Protein- and Carbohydrate-Rich Supplements in Feeding Adult Black Soldier Flies (Hermetia illucens) Affect Life History Traits and Egg Productivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Feeding BSF Adults: Experimental Design and Influence on Life History Traits
2.3. Data Processing and Statistics
3. Results
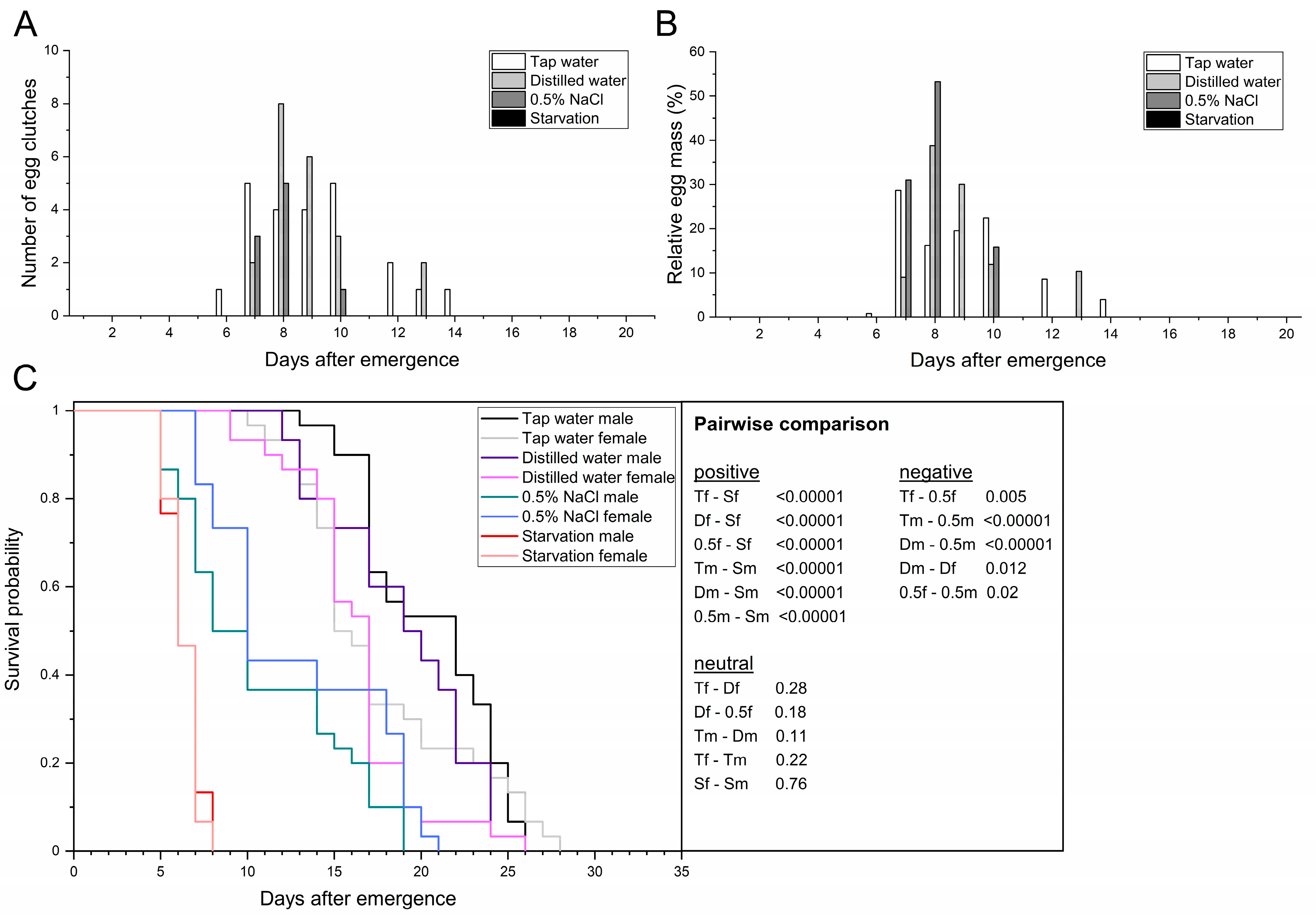
3.1. Providing the Adult BSF Different Types of Water
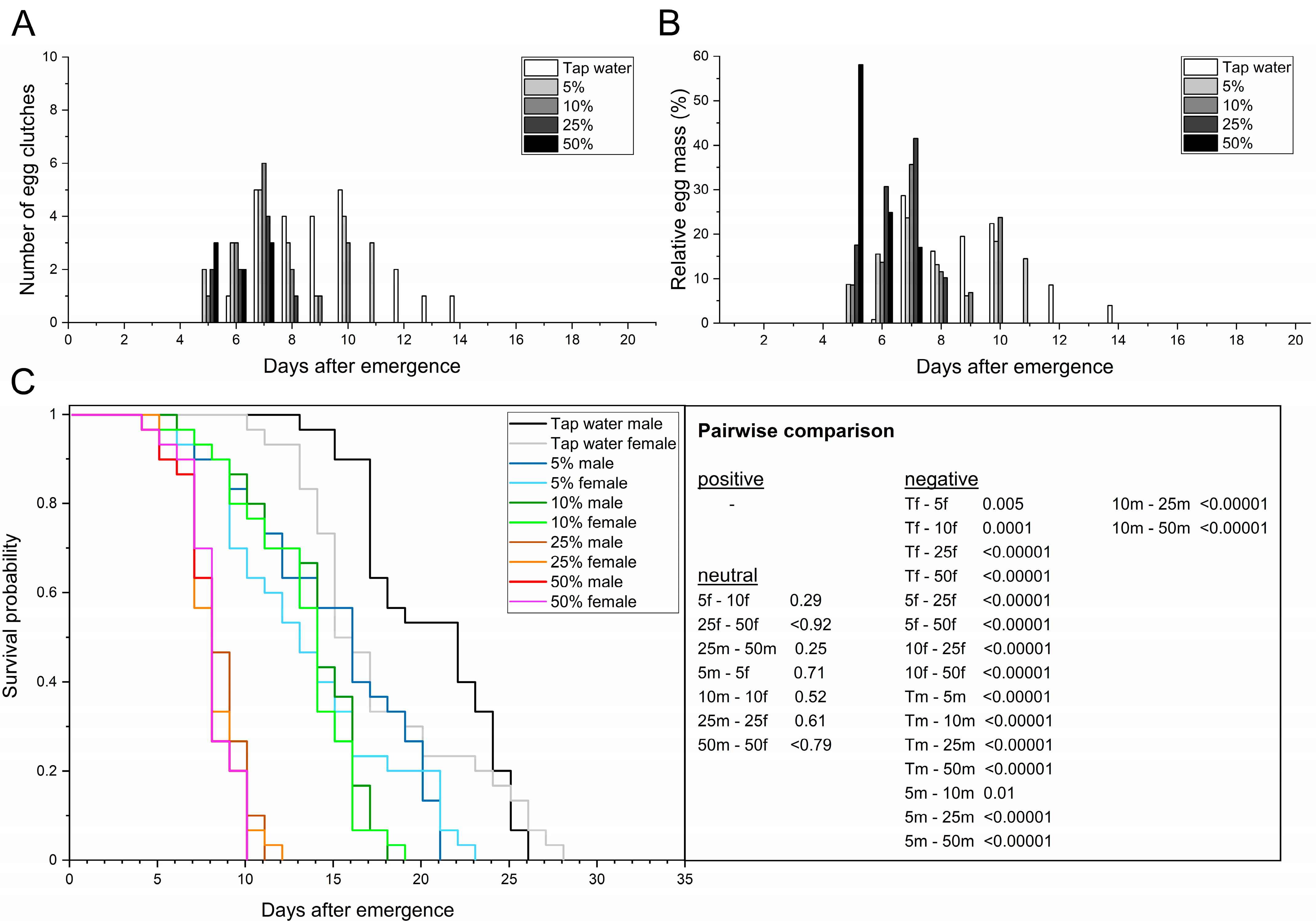
3.2. Feeding the Adult BSF Carbohydrate-Rich Solutions
3.2.1. Organic Honey
3.2.2. d-Glucose
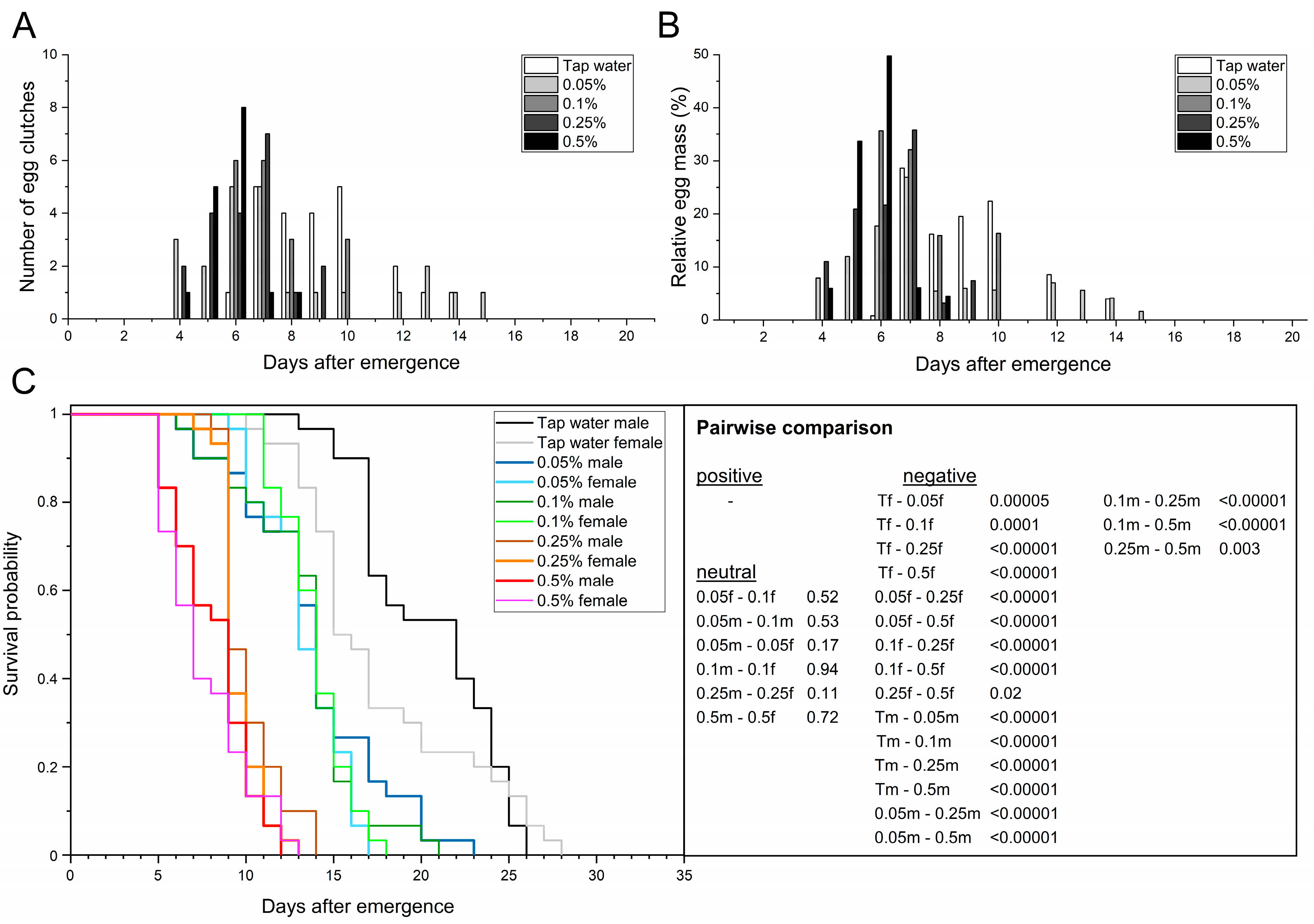
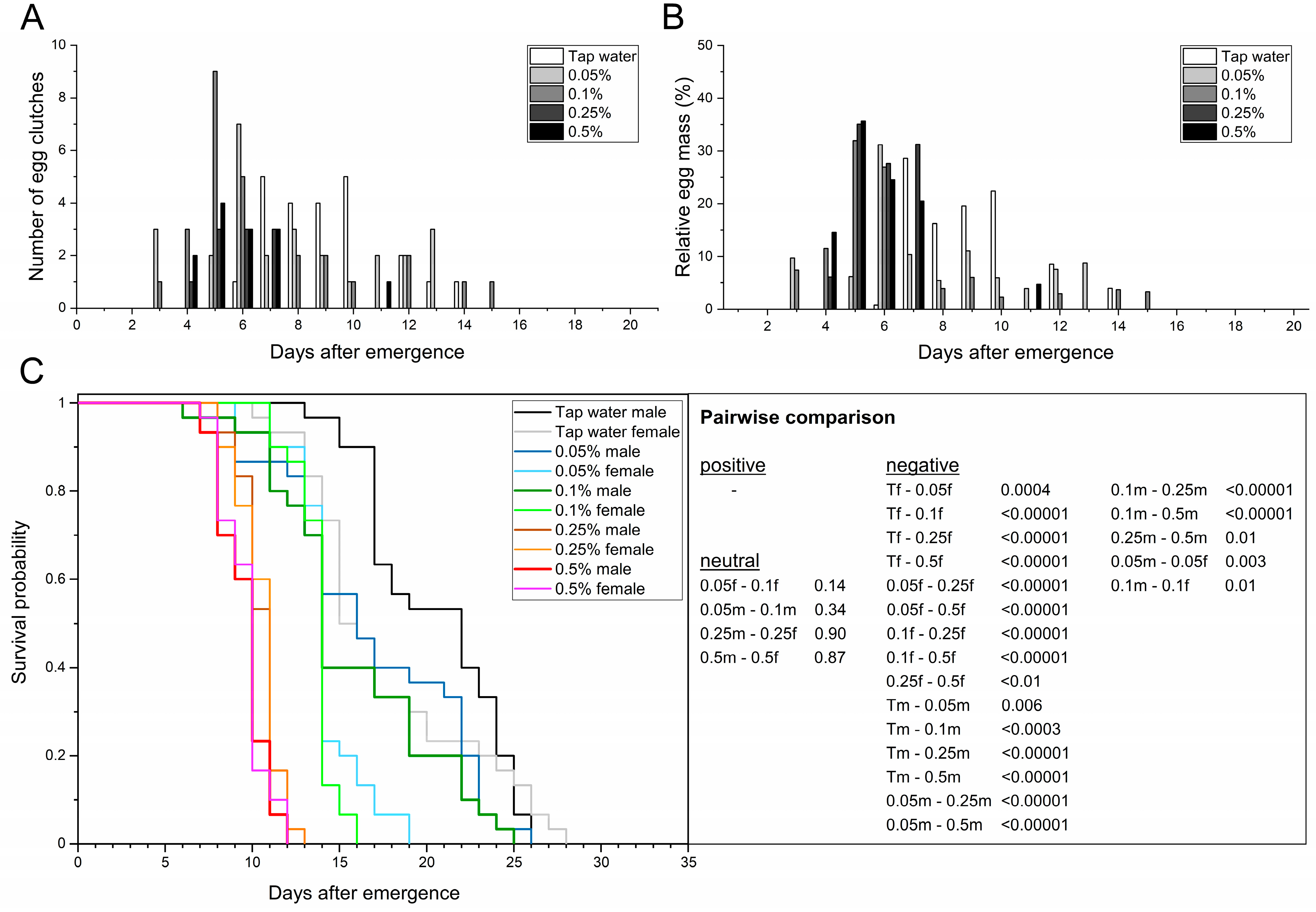
3.3. Feeding the Adult BSF Protein-Rich Solutions
3.3.1. Spirulina Powder
3.3.2. Chlorella Powder
3.4. Female Longevity after Oviposition
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. 2017 Revision of World Population Prospects. 2017. Available online: https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/files/documents/2020/Jan/un_2017_world_population_prospects-2017_revision_databooklet.pdf (accessed on 22 February 2022).
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future protein supply and demand: Strategies and factors influencing a sustainable equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Organization for Economic Cooperation and Development; Food and Agriculture Organization of the United Nations. OECD-FAO Agricultural Outlook 2018–2027. 2018. Available online: https://doi.org/10.1787/agr_outlook-2018-en (accessed on 25 February 2022).
- Joseph, P.; Searing, A.; Watson, C.; McKeague, J. Alternative proteins: Market research on consumer trends and emerging landscape. Meat Muscle Biol. 2020, 4, 1–11. [Google Scholar] [CrossRef]
- Lalander, C.H.; Fidjeland, J.; Diener, S.; Eriksson, S.; Vinnerås, B. High waste-to-biomass conversion and efficient Salmonella spp. reduction using black soldier fly for waste recycling. Agron. Sustain. Dev. 2015, 35, 261–271. [Google Scholar] [CrossRef] [Green Version]
- Barragan-Fonseca, K.B.; Dicke, M.; Van Loon, J.J.A. Influence of larval density and dietary nutrient concentration on performance, body protein, and fat contents of black soldier fly larvae (Hermetia illucens). Entomol. Exp. Appl. 2018, 166, 761–770. [Google Scholar] [CrossRef] [Green Version]
- Bertinetti, C.; Samayoa, A.C.; Hwang, S.-Y. Effects of feeding adults of Hermetia illucens (Diptera: Stratiomyidae) on longevity, oviposition, and egg hatchability: Insights into optimizing egg production. J. Insect Sci. 2019, 19, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Lemke, N.B.; Dickerson, A.J.; Tomberlin, J.K. No neonates without adults: A review of adult black soldier fly biology, Hermetia illucens (Diptera: Stratiomyidae). Bioessays 2022, 45, e2200162. [Google Scholar] [CrossRef]
- Meneguz, M.; Miranda, C.D.; Cammack, J.A.; Tomberlin, J.K. Adult behavior as the next frontier for optimizing industrial production of the black soldier fly Hermetia illucens (L.) (Diptera: Stratiomyidae). J. Insects Food Feed. 2022, 8, 1–16. [Google Scholar]
- Tomberlin, J.K.; Sheppard, D.C. Factors influencing mating and oviposition of black soldier flies (Diptera: Stratiomyidae) in a colony. J. Entomol. Sci. 2002, 37, 345–352. [Google Scholar] [CrossRef]
- Hall, D.C.; Gerhardt, R.R. Flies (Diptera). In Medical and Veterinary Entomology; Mullen, G., Durden, L., Eds.; Academic Press: San Diego, CA, USA, 2002; pp. 127–161. [Google Scholar]
- Bruno, D.; Bonelli, M.; Cadamuro, A.G.; Reguzzoni, M.; Grimaldi, A.; Casartelli, M.; Tettamanti, G. The digestive system of the adult Hermetia illucens (Diptera: Stratiomyidae): Morphological features and functional properties. Cell Tissue Res. 2019, 378, 221–238. [Google Scholar] [CrossRef]
- Tettamanti, G.; van Campenhout, L.; Casartelli, M. A hungry need for knowledge on the black soldier fly digestive system. J. Insects Food Feed. 2022, 8, 217–222. [Google Scholar] [CrossRef]
- Nakamura, S.; Ichiki, R.T.; Shimoda, M.; Morioka, S. Small-scale rearing of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae), in the laboratory: Low-cost and year-round rearing. Appl. Entomol. Zool. 2016, 51, 161–166. [Google Scholar] [CrossRef]
- Macavei, L.I.; Benassi, G.; Stoian, V.; Maistrello, L. Optimization of Hermetia illucens (L.) egg laying under different nutrition and light conditions. PLoS ONE 2020, 15, e0232144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oonincx, D.G.A.B.; Volk, N.; Diehl, J.J.E.; Van Loon, J.J.A.; Belušič, G. Photoreceptor spectral sensitivity of the compound eyes of black soldier fly (Hermetia illucens) informing the design of LED-based illumination to enhance indoor reproduction. J. Insect Physiol. 2016, 95, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Kim, W.; Kim, E.; Choi, J.-Y.; Kim, S.-H. Effect of adult population density on egg production in the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae). Int. J. Indust. Entomol. 2016, 33, 92–95. [Google Scholar] [CrossRef] [Green Version]
- Hoc, B.; Noël, G.; Carpentier, J.; Francis, F.; Caparros Megido, R. Optimization of black soldier fly (Hermetia illucens) artificial reproduction. PloS ONE 2019, 14, e0216160. [Google Scholar] [CrossRef] [Green Version]
- Chia, S.Y.; Tanga, C.M.; Khamis, F.M.; Mohamed, S.A.; Salifu, D.; Sevgan, S.; Fiaboe, K.K.M.; Niassy, S.; Van Loon, J.J.A.; Dicke, M.; et al. Threshold temperatures and thermal requirements of black soldier fly Hermetia illucens: Implications for mass production. PLoS ONE 2018, 13, e0206097. [Google Scholar] [CrossRef] [Green Version]
- Holmes, L.A.; Vanlaerhoven, S.L.; Tomberlin, J.K. Relative humidity effects on the life history of Hermetia illucens (Diptera: Stratiomyidae). Environ. Entomol. 2012, 41, 971–978. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Crippen, T.L.; Holmes, L.; Singh, B.; Pimsler, M.L.; Benbow, M.E.; Tarone, A.M.; Dowd, S.; Yu, Z.; Vanlaerhoven, S.L.; et al. Bacteria mediate oviposition by the black soldier fly, Hermetia illucens (L.), (Diptera: Stratiomyidae). Sci. Rep. 2013, 3, 2563. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Huang, L.; He, J.; Tomberlin, J.K.; Li, J.; Lei, C.; Sun, M.; Liu, Z.; Yu, Z. An artificial light source influences mating and oviposition of black soldier flies, Hermetia illucens. J. Insect Sci. 2010, 10, 202. [Google Scholar] [CrossRef] [Green Version]
- Heussler, C.D.; Walter, A.; Oberkofler, H.; Insam, H.; Arthofer, W.; Schlick-Steiner, B.C.; Steiner, F.M. Influence of three artificial light sources on oviposition and half-life of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae): Improving small-scale indoor rearing. PLoS ONE 2018, 13, e0197896. [Google Scholar] [CrossRef] [Green Version]
- Schneider, J.C. Effects of light intensity on mating of the black soldier fly (Hermetia illucens, Diptera: Stratiomyidae). J. Insects Food Feed. 2019, 6, 111–119. [Google Scholar] [CrossRef]
- Liu, Z.; Najar-Rodriguez, A.J.; Minor, M.A.; Hedderley, D.I.; Morel, P.C.H. Mating success of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae), under four artificial light sources. J. Photochem. Photobiol. B Biol. 2020, 205, 111815. [Google Scholar] [CrossRef] [PubMed]
- Bava, L.; Jucker, C.; Gislon, G.; Lupi, D.; Savoldelli, S.; Zucali, M.; Colombini, S. Rearing of Hermetia illucens on different organic by-products: Influence on growth, waste reduction, and environmental impact. Animals 2019, 9, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klüber, P.; Bakonyi, D.; Zorn, H.; Rühl, M. Does light color temperature influence aspects of oviposition by the black soldier fly (Diptera: Stratiomyidae)? J. Econ. Entomol. 2020, 113, 2549–2552. [Google Scholar] [CrossRef] [PubMed]
- Tegtmeier, D.; Hurka, S.; Klüber, P.; Brinkrolf, K.; Heise, P.; Vilcinskas, A. Cottonseed press cake as a potential diet for industrially farmed black soldier fly larvae triggers adaptations of their bacterial and fungal gut microbiota. Front. Microbiol. 2021, 12, 634503. [Google Scholar] [CrossRef] [PubMed]
- Holmes, L. Role of abiotic factors on the development and life history of the black soldier fly, Hermetia illucens (L.) (Diptera: Stratiomyidae). Ph.D. Thesis, University of Windsor, Windsor, ON, Canada, 2010. [Google Scholar]
- Rozkošný, R. A Biosystematic Study of the European Stratiomyidae (Diptera): Clitellariinae, Hermediinae, Pachygasterinae and Bibliography; Springer: Dordrecht, The Netherlands, 1983. [Google Scholar]
- Klüber, P.; Tegtmeier, D.; Hurka, S.; Pfeiffer, J.; Vilcinskas, A.; Rühl, M.; Zorn, H. Diet fermentation leads to microbial adaptation in black soldier fly (Hermetia illucens; Linnaeus, 1758) larvae reared on palm oil side streams. Sustainability 2022, 14, 5626. [Google Scholar] [CrossRef]
- Holmes, S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]
- Hilgers, R.D.; Heussen, N.; Stanzel, S. Korrelationskoeffizient nach Pearson. In Lexikon der Medizinischen Laboratoriumsdiagnostik, 1st ed.; Gressner, A.M., Arndt, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1305–1417. [Google Scholar]
- Barbi, S.; Messori, M.; Manfredini, T.; Pini, M.; Montorsi, M. Rational design and characterization pf bioplastics from Hermetia illucens prepupae proteins. Biopolymers 2018, 110, e23250. [Google Scholar] [CrossRef]
- Samayoa, A.C.; Chen, W.-T.; Hwang, S.-Y. Survival and development of Hermetia illucens (Diptera: Stratiomyidae): A biodegradation agent of organic waste. J. Econ. Entomol. 2016, 109, 2580–2585. [Google Scholar] [CrossRef]
- Čičková, H.; Newton, G.L.; Lacy, R.C.; Kozánek, M. The use of fly larvae for organic waste treatment. Waste Manag. 2015, 35, 68–80. [Google Scholar] [CrossRef]
- Sheppard, D.C.; Tomberlin, J.K.; Joyce, J.A.; Kiser, B.C.; Sumner, S.M. Rearing methods for the black soldier fly (Diptera: Stratiomyidae). J. Med. Entomol. 2002, 39, 695–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovacs, F.; Medveczky, I.; Papp, L.; Gondar, E. Role of prestomal teeth in feeding of the house fly Musca domestica (Diptera, Muscidae). Med. Vet. Entomol. 1990, 4, 331–335. [Google Scholar] [PubMed]
- Fujita, T.; Kobayashi, S. Structure and function of gut endocrine cells. Int. Rev. Cytol. Suppl. 1977, 6, 187–233. [Google Scholar]
- Takashima, S.; Younossi-Hartenstein, A.; Ortiz, P.A.; Hartenstein, V. A novel tissue in an established model system: The Drosophila pupal midgut. Dev. Genes Evol. 2011, 221, 69–81. [Google Scholar] [CrossRef] [Green Version]
- Lupi, D.; Savoldelli, S.; Leonardi, M.G.; Jucker, C. Feeding in the adult Hermetia illucens (Diptera Stratiomyidae): Reality or fiction? J. Entomol. Acarol. Res. 2019, 51, 27–32. [Google Scholar] [CrossRef]
- Tomberlin, J.K.; Adler, P.H.; Myers, H.M. Development of the black soldier fly (Diptera: Stratiomyidae) in relation to temperature. Environ. Entomol. 2009, 38, 930–934. [Google Scholar] [CrossRef]
- Bradley, T.J.; Strange, K.; Phillips, J.E. Osmotic and ionic regulation in saline-water mosquito larvae. In Osmoregulation in Estuarine and Marine Animals. Lecture Notes on Coastal and Estuarine Studies; Pequeux, A., Gilles, R., Bolis, L., Eds.; Springer: Berlin/Heidelberg, Germany, 1984; Volume 9, pp. 35–50. [Google Scholar]
- Vatandoost, H. Mechanism of survival of arthropods to saline-water habitat, osmoregulation habit. Int. J. Fish. Aquat. Stud. 2021, 9, 135–138. [Google Scholar]
- Romano, N.; Fischer, H.; Egnew, N. Color and sugar preferences of adult black soldier fly (Hermetia illucens) (Diptera: Stratiomyidae) for feeding and oviposition. J. Environ. Biol. 2020, 41, 1132–1137. [Google Scholar] [CrossRef]
- Furman, D.P.; Young, R.D.; Catts, P.E. Hermetia illucens (Linnaeus) as a factor in the natural control of Musca domestica (Linnaeus). J. Econ. Entomol. 1959, 52, 917–921. [Google Scholar] [CrossRef]
- Stephens, C.S. Hermetia illucens (Diptera: Stratiomyidae) as a banana pest in Panama. Trop. Agric. 1975, 52, 173–178. [Google Scholar]
- Doner, L.W. The sugars of honey—A review. J. Sci. Food Agric. 1977, 28, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Anjum, S.I.; Rahman, K.; Ansari, M.J.; Khan, W.U.; Kamal, S.; Khattak, B.; Muhammad, A.; Khan, H.U. Honey: Single food stuff comprises many drugs. Saudi J. Biol. Sci. 2018, 25, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, H.; Giordanengo, P.; Wäckers, F.L.; Kaiser, L. Effects of feeding frequency and sugar concentration on behavior and longevity of the adult aphid parasitoid: Aphidius ervi (Haliday) (Hymenoptera: Braconidae). Biol. Control 2004, 31, 445–452. [Google Scholar] [CrossRef]
- Edward, D.A.; Chapman, T. Mechanisms underlying reproductive trade-offs: Costs of reproduction. In Mechanisms of Life History Evolution: The Genetics and Physiology of Life History Traits and Trade-offs; Flatt, T., Heyland, A., Eds.; Oxford University Press: Oxford, UK, 2011; pp. 137–152. [Google Scholar]
- Blacher, P.; Huggins, T.J.; Bourke, F.G. Evolution of ageing, costs of reproduction and the fecundity-longevity trade-off in eusocial insects. Proc. R. Soc. B. 2017, 284, 20170380. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, A.; Heinze, J. Lay eggs, live longer: Division of labor and life span in a clonal ant species. Evolution 2003, 57, 2424–2429. [Google Scholar]
- Giunti, G.; Campolo, O.; Laudani, F.; Vincenzo, P. Male courtship behavior and potential for female mate choice in the black soldier fly Hermetia illucens L. (Diptera: Stratiomyidae). Entomol. Gen. 2018, 38, 29–46. [Google Scholar] [CrossRef]
- Hosikian, A.; Lim, S.; Halim, R.; Danquah, M.K. Chlorophyll extraction from microalgae: A review on the process engineering aspects. Int. J. Chem. Eng. 2010, 2010, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Azizullah, A.; Rehman, Z.U.; Ali, I.; Murad, W.; Muhammad, N.; Ullah, W.; Häder, D.-P. Chlorophyll derivatives can be an efficient weapon in the fight against dengue. Parasitol. Res. 2014, 113, 4321–4326. [Google Scholar] [CrossRef]
- Wohllebe, S.; Richter, R.; Richter, P.R.; Häder, D.-P. Photodynamic control of human pathogenic parasites in aquatic ecosystems using chlorophyllin and pheophorbid as photodynamic substances. Parasitol. Res. 2009, 104, 593–600. [Google Scholar] [CrossRef]
- Erzinger, G.S.; Häder, D.-P.; Richter, P.; Carolina, S.; Wohllebe, S. New perspectives for the control of mosquito larvae using chlorophyll derivatives as photosensitizers. In Proceedings of the Environment and Health Conference, Basel, Switzerland, 19–23 August 2013; Environmental Health Perspectives: Durham, NC, USA, 2013. [Google Scholar]
- Wohllebe, S.; Ulbrich, C.; Grimm, D.; Pietsch, J.; Erzinger, G.; Richter, R.; Häder, D.-P. Photodynamic treatment of Chaoborus crystallinus larvae with chlorophyllin induces necrosis and apoptosis. Photochem. Photobiol. 2011, 87, 1113–1122. [Google Scholar] [CrossRef]
- Mair, W.; Piper, M.D.W.; Partridge, L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 2005, 3, e223. [Google Scholar] [CrossRef] [Green Version]
- Bruce, K.D.; Hoxha, S.; Carvalho, G.B.; Yamada, R.; Wang, H.-D.; Karayan, P.; He, S.; Brummel, T.; Kapahi, P.; Ja, W.W. High carbohydrate-low protein consumption maximizes Drosophila lifespan. Exp. Gerontol. 2013, 48, 1129–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bleakley, S.; Hayes, M. Algal proteins: Extraction, application, and challenges concerning production. Foods 2017, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Volkmann, H.; Imianovsky, U.; Oliveira, J.L.B.; Sant’anna, E.S. Cultivation of Arthrospira (spirulina) platensis in desalinator wastewater and salinated synthetic medium: Protein content and amino acid profile. Braz. J. Microbiol. 2008, 39, 98–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.P.; Simpson, S.J.; Clissold, F.J.; Brooks, R.; Ballard, J.W.; Taylor, P.W.; Soran, N.; Raubenheimer, D. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc. Natl. Acad. Sci. USA 2008, 105, 2498–2503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, D.M. Fuel use in flight and its dependence on nectar feeding in the hawkmoth Amphion floridensis. J. Exp. Biol. 1999, 202, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Carey, J.R.; Harshman, L.G.; Liedo, P.; Muller, H.G.; Wang, J.L.; Zhang, Z. Longevity-fertility trade-offs in the tephritid fruit fly, Anastrepha ludens, across dietary-restriction gradients. Aging Cell 2008, 7, 470–477. [Google Scholar] [CrossRef] [Green Version]
- Fanson, B.G.; Weldon, C.W.; Perez-Staples, D.; Simpson, S.J.; Taylor, P.W. Nutrients, not caloric restriction, extend lifespan in Queensland fruit flies (Bactrocera tryoni). Aging Cell 2009, 8, 514–523. [Google Scholar] [CrossRef]


| Chlorella | Spirulina | Honey | d-Glucose | |
|---|---|---|---|---|
| Product name | Bio Chlorella powder | Bio Spirulina powder | Bio forest honey | d (+)-glucose monohydrate |
| Species | Chlorella vulgaris | Arthrospira sp. | (Apis mellifera) | - |
| Company | Narayana Verlag, Kandern, Germany | Piura, Dublin, Ireland | Alnatura, Darmstadt, Germany | Carl Roth, Karlsruhe, Germany |
| Protein (g /100 g) | 66.0 | 67.0 | 0.4 | - |
| Fat (g /100 g) | 1.8 | 0.7 | <0.1 | - |
| Saturated fatty acids (g/100 g) | 0.6 | 0.5 | <0.1 | - |
| Carbohydrates (g/100 g) | 12.0 | 13.0 | 77.9 | 91.8 |
| Fiber (g/100 g) | 14.0 | 5.8 | 0.0 | - |
| Gross energy (kcal/100 g) | 340 | 339 | 313 | 343 |
| Sodium chloride (g/100 g) | 0.2 | 2.2 | <0.1 | - |
| Iron (mg/100 g) | 76 | 53 | - | - |
| Phosphorus (mg/100 g) | 1100 | - | - | - |
| Manganese (mg/100 g) | 6 | - | - | - |
| Zinc (mg/100 g) | 2.6 | - | - | - |
| Vitamin A (µg/100 g) | 1440 | 1204 | - | - |
| Vitamin B2 (mg/100 g) | 0.4 | - | - | - |
| Vitamin B6 (mg/100 g) | - | 0.71 | - | - |
| Vitamin B12 (µg/100 g) | 60 | - | - | - |
| Parameters | n | Tap Water | Distilled Water | 0.5% NaCl | Starvation | |
|---|---|---|---|---|---|---|
| Adult preoviposition period (d) | n = 30 | 9.1 ± 1.1 a | 9.0 ± 0.7 a | 7.9 ± 0.4 a | no eggs | |
| Oviposition period (d) | n = 30 | 5.0 ± 0.8 a | 4.0 ± 2.2 a | 1.7 ± 0.9 a | no eggs | |
| Oviposition peak (d) | n = 30 | 8.3 ± 1.2 a | 9.0 ± 0.8 a | 7.8 ± 0.6 a | no eggs | |
| Oviposition span (min–max d) | n = 30 | 6–14 | 7–13 | 7–10 | no eggs | |
| Adult longevity (d) | ♂ | n = 30 | 20.5 ± 1.3 a | 19.0 ± 2.0 a | 10.7 ± 0.6 b | 6.4 ± 0.1 c |
| ♀ | n = 30 | 17.6 ± 1.0 a | 16.2 ± 1.3 ab | 12.9 ± 2.2 b | 6.3 ± 0.4 c | |
| total | n = 60 | 19.1 ± 1.1 a | 17.6 ± 1.2 b | 11.8 ± 1.3 c | 6.4 ± 0.1 d | |
| Adult longevity (min–max d) | ♂ | n = 30 | 13–26 | 12–26 | 5–19 | 5–8 |
| ♀ | n = 30 | 10–28 | 9–26 | 7–21 | 5–8 | |
| total | n = 60 | 10–28 | 9–26 | 5–21 | 5–8 | |
| AL50 (d) | n = 60 | 19.0 ± 2.8 a | 16.3 ± 0.9 ab | 11.3 ± 1.9 bc | 6.3 ± 0.5 c | |
| Female longevity after oviposition (d) | n = 30 | 6.7 ± 1.8 a | 6.1 ± 0.1 a | 4.8 ± 2.9 a | no eggs | |
| Fecundity (egg clutches/10 females) | n = 30 | 7.0 ± 0.8 a | 6.7 ± 1.2 a | 3.0 ± 0.0 b | 0.0 ± 0.0 c | |
| Egg clutch weight (mg) | n = 30 | 15.0 ± 2.1 a | 15.6 ± 0.2 a | 12.8 ± 2.0 a | no eggs | |
| Span of egg clutch weight (min–max mg) | n = 30 | 11.3–31.8 | 12.4–20.1 | 7.9–18.2 | no eggs | |
| Mean egg yield (mg eggs/10 females) | n = 30 | 106.9 ± 26.9 a | 104.0 ± 18.3 a | 38.5 ± 6.1 b | 0.0 ± 0.0 b | |
| Total egg yield (mg eggs) | n = 30 | 320.8 | 312.0 | 115.4 | 0.0 ± 0.0 | |
| Egg clutch size (eggs/clutch) | n = 30 | 626.5 ± 86.1 a | 651.4 ± 9.5 a | 534.3 ± 84.7 a | no eggs | |
| Span of egg clutch size (min–max eggs) | n = 30 | 471–1325 | 517–838 | 329–758 | no eggs | |
| Multiple oviposition events (yes/no (number)) | n = 30 | yes (2) | yes (1) | no | no | |
| No oviposition (yes/no (number)) | n = 30 | yes (9) | yes (10) | yes (21) | yes (30) | |
| Parameters | n | Tap Water | 5% Honey | 10% Honey | 25% Honey | 50% Honey | |
|---|---|---|---|---|---|---|---|
| Adult preoviposition period (d) | n = 30 | 9.1 ± 1.1 a | 9.2 ± 1.1 a | 9.0 ± 1.4 a | 8.4 ± 0.3 a | 8.0 ± 0.3 a | |
| Oviposition period (d) | n = 30 | 5.0 ± 0.8 a | 7.3 ± 3.4 a | 8.0 ± 3.6 a | 4.0 ± 0.8 a | 1.7 ± 0.5 a | |
| Oviposition peak (d) | n = 30 | 8.3 ± 1.2 a | 7.3 ± 0.5 a | 8.6 ± 1.7 a | 8.7 ± 0.5 a | 7.7 ± 0.5 a | |
| Oviposition span (min–max d) | n = 30 | 6–14 | 6–19 | 5–20 | 6–15 | 7–9 | |
| Adult longevity (d) | ♂ | n = 30 | 20.5 ± 1.3 a | 21.4 ± 1.2 ab | 18.9 ± 0.7 ac | 14.7 ± 0.9 d | 10.5 ± 0.8 e |
| ♀ | n = 30 | 17.6 ± 1.0 a | 20.8 ± 0.8 b | 17.6 ± 0.9 ac | 13.1 ± 0.2 d | 10.1 ± 0.2 e | |
| total | n = 60 | 19.1 ± 1.1 a | 21.1 ± 1.0 b | 18.3 ± 0.7 ac | 13.9 ± 0.6 d | 10.3 ± 0.3 e | |
| Adult longevity (min–max d) | ♂ | n = 30 | 13–26 | 14–34 | 14–27 | 10–18 | 7–17 |
| ♀ | n = 30 | 10–28 | 13–33 | 13–25 | 9–17 | 7–15 | |
| total | n = 60 | 10–28 | 13–34 | 13–27 | 9–18 | 7–17 | |
| AL50 (d) | n = 60 | 19.0 ± 2.8 a | 20.0 ± 1.4 a | 17.7 ± 0.5 ab | 14.3 ± 0.5 b | 9.3 ± 0.5 c | |
| Female longevity after oviposition (d) | n = 30 | 6.7 ± 1.8 a | 11.5 ± 0.6 b | 8.4 ± 2.2 ac | 4.8 ± 0.4 ad | 2.4 ± 0.4 e | |
| Fecundity (egg clutches/10 females) | n = 30 | 7.0 ± 0.8 a | 9.0 ± 0.8 ab | 9.3 ± 0.5 ab | 6.3 ± 0.9 ac | 4.0 ± 0.8 c | |
| Egg clutch weight (mg) | n = 30 | 15.0 ± 2.1 a | 20.3 ± 1.0 a | 18.0 ± 3.3 a | 15.2 ± 2.9 a | 12.7 ± 2.9 a | |
| Span of egg clutch weight (min–max mg) | n = 30 | 11.3–31.8 | 10.7–33.0 | 5.8–41.7 | 3.4–42.0 | 8.7–22.1 | |
| Mean egg yield (mg eggs/10 females) | n = 30 | 106.9 ± 26.9 a | 182.4 ± 10.7 b | 167.7 ± 27.8 ab | 94.6 ± 16.4 ac | 52.5 ± 22.3 ac | |
| Total egg yield (mg eggs) | n = 30 | 320.8 | 489.5 | 487.0 | 308.9 | 157.6 | |
| Egg clutch size (eggs/clutch) | n = 30 | 626.5 ± 86.1 a | 847.4 ± 41.0 a | 751.1 ± 136.7 a | 633.8 ± 122.2 a | 527.8 ± 122.8 a | |
| Span of egg clutch size (min–max eggs) | n = 30 | 471–1325 | 271–1375 | 242–1738 | 163–1750 | 363–921 | |
| Multiple oviposition events (yes/no (number)) | n = 30 | yes (2) | yes (15) | yes (9) | yes (6) | yes (1) | |
| No oviposition (yes/no (number)) | n = 30 | yes (9) | yes (3) | yes (2) | yes (11) | yes (18) | |
| Parameters | n | Tap Water | 5% Glucose | 10% Glucose | 25% Glucose | 50% Glucose | |
|---|---|---|---|---|---|---|---|
| Adult preoviposition period (d) | n = 30 | 9.1 ± 1.1 a | 7.9 ± 1.5 ab | 7.6 ± 0.8 ab | 6.4 ± 0.3 b | 6.1 ± 0.3 b | |
| Oviposition period (d) | n = 30 | 5.0 ± 0.8 a | 3.3 ± 1.9 a | 3.3 ± 1.2 a | 2.0 ± 0.8 a | 1.7 ± 0.5 a | |
| Oviposition peak (d) | n = 30 | 8.3 ± 1.2 a | 8.3 ± 1.2 a | 8.0 ± 1.4 a | 6.4 ± 0.3 a | 5.8 ± 0.6 a | |
| Oviposition span (min–max d) | n = 30 | 6–14 | 5–11 | 5–10 | 5–8 | 5–7 | |
| Adult longevity (d) | ♂ | n = 30 | 20.5 ± 1.3 a | 15.0 ± 0.6 b | 13.6 ± 0.4 c | 8.2 ± 0.5 d | 7.8 ± 0.4 d |
| ♀ | n = 30 | 17.6 ± 1.0 a | 13.6 ± 1.6 b | 13.1 ± 0.4 b | 8.0 ± 0.7 c | 8.0 ± 0.4 c | |
| total | n = 60 | 19.1 ± 1.1 a | 14.3 ± 1.1 b | 13.3 ± 0.4 c | 8.1 ± 0.6 d | 7.9 ± 0.4 d | |
| Adult longevity (min–max d) | ♂ | n = 30 | 13–26 | 6–21 | 6–18 | 5–11 | 4–10 |
| ♀ | n = 30 | 10–28 | 6–23 | 5–18 | 5–12 | 4–10 | |
| total | n = 60 | 10–28 | 6–23 | 5–18 | 5–12 | 4–10 | |
| AL50 (d) | n = 60 | 19.0 ± 2.8 a | 13.7 ± 1.7 b | 14.0 ± 0.0 b | 8.0 ± 0.8 c | 7.7 ± 0.5 c | |
| Female longevity after oviposition (d) | n = 30 | 6.7 ± 1.8 a | 5.6 ± 2.1 a | 6.1 ± 1.1 a | 2.0 ± 1.0 b | 2.1 ± 0.5 b | |
| Fecundity (egg clutches/10 females) | n = 30 | 7.0 ± 0.8 a | 5.7 ± 0.5 ab | 4.7 ± 0.5 bc | 3.0 ± 0.8 c | 2.7 ± 0.5 c | |
| Egg clutch weight (mg) | n = 30 | 15.0 ± 2.1 a | 14.2 ± 0.2 a | 15.2 ± 1.5 a | 12.3 ± 1.7 a | 11.8 ± 2.2 a | |
| Span of egg clutch weight (min–max mg) | n = 30 | 11.3–31.8 | 5.8–21.8 | 9.5–22.3 | 4.2–22.5 | 3.9–20.3 | |
| Mean egg yield (mg eggs/10 females) | n = 30 | 106.9 ± 26.9 a | 80.7 ± 6.3 ab | 71.7 ± 13.7 ab | 38.2 ± 14.4 b | 32.3 ± 10.6 b | |
| Total egg yield (mg eggs) | n = 30 | 320.8 | 241.0 | 215.1 | 114.6 | 96.9 | |
| Egg clutch size (eggs/clutch) | n = 30 | 626.5 ± 86.1 a | 593.4 ± 7.6 a | 633.8 ± 63.0 a | 512.8 ± 72.6 a | 490.0 ± 91.4 a | |
| Span of egg clutch size (min–max eggs) | n = 30 | 471–1325 | 242–908 | 396–929 | 175–938 | 163–846 | |
| Multiple oviposition events (yes/no (number)) | n = 30 | yes (2) | yes (4) | yes (2) | no | no | |
| No oviposition (yes/no (number)) | n = 30 | yes (9) | yes (13) | yes (16) | yes (21) | yes (22) | |
| Parameters | n | Tap Water | 0.05% Spirulina | 0.1% Spirulina | 0.25% Spirulina | 0.5% Spirulina | |
|---|---|---|---|---|---|---|---|
| Adult preoviposition period (d) | n = 30 | 9.1 ± 1.1 a | 7.7 ± 0.9 ab | 7.3 ± 0.5 ab | 6.3 ± 0.5 b | 5.7 ± 0.6 b | |
| Oviposition period (d) | n = 30 | 5.0 ± 0.8 a | 8.7 ± 1.7 b | 3.3 ± 0.9 a | 3.0 ± 0.8 a | 1.7 ± 0.5 a | |
| Oviposition peak (d) | n = 30 | 8.3 ± 1.2 a | 5.7 ± 1.2 a | 6.3 ± 0.5 a | 6.7 ± 0.5 a | 5.7 ± 0.5 a | |
| Oviposition span (min–max d) | n = 30 | 6–14 | 4–15 | 6–10 | 4–9 | 4–8 | |
| Adult longevity (d) | ♂ | n = 30 | 20.5 ± 1.3 a | 13.8 ± 1.3 b | 13.3 ± 1.5 b | 10.1 ± 0.8 c | 8.1 ± 0.5 d |
| ♀ | n = 30 | 17.6 ± 1.0 a | 13.4 ± 0.5 b | 13.9 ± 0.2 b | 9.6 ± 0.4 c | 7.6 ± 0.6 d | |
| total | n = 60 | 19.1 ± 1.1 a | 13.6 ± 0.9 b | 13.6 ± 0.8 b | 9.9 ± 0.6 c | 7.9 ± 0.3 d | |
| Adult longevity (min–max d) | ♂ | n = 30 | 13–26 | 6–23 | 6–21 | 8–14 | 5–12 |
| ♀ | n = 30 | 10–28 | 9–17 | 11–18 | 7–13 | 5–13 | |
| total | n = 60 | 10–28 | 6–23 | 6–21 | 7–14 | 5–13 | |
| AL50 (d) | n = 60 | 19.0 ± 2.8 a | 13.7 ± 0.5 b | 14.0 ± 0.8 b | 9.3 ± 0.5 c | 7.3 ± 0.5 c | |
| Female longevity after oviposition (d) | n = 30 | 6.7 ± 1.8 a | 5.5 ± 0.9 a | 6.4 ± 0.6 a | 3.4 ± 0.7 b | 1.7 ± 0.3 c | |
| Fecundity (egg clutches/ 10 females) | n = 30 | 7.0 ± 0.8 a | 7.0 ± 0.8 a | 6.0 ± 0.8 a | 6.7 ± 0.9 a | 5.0 ± 0.8 a | |
| Egg clutch weight (mg) | n = 30 | 15.0 ± 2.1 a | 15.8 ± 3.7 a | 15.0 ± 0.9 a | 16.2 ± 2.0 a | 15.5 ± 1.6 a | |
| Span of egg clutch weight (min–max mg) | n = 30 | 11.3–31.8 | 3.8–29.2 | 4.8–23.1 | 9.2–23.6 | 9.8–21.5 | |
| Mean egg yield (mg eggs/10 females) | n = 30 | 106.9 ± 26.9 a | 107.7 ± 13.4 a | 90.4 ± 16.4 a | 106.0 ± 0.5 a | 76.4 ± 6.6 a | |
| Total egg yield (mg eggs) | n = 30 | 320.8 | 323.1 | 271.1 | 318.1 | 229.2 | |
| Egg clutch size (eggs/clutch) | n = 30 | 626.5 ± 86.1 a | 659.0 ± 154.0 a | 623.6 ± 39.4 a | 674.7 ± 84.9 a | 646.5 ± 68.1 a | |
| Span of egg clutch size (min–max eggs) | n = 30 | 471–1325 | 158–1217 | 200–963 | 383–983 | 408–896 | |
| Multiple oviposition events (yes/no (number)) | n = 30 | yes (2) | yes (2) | no | no | yes (1) | |
| No oviposition (yes/no (number)) | n = 30 | yes (9) | yes (9) | yes (12) | yes (10) | yes (15) | |
| Parameters | n | Tap Water | 0.05% Chlorella | 0.1% Chlorella | 0.25% Chlorella | 0.5% Chlorella | |
|---|---|---|---|---|---|---|---|
| Adult preoviposition period (d) | n = 30 | 9.1 ± 1.1 a | 7.4 ± 0.9 ab | 6.4 ± 1.2 b | 5.7 ± 0.5 b | 6.0 ± 0.6 b | |
| Oviposition period (d) | n = 30 | 5.0 ± 0.8 a | 7.7 ± 0.5 a | 6.7 ± 3.3 a | 2.0 ± 0.8 a | 3.7 ± 2.4 a | |
| Oviposition peak (d) | n = 30 | 8.3 ± 1.2 a | 5.0 ± 1.4 a | 5.3 ± 0.5 a | 6.0 ± 0.8 a | 6.2 ± 0.3 a | |
| Oviposition span (min–max d) | n = 30 | 6–14 | 3–13 | 3–15 | 4–7 | 4–11 | |
| Adult longevity (d) | ♂ | n = 30 | 20.5 ± 1.3 a | 16.9 ± 1.2 b | 15.7 ± 0.1 b | 10.5 ± 0.3 c | 9.5 ± 0.2 d |
| ♀ | n = 30 | 17.6 ± 1.0 a | 14.1 ± 0.5 b | 13.7 ± 0.1 b | 10.5 ± 0.6 c | 9.6 ± 0.4 d | |
| total | n = 60 | 19.1 ± 1.1 a | 15.5 ± 0.8 b | 14.7 ± 0.1 b | 10.5 ± 0.4 c | 9.6 ± 0.3 d | |
| Adult longevity (min–max d) | ♂ | n = 30 | 13–26 | 6–26 | 6–25 | 8–13 | 7–12 |
| ♀ | n = 30 | 10–28 | 9–19 | 11–16 | 8–13 | 7–12 | |
| total | n = 60 | 10–28 | 6–26 | 6–25 | 8–13 | 7–12 | |
| AL50 (d) | n = 60 | 19.0 ± 2.8 a | 14.0 ± 0.0 b | 14.0 ± 0.0 b | 10.7 ± 0.5 bc | 9.3 ± 0.9 c | |
| Female longevity after oviposition (d) | n = 30 | 6.7 ± 1.8 a | 6.6 ± 0.3 a | 7.4 ± 1.5 a | 4.9 ± 1.3 b | 4.0 ± 0.9 b | |
| Fecundity (egg clutches/10 females) | n = 30 | 7.0 ± 0.8 a | 7.3 ± 0.5 a | 7.3 ± 0.9 a | 3.3 ± 1.2 b | 4.0 ± 0.8 b | |
| Egg clutch weight (mg) | n = 30 | 15.0 ± 2.1 a | 16.7 ± 0.8 a | 15.0 ± 0.7 a | 15.8 ± 1.9 a | 14.4 ± 2.4 a | |
| Span of egg clutch weight (min–max mg) | n = 30 | 11.3–31.8 | 5.1–24.5 | 6.2–27.3 | 9.2–20.2 | 7.9–21.3 | |
| Mean egg yield (mg eggs/10 females) | n = 30 | 106.9 ± 26.9 a | 122.5 ± 13.4 a | 109.0 ± 10.0 a | 50.3 ± 12.4 b | 55.8 ± 5.2 b | |
| Total egg yield (mg eggs) | n = 30 | 320.8 | 367.6 | 327.1 | 150.8 | 167.4 | |
| Egg clutch size (eggs/clutch) | n = 30 | 626.5 ± 86.1 a | 694.3 ± 32.3 a | 623.1 ± 31.1 a | 658.3 ± 80.2 a | 600.6 ± 101.4 a | |
| Span of egg clutch size (min–max eggs) | n = 30 | 471–1325 | 213–1021 | 258–1138 | 383–842 | 329–888 | |
| Multiple oviposition events (yes/no (number)) | n = 30 | yes (2) | yes (5) | yes (5) | no | yes (1) | |
| No oviposition (yes/no (number)) | n = 30 | yes (9) | yes (8) | yes (8) | yes (20) | yes (18) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klüber, P.; Arous, E.; Zorn, H.; Rühl, M. Protein- and Carbohydrate-Rich Supplements in Feeding Adult Black Soldier Flies (Hermetia illucens) Affect Life History Traits and Egg Productivity. Life 2023, 13, 355. https://doi.org/10.3390/life13020355
Klüber P, Arous E, Zorn H, Rühl M. Protein- and Carbohydrate-Rich Supplements in Feeding Adult Black Soldier Flies (Hermetia illucens) Affect Life History Traits and Egg Productivity. Life. 2023; 13(2):355. https://doi.org/10.3390/life13020355
Chicago/Turabian StyleKlüber, Patrick, Emna Arous, Holger Zorn, and Martin Rühl. 2023. "Protein- and Carbohydrate-Rich Supplements in Feeding Adult Black Soldier Flies (Hermetia illucens) Affect Life History Traits and Egg Productivity" Life 13, no. 2: 355. https://doi.org/10.3390/life13020355
APA StyleKlüber, P., Arous, E., Zorn, H., & Rühl, M. (2023). Protein- and Carbohydrate-Rich Supplements in Feeding Adult Black Soldier Flies (Hermetia illucens) Affect Life History Traits and Egg Productivity. Life, 13(2), 355. https://doi.org/10.3390/life13020355





