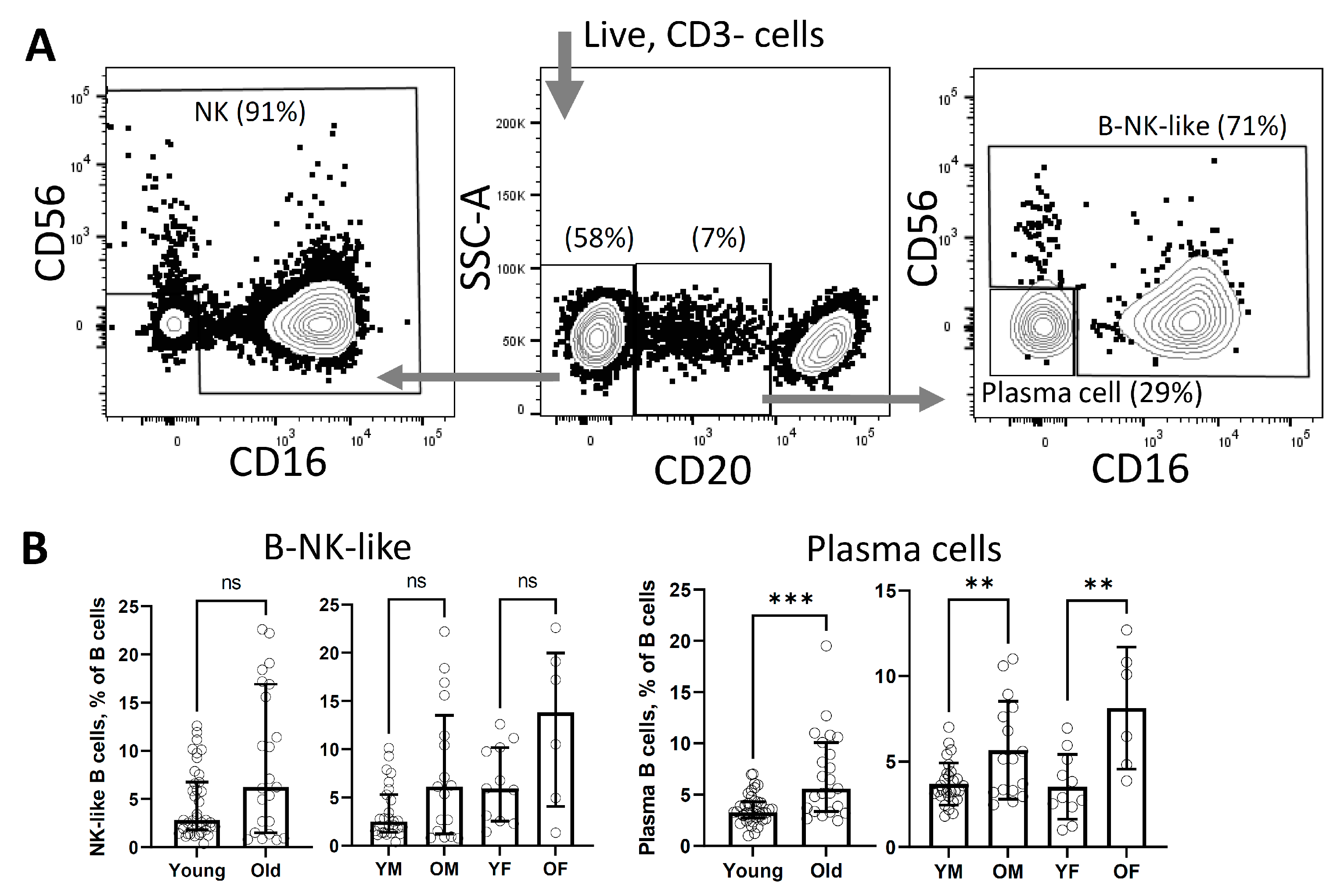
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Haemoanalyser populations | |
| EO | percentage of eosinophils in the blood |
| BA | Basophils |
| Mo | Monocytes |
| PLT × 109/L | Platelets |
| PCT, % | percentage of platelets in the blood |
| HCT, % | Haematocrit |
| HGB, g/L | Haemoglobin |
| MCHC g/L index | concentration of haemoglobin in a single erythrocyte |
| RDW-CV, % | Erythrocyte width variation coefficient |
| Flow cytometry populations | |
| CTL % of T-cells | percentage of CD4-CD8+ cells (Cytotoxic Lymphocytes) in CD3+ cell population |
| DP % of T-cells | percentage of Double Positive CD4+CD8+ cells in CD3+ cell population |
| Th, % of T-cells | percentage of CD4+CD8- cells (T-helpers) in CD3+ cell population |
| Act T, % | percentage of CD3+CD20+ cells (activated T-cells) in CD3+ cell population |
| Th/CTL index | CD3+CD4-CD8+/CD3+CD4+CD8- ratio |
| T NK-like, % | percentage of CD3+CD20-CD16&CD56+ cells in lymphocyte gate |
| B, % | percentage of CD20+CD3-CD16-CD56- cells in lymphocyte gate |
| B-NK-like, % | percentage of CD20loCD3-CD16&CD56+ cells in lymphocyte gate |
| Plasma cells, % | percentage of CD20loCD3-CD16-CD56- cells in lymphocyte gate |
References
- Zhang, W.; Zhang, S.; Yan, P.; Ren, J.; Song, M.; Li, J.; Lei, J.; Pan, H.; Wang, S.; Ma, X.; et al. A single-cell transcriptomic landscape of primate arterial aging. Nat. Commun. 2020, 11, 2202. [Google Scholar] [CrossRef] [PubMed]
- Rybtsova, N.N.; Berezina, T.N.; Kagansky, A.M.; Rybtsov, S.A. Can blood-circulating factors unveil and delay your biological aging? Biomedicines 2020, 8, 615. [Google Scholar] [CrossRef] [PubMed]
- Jonker, M.J.; Melis, J.P.; Kuiper, R.V.; van der Hoeven, T.V.; Wackers, P.F.K.; Robinson, J.; van der Horst, G.T.; Dollé, M.E.; Vijg, J.; Breit, T.M.; et al. Life spanning murine gene expression profiles in relation to chronological and pathological aging in multiple organs. Aging Cell 2013, 12, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Friedman, H.; Ator, N.; Haigwood, N.; Newsome, W.; Allan, J.S.; Golos, T.G.; Kordower, J.H.; Shade, R.E.; Goldberg, M.E.; Bailey, M.R.; et al. The critical role of nonhuman primates in medical research. Pathog. Immun. 2017, 2, 352–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colman, R.J. Non-human primates as a model for aging. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864 Pt A, 2733–2741. [Google Scholar] [CrossRef]
- Xie, L.; Xu, F.; Liu, S.; Ji, Y.; Zhou, Q.; Wu, Q.; Gong, W.; Cheng, K.; Li, J.; Li, L.; et al. Age- and sex-based hematological and biochemical parameters for Macaca fascicularis. PLoS ONE 2013, 8, e64892. [Google Scholar] [CrossRef] [Green Version]
- Drevon-Gaillot, E.; Perron-Lepage, M.F.; Clément, C.; Burnett, R. A review of background findings in cynomolgus monkeys (Macaca fascicularis) from three different geographical origins. Exp. Toxicol. Pathol. 2006, 58, 77–88. [Google Scholar] [CrossRef]
- Dewi, F.N.; Cline, J.M. Nonhuman primate model in mammary gland biology and neoplasia research. Lab. Anim. Res. 2021, 37, 3. [Google Scholar] [CrossRef]
- Mubiru, J.N.; Garcia-Forey, M.; Higgins, P.B.; Hemmat, P.; Cavazos, N.E.; Dick, E.J., Jr.; Owston, M.A.; Bauer, C.A.; Shade, R.E.; Comuzzie, A.G.; et al. A preliminary report on the feeding of cynomolgus monkeys (Macaca fascicularis) with a high-sugar high-fat diet for 33 weeks. J. Med. Primatol. 2011, 40, 335–341. [Google Scholar] [CrossRef] [Green Version]
- Kimura, N.; Tanemura, K.; Nakamura, S.; Takashima, A.; Ono, F.; Sakakibara, I.; Ishii, Y.; Kyuwa, S.; Yoshikawa, Y. Age-related changes of Alzheimer’s disease-associated proteins in cynomolgus monkey brains. Biochem. Biophys. Res. Commun. 2003, 310, 303–311. [Google Scholar] [CrossRef]
- Estes, J.D.; Wong, S.W.; Brenchley, J.M. Nonhuman primate models of human viral infections. Nat. Rev. Immunol. 2018, 18, 390–404. [Google Scholar] [CrossRef] [PubMed]
- Watowich, M.M.; Chiou, K.L.; Montague, M.J.; Simons, N.D.; Horvath, J.E.; Ruiz-Lambides, A.V.; Martínez, M.I.; Higham, J.P.; Brent, L.J.N.; Platt, M.L.; et al. Natural disaster and immunological aging in a nonhuman primate. Proc. Natl. Acad. Sci. USA 2022, 119, 3119. [Google Scholar] [CrossRef] [PubMed]
- Van Gassen, S.; Callebaut, B.; Van Helden, M.J.; Lambrecht, B.N.; Demeester, P.; Dhaene, T.; Saeys, Y. FlowSOM: Using self-organizing maps for visualization and interpretation of cytometry data. Cytom. A 2015, 87, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Schuurman, H.J.; Smith, H.T. Reference values for clinical chemistry and clinical hematology parameters in cynomolgus monkeys. Xenotransplantation 2005, 12, 72–75. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Hanari, K.; Narita, H.; Honjo, S. Normal hematologic values in the cynomolgus monkeys aged from 1 to 18 years. Jikken Dobutsu 1986, 35, 443–447. [Google Scholar] [CrossRef] [Green Version]
- Montenont, E.; Rondina, M.T.; Campbell, R.A. Altered functions of platelets during aging. Curr Opin Hematol 2019, 26, 336–342. [Google Scholar] [CrossRef]
- Choi, S.M.; Park, H.J.; Choi, E.A.; Jung, K.C.; Lee, J.I. Cellular heterogeneity of circulating CD4(+)CD8(+) double-positive T cells characterized by single-cell RNA sequencing. Sci. Rep. 2021, 11, 23607. [Google Scholar] [CrossRef]
- Choi, S.M.; Park, H.J.; Choi, E.A.; Jung, K.C.; Lee, J.I. Heterogeneity of circulating CD4(+)CD8(+) double-positive T cells characterized by scRNA-seq analysis and trajectory inference. Sci. Rep. 2022, 12, 14111. [Google Scholar] [CrossRef]
- Parel, Y.; Chizzolini, C. CD4+ CD8+ double positive (DP) T cells in health and disease. Autoimmun. Rev. 2004, 3, 215–220. [Google Scholar] [CrossRef]
- Shevyrev, D.; Tereshchenko, V.; Kozlov, V. Immune Equilibrium Depends on the Interaction Between Recognition and Presentation Landscapes. Front. Immunol. 2021, 12, 706136. [Google Scholar] [CrossRef]
- Macosko, E.Z.; Basu, A.; Satija, R.; Nemesh, J.; Shekhar, K.; Goldman, M.; Tirosh, I.; Bialas, A.R.; Kamitaki, N.; Martersteck, E.M.; et al. Highly Parallel Genome-wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell 2015, 161, 1202–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desfrançois, J.; Moreau-Aubry, A.; Vignard, V.; Godet, Y.; Khammari, A.; Dréno, B.; Jotereau, F.; Gervois, N. Double positive CD4CD8 alphabeta T cells: A new tumor-reactive population in human melanomas. PLoS ONE 2010, 5, e8437. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.W.; Nam, K.H.; Terao, K.; Akari, H.; Yoshikawa, Y. Age-related increase of peripheral CD4+ CD8+ double-positive T lymphocytes in cynomolgus monkeys: Longitudinal study in relation to thymic involution. Immunology 2003, 109, 217–225. [Google Scholar] [CrossRef]
- Chen, Q.; Yuan, S.; Sun, H.; Peng, L. CD3(+)CD20(+) T cells and their roles in human diseases. Hum. Immunol. 2019, 80, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Gubbels Bupp, M.R.; Potluri, T.; Fink, A.L.; Klein, S.L. The Confluence of Sex Hormones and Aging on Immunity. Front. Immunol. 2018, 9, 1269. [Google Scholar] [CrossRef] [PubMed]
- Márquez, E.J.; Chung, C.-h.; Marches, R.; Rossi, R.J.; Nehar-Belaid, D.; Eroglu, A.; Mellert, D.J.; Kuchel, G.A.; Banchereau, J.; Ucar, D. Sexual-dimorphism in human immune system aging. Nat. Commun. 2020, 11, 751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Yao, D.; Zeng, X.; Kasakovski, D.; Zhang, Y.; Chen, S.; Zha, X.; Li, Y.; Xu, L. Age related human T cell subset evolution and senescence. Immun. Ageing 2019, 16, 24. [Google Scholar] [CrossRef] [Green Version]
- McBride, J.A.; Striker, R. Imbalance in the game of T cells: What can the CD4/CD8 T-cell ratio tell us about HIV and health? PLoS Pathog. 2017, 13, e1006624. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.M.; Liu, C.Y.; Shao, Z.H. Advances in the role of helper T cells in autoimmune diseases. Chin. Med. J. 2020, 133, 968–974. [Google Scholar] [CrossRef]
- Shevyrev, D.; Tereshchenko, V.; Blinova, E.; Knauer, N.; Pashkina, E.; Sizikov, A.; Kozlov, V. Regulatory T Cells Fail to Suppress Fast Homeostatic Proliferation In Vitro. Life 2021, 11, 245. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Uppal, S.S.; Verma, S.; Dhot, P.S. Normal values of CD4 and CD8 lymphocyte subsets in healthy indian adults and the effects of sex, age, ethnicity, and smoking. Cytom. B Clin. Cytom. 2003, 52, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Dogan, E.; Erkoc, R.; Demir, C.; Sayarlioglu, H.; Dilek, I.; Sayarlioglu, M. Effect of hormone replacement therapy on CD4+ and CD8+ numbers, CD4+/CD8+ ratio, and immunoglobulin levels in hemodialysis patients. Ren. Fail. 2005, 27, 421–424. [Google Scholar] [PubMed]
- Wong, W.S.; Lo, A.W.; Siu, L.P.; Leung, J.N.; Tu, S.P.; Tai, S.W.; Lam, S.C.; Wong, K.F. Reference ranges for lymphocyte subsets among healthy Hong Kong Chinese adults by single-platform flow cytometry. Clin. Vaccine Immunol. 2013, 20, 602–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bynoe, M.S.; Grimaldi, C.M.; Diamond, B. Estrogen up-regulates Bcl-2 and blocks tolerance induction of naive B cells. Proc. Natl. Acad. Sci. USA 2000, 97, 2703–2708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubtsova, K.; Rubtsov, A.V.; Thurman, J.M.; Mennona, J.M.; Kappler, J.W.; Marrack, P. B cells expressing the transcription factor T-bet drive lupus-like autoimmunity. J. Clin. Investig. 2017, 127, 1392–1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kläsener, K.; Jellusova, J.; Andrieux, G.; Salzer, U.; Böhler, C.; Steiner, S.N.; Albinus, J.B.; Cavallari, M.; Süß, B.; Voll, R.E.; et al. CD20 as a gatekeeper of the resting state of human B cells. Proc. Natl. Acad. Sci. USA 2021, 118, 2118. [Google Scholar] [CrossRef]
- Delaloy, C.; Schuh, W.; Jäck, H.M.; Bonaud, A.; Espéli, M. Single-cell resolution of plasma cell fate programming in health and disease. Eur. J. Immunol. 2022, 52, 10–23. [Google Scholar] [CrossRef]
- Gill, H.S.; Lau, W.H.; Chan, A.C.; Leung, R.Y.; Khong, P.L.; Leung, A.Y.; Kwong, Y.L. CD20 expression in natural killer T cell lymphoma. Histopathology 2010, 57, 157–159. [Google Scholar] [CrossRef]
- Milman, N.; Pedersen, A.N.; Ovesen, L.; Schroll, M. Hemoglobin concentrations in 358 apparently healthy 80-year-old Danish men and women. Should the reference interval be adjusted for age? Aging Clin. Exp. Res. 2008, 20, 8–14. [Google Scholar] [CrossRef]
- Berezina, T.N.; Rybtsov, S. Acceleration of Biological Aging and Underestimation of Subjective Age Are Risk Factors for Severe COVID-19. Biomedicines 2021, 9, 913. [Google Scholar] [CrossRef] [PubMed]
- Drew, L. Turning back time with epigenetic clocks. Nature 2022, 601, S20–S22. [Google Scholar] [CrossRef] [PubMed]
- Linhares, Y.; Kaganski, A.; Agyare, C.; Kurnaz, I.A.; Neergheen, V.; Kolodziejczyk, B.; Kędra, M.; Wahajuddin, M.; El-Youssf, L.; de la Cruz, T.E.; et al. Biodiversity: The overlooked source of human health. Trends Mol. Med. 2022. [Google Scholar] [CrossRef] [PubMed]
- Mattison, J.A.; Vaughan, K.L. An overview of nonhuman primates in aging research. Exp. Gerontol 2017, 94, 41–45. [Google Scholar] [CrossRef]
- Ahuja, A.; Ikladios, O. Progesterone as a cause of eosinophilic pneumonia after in vitro fertilization. J. Community Hosp. Intern. Med. Perspect. 2017, 7, 366–368. [Google Scholar] [CrossRef] [Green Version]
- Murphy, W.G. The sex difference in haemoglobin levels in adults-mechanisms, causes, and consequences. Blood Rev. 2014, 28, 41–47. [Google Scholar] [CrossRef]
- Bertolone, L.; Shin, H.K.; Stefanoni, D.; Baek, J.H.; Gao, Y.; Morrison, E.J.; Nemkov, T.; Thomas, T.; Francis, R.O.; Hod, E.A.; et al. ZOOMICS: Comparative Metabolomics of Red Blood Cells From Old World Monkeys and Humans. Front. Physiol. 2020, 11, 593841. [Google Scholar] [CrossRef]
- Patin, E.; Hasan, M.; Bergstedt, J.; Rouilly, V.; Libri, V.; Urrutia, A.; Alanio, C.; Scepanovic, P.; Hammer, C.; Jönsson, F.; et al. Natural variation in the parameters of innate immune cells is preferentially driven by genetic factors. Nat. Immunol. 2018, 19, 302–314. [Google Scholar] [CrossRef]
- Abdullah, M.; Chai, P.S.; Chong, M.Y.; Tohit, E.R.; Ramasamy, R.; Pei, C.P.; Vidyadaran, S. Gender effect on in vitro lymphocyte subset levels of healthy individuals. Cell Immunol. 2012, 272, 214–219. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karal-ogly, D.D.; Shumeev, A.N.; Keburiya, V.V.; Mintel, M.V.; Rybtsov, S.A. Age-Related Changes in the Clustering of Blood Populations in Cynomolgus Monkeys Depend on Sex and Immune Status. Life 2023, 13, 316. https://doi.org/10.3390/life13020316
Karal-ogly DD, Shumeev AN, Keburiya VV, Mintel MV, Rybtsov SA. Age-Related Changes in the Clustering of Blood Populations in Cynomolgus Monkeys Depend on Sex and Immune Status. Life. 2023; 13(2):316. https://doi.org/10.3390/life13020316
Chicago/Turabian StyleKaral-ogly, Dzhina D., Alexander N. Shumeev, Viktoria V. Keburiya, Marina V. Mintel, and Stanislav A. Rybtsov. 2023. "Age-Related Changes in the Clustering of Blood Populations in Cynomolgus Monkeys Depend on Sex and Immune Status" Life 13, no. 2: 316. https://doi.org/10.3390/life13020316
APA StyleKaral-ogly, D. D., Shumeev, A. N., Keburiya, V. V., Mintel, M. V., & Rybtsov, S. A. (2023). Age-Related Changes in the Clustering of Blood Populations in Cynomolgus Monkeys Depend on Sex and Immune Status. Life, 13(2), 316. https://doi.org/10.3390/life13020316





