Anti-Arthritic and Anti-Cancer Activities of Polyphenols: A Review of the Most Recent In Vitro Assays
Abstract
1. Introduction
2. Methodology
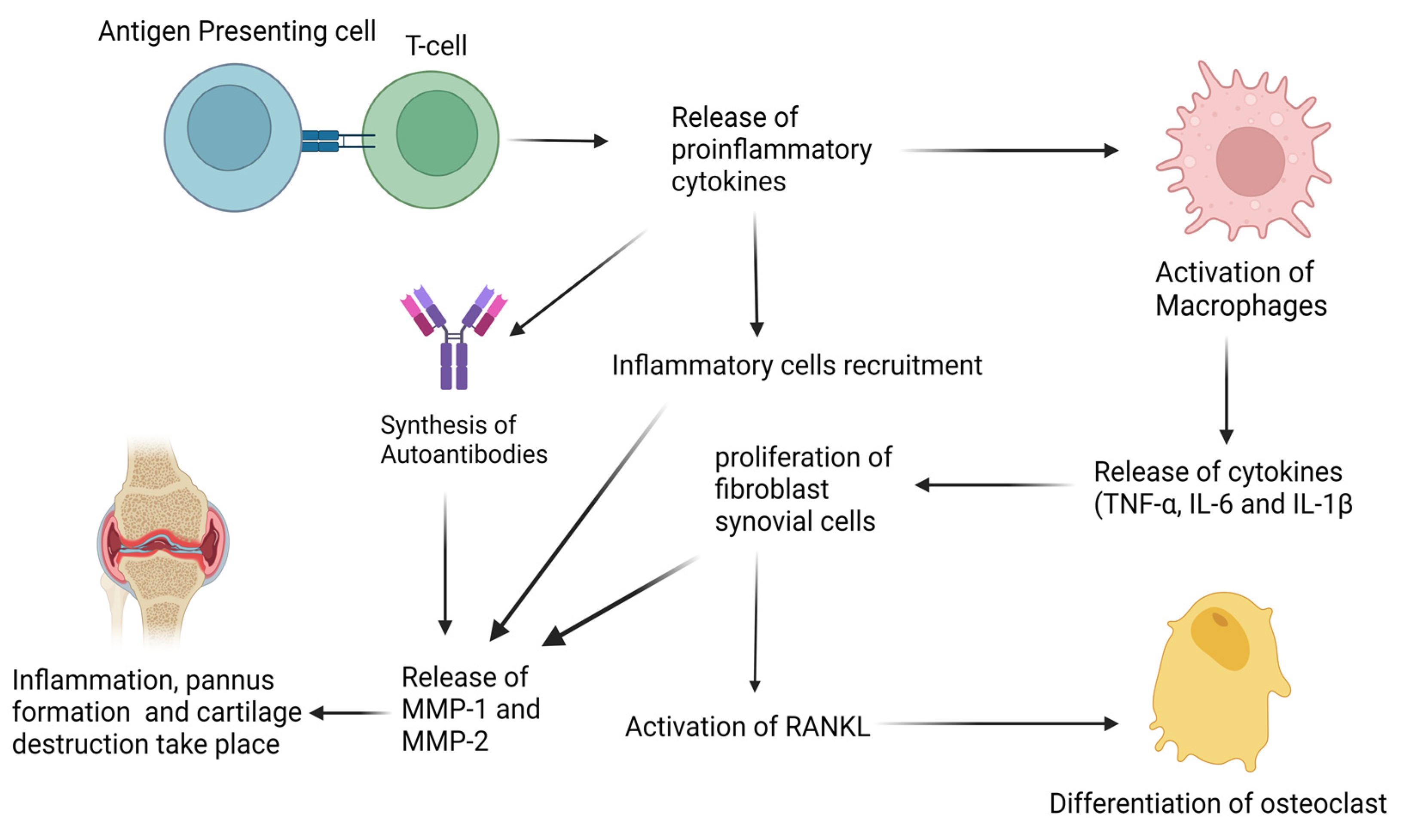
3. Biological Basis of Rheumatoid Arthritis
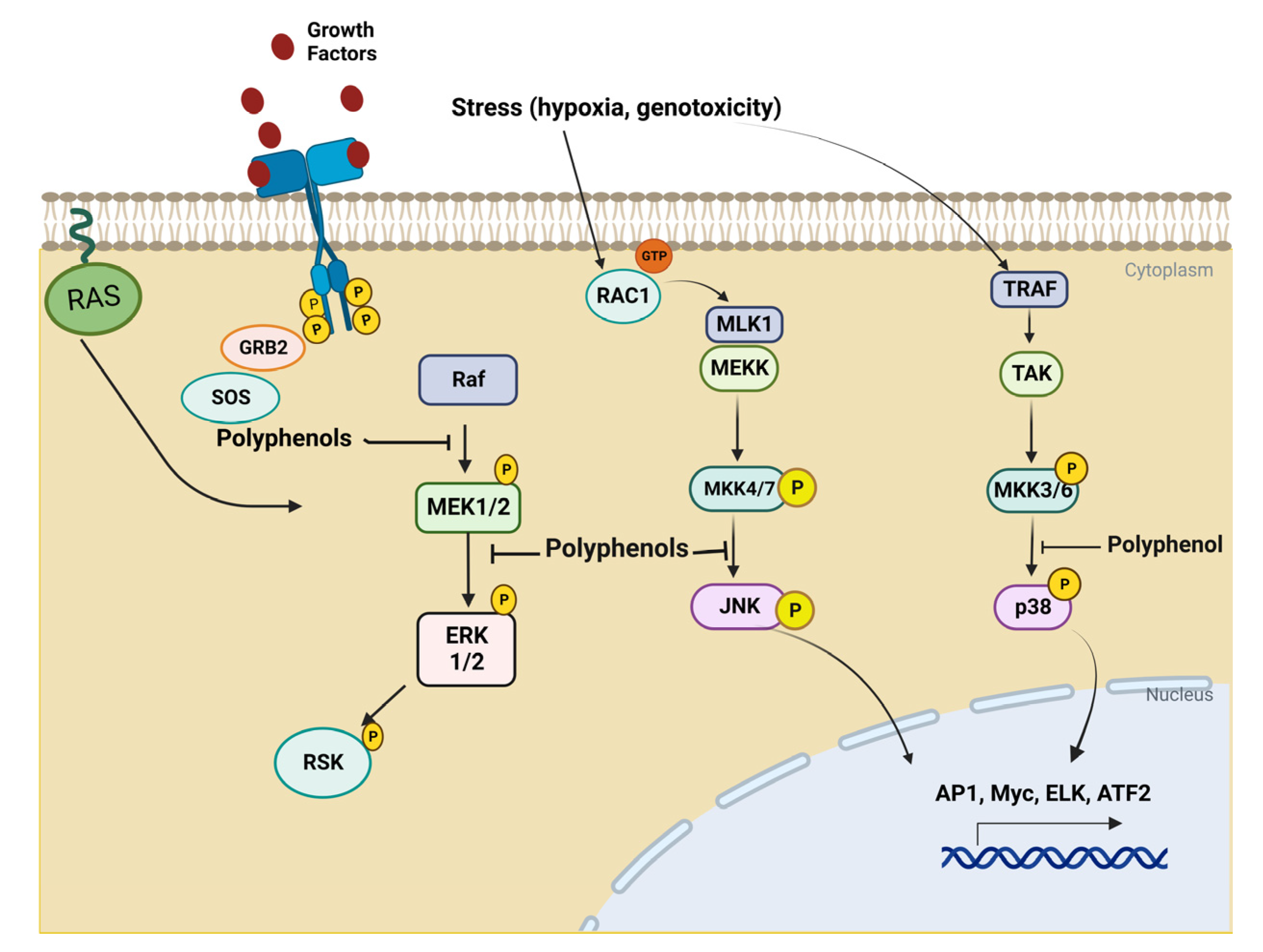
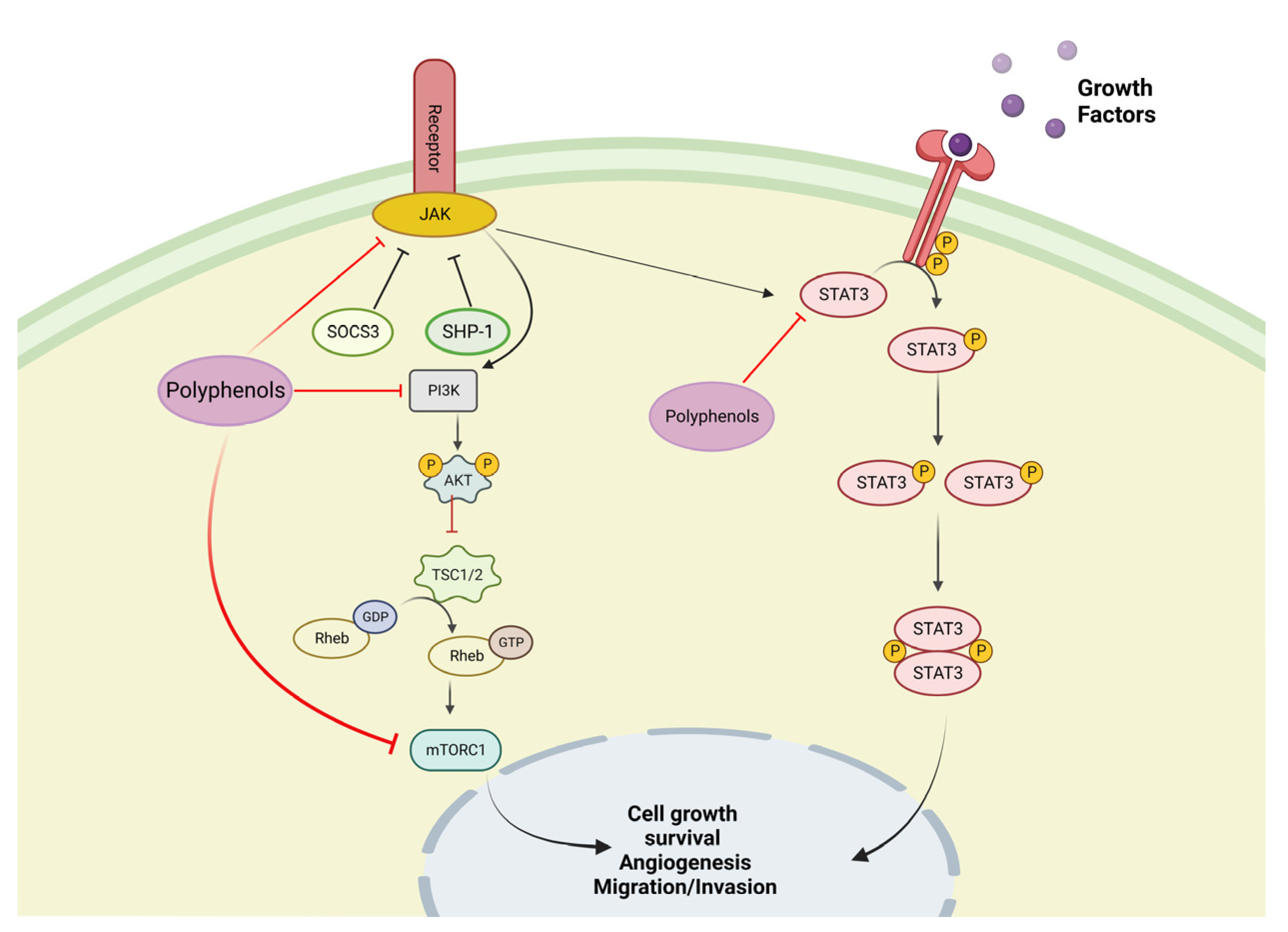
4. Biological Basis of Cancer Disease
5. In Vitro Test with Polyphenols
5.1. In Vitro Test with Olive Oil Polyphenols
5.1.1. In Vitro Test with Tyrosol on Rheumatoid Arthritis Cellular Models
5.1.2. In Vitro Test with Oleocanthal on Rheumatoid Arthritis Cellular Models
5.1.3. In Vitro Test with Oleuropein on Rheumatoid Arthritis Cellular Models
5.1.4. In Vitro Test with Hydroxytyrosol on Cancer Cell Lines
5.1.5. In Vitro Test with Luteolin on Cancer Cell Lines
5.2. In Vitro Test with Spice Polyphenols
5.2.1. In Vitro Test with Curcumin Polyphenols on Rheumatoid Arthritis Cellular Models
5.2.2. In Vitro Test with Curcumin Polyphenols on Cancer Cell Lines
5.2.3. In Vitro Test with Ginger Polyphenols on Cancer Cell Lines
5.2.4. In Vitro Test with Stilbenes on Rheumatoid Arthritis Cellular Models
5.3. In Vitro Test with Grapes Polyphenols
In Vitro Test with Resveratrol on Cancer Cell Lines
| Polyphenols | Cell Lines | Major Findings | References |
|---|---|---|---|
| Rheumatoid Arthitis | |||
| Hydroxytyrosol | SW982 | Decrease the expression of TNF, MMP and IL-6 | [114] |
| Tyrosol | RAW 264.7 macrophages | Decrease the release of inducible iNOS, COX-2, and phosphorylated-IκBα | [67] |
| Oleocanthal | J774 macrophages and ATDC5 chondrocytes | Inhibition of expression of iNOS and IL-6, and suppression of the expression of macrophage inflammatory protein-1 | [68] |
| Oleuropein | SW982 | Inhibition of cytokines IL-6, TNF-α, MMP-1, MMP-3, mPGES-1, and COX-2 expression | [69] |
| Curcumin | MH7A, FLS | Decrease in the production of VEGF and IL-6; inhibition of the ERK1/2 and NF-κB inflammatory pathways | [79] |
| Resveratrol | RA-FLS, MH-7A | Inhibition of the TNF—α—stimulated production of P-Akt, activation of NF-jB expression, increase in the expression of HO-1and NRF2 Downregulation of the expression of kelch-like ECH-related protein 1 (keap1), ROS, and MDA. Arrest of the expression NF-κB p65 and enhancement of Bcl-2/Bax expression. Activation of caspase-9 and caspase-3. Inhibition of the of MMP-3 and IL-1b protein expression. | [11,104,105] |
| Kaempferitrin | MH7A, RASFs | Decrease of IL-6, IL-1 β, MMP-1, MMP-3, COX-2, PGE2, and TNF-α levels. Inhibition of the activation of protein kinase B and NF-κB., NF-jB, and MAPK. | [65,106] |
| Gallicacid | RA-FLS | Inhibition of the expression of different chemokines, proinflammatory cytokines, MMP-9, and COX-2, as well as induction of cell apoptosis by regulation of Bcl-2, caspase-3, p53, and Bax. | [97] |
| Quercetin | RASFs | Inhibition of the expression of PGE2, COX-2, and HO-1 genes, as well as MMPs, by inhibiting various signaling pathways, including ERK1/2, p-38, JNK, NF-kB, and MAPK. | [108,113] |
| Cancer Disease | |||
| Hydroxytyrosol | LS180 MCF-7 | Upregulating pro-apoptotic genes such as BAX, CASP3, and P53, as well as increasing the BAX: BCL2 ratio and decreasing NFE2L2 expression, lowering oxidative stress, and inhibiting the P13K/Akt/mTOR pathway. | [80,82] |
| Luteolin | MCF-7 | Inhibition of MDA-MB-231, activation of the MAPK signaling pathway, expression of Notch signaling-related protein and mRNAs. Inhibition of ERK via Akt inactivation and others; inhibition of 9 in TPA. | [62,63,64] |
| Curcumin | T47D, MCF7, MDA-MB-231, and MDA-MB-468 | Suppresses of protein kinase B (Akt)/mTOR phosphorylation, decreased BCL2, and enhancement of BCL-2-associated X protein (BAX) and caspase 3 cleavage. Triggers G2/M phase cell cycle arrest and apoptosis, increasex P21 protein levels, suppresses Akt/mTOR phosphorylation, and stimulates the mitochondrial apoptotic pathway. | [73,96] |
| Gingerol | Human osteosarcoma cell line 143B. | Modulation of various signal pathways in cancerous cells, e.g., NF-KB, STAT3AP-1EGFR, VEGFRMAPK, and pro-inflammatory mediators. Activation of the AMP-activated protein kinase pathways and caspase cascades, and regulation of Bcl2 and Bax levels, resulting in the apoptosis of cells. | [77,100] |
| Resveratrol | LNCaP, HT29, SW480, and HCA-17 cell lines | Promotion of the accumulation of cells in S-phase, apoptosis process, and DNA damage. Inhibition of the receptor expression of XRCC1. | [106,107] |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Akanda, M.R.; Uddin, M.N.; Kim, I.-S.; Ahn, D.; Tae, H.-J.; Park, B.-Y. The Biological and Pharmacological Roles of Polyphenol Flavonoid Tilianin. Eur. J. Pharm. 2019, 842, 291–297. [Google Scholar] [CrossRef]
- Avtanski, D.; Poretsky, L. Phyto-Polyphenols as Potential Inhibitors of Breast Cancer Metastasis. Mol. Med. 2018, 24, 29. [Google Scholar] [CrossRef]
- Moalin, M.; van Strijdonck, G.P.F.; Beckers, M.; Hagemen, G.J.; Borm, P.J.; Bast, A.; Haenen, G.R.M.M. A Planar Conformation and the Hydroxyl Groups in the B and C Rings Play a Pivotal Role in the Antioxidant Capacity of Quercetin and Quercetin Derivatives. Molecules 2011, 16, 9636–9650. [Google Scholar] [CrossRef]
- Mateen, S.; Moin, S.; Zafar, A.; Khan, A.Q. Redox Signaling in Rheumatoid Arthritis and the Preventive Role of Polyphenols. Clin. Chim. Acta 2016, 463, 4–10. [Google Scholar] [CrossRef]
- Li, S.; Hu, T.; Yuan, T.; Cheng, D.; Yang, Q. Nucleoside Diphosphate Kinase B Promotes Osteosarcoma Proliferation through C-Myc. Cancer Biol. Ther. 2018, 19, 565–572. [Google Scholar] [CrossRef]
- Behl, T.; Sharma, A.; Sharma, L.; Sehgal, A.; Singh, S.; Sharma, N.; Zengin, G.; Bungau, S.; Toma, M.M.; Gitea, D.; et al. Current Perspective on the Natural Compounds and Drug Delivery Techniques in Glioblastoma Multiforme. Cancers 2021, 13, 2765. [Google Scholar] [CrossRef]
- Hazafa, A.; Rehman, K.U.; Jahan, N.; Jabeen, Z. The Role of Polyphenol (Flavonoids) Compounds in the Treatment of Cancer Cells. Nutr. Cancer 2020, 72, 386–397. [Google Scholar] [CrossRef]
- Vetal, S.; Bodhankar, S.L.; Mohan, V.; Thakurdesai, P.A. Anti-Inflammatory and Anti-Arthritic Activity of Type-A Procyanidine Polyphenols from Bark of Cinnamomum Zeylanicum in Rats. Food Sci. Hum. Wellness 2013, 2, 59–67. [Google Scholar] [CrossRef]
- Silva, S.; Sepodes, B.; Rocha, J.; Direito, R.; Fernandes, A.; Brites, D.; Freitas, M.; Fernandes, E.; Bronze, M.R.; Figueira, M.E. Protective Effects of Hydroxytyrosol-Supplemented Refined Olive Oil in Animal Models of Acute Inflammation and Rheumatoid Arthritis. J. Nutr. Biochem. 2015, 26, 360–368. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, Q. Prevalence Trend and Disparities in Rheumatoid Arthritis among US Adults, 2005–2018. J. Clin. Med. 2021, 10, 3289. [Google Scholar] [CrossRef]
- Tian, J.; Chen, J.; Gao, J.; Li, L.; Xie, X. Resveratrol Inhibits TNF-α-Induced IL-1β, MMP-3 Production in Human Rheumatoid Arthritis Fibroblast-like Synoviocytes via Modulation of PI3kinase/Akt Pathway. Rheumatol. Int. 2013, 33, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Deligiannidou, G.-E.; Gougoula, V.; Bezirtzoglou, E.; Kontogiorgis, C.; Constantinides, T.K. The Role of Natural Products in Rheumatoid Arthritis: Current Knowledge of Basic In Vitro and In Vivo Research. Antioxidants 2021, 10, 599. [Google Scholar] [CrossRef]
- Kurowska, W.; Kuca-Warnawin, E.H.; Radzikowska, A.; Maśliński, W. The Role of Anti-Citrullinated Protein Antibodies (ACPA) in the Pathogenesis of Rheumatoid Arthritis. Cent. Eur. J. Immunol. 2017, 42, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Bizzaro, N.; Bartoloni, E.; Morozzi, G.; Manganelli, S.; Riccieri, V.; Sabatini, P.; Filippini, M.; Tampoia, M.; Afeltra, A.; Sebastiani, G.; et al. Anti-Cyclic Citrullinated Peptide Antibody Titer Predicts Time to Rheumatoid Arthritis Onset in Patients with Undifferentiated Arthritis: Results from a 2-Year Prospective Study. Arthritis Res. Ther. 2013, 15, R16. [Google Scholar] [CrossRef] [PubMed]
- Klareskog, L.; Stolt, P.; Lundberg, K.; Källberg, H.; Bengtsson, C.; Grunewald, J.; Rönnelid, J.; Erlandsson Harris, H.; Ulfgren, A.-K.; Rantapää-Dahlqvist, S.; et al. A New Model for an Etiology of Rheumatoid Arthritis: Smoking May Trigger HLA–DR (Shared Epitope)–Restricted Immune Reactions to Autoantigens Modified by Citrullination. Arthritis Rheum. 2006, 54, 38–46. [Google Scholar] [CrossRef]
- Konig, M.F.; Abusleme, L.; Reinholdt, J.; Palmer, R.J.; Teles, R.P.; Sampson, K.; Rosen, A.; Nigrovic, P.A.; Sokolove, J.; Giles, J.T.; et al. Aggregatibacter Actinomycetemcomitans–Induced Hypercitrullination Links Periodontal Infection to Autoimmunity in Rheumatoid Arthritis. Sci. Transl. Med. 2016, 8, 369. [Google Scholar] [CrossRef]
- Wegner, N.; Wait, R.; Sroka, A.; Eick, S.; Nguyen, K.-A.; Lundberg, K.; Kinloch, A.; Culshaw, S.; Potempa, J.; Venables, P.J. Peptidylarginine Deiminase from Porphyromonas Gingivalis Citrullinates Human Fibrinogen and α-Enolase: Implications for Autoimmunity in Rheumatoid Arthritis. Arthritis Rheum. 2010, 62, 2662–2672. [Google Scholar] [CrossRef]
- Firestein, G.S.; McInnes, I.B. Immunopathogenesis of Rheumatoid Arthritis. Immunity 2017, 46, 183–196. [Google Scholar] [CrossRef]
- Kakkar, M.; Behl, T.; Nijhawan, P.; Grover, M.; Jaglan, D.; Pandey, R.K.; Binjam, K.R. Exploration of Combined Effect of Peroxisome Proliferator Activated Receptor (PPAR) Agonist and Retinoic Acid Receptor (RAR) Agonist on Experimental Models of Inflammation in Rats. Obes. Med. 2020, 19, 100260. [Google Scholar] [CrossRef]
- Rocha, J.; Sepodes, B.; Eduardo-Figueira, M. Phenolic Compounds Impact on Rheumatoid Arthritis, Inflammatory Bowel Disease and Microbiota Modulation. Pharmaceutics 2021, 13, 145. [Google Scholar] [CrossRef]
- Marino, A.; Paterniti, I.; Cordaro, M.; Morabito, R.; Campolo, M.; Navarra, M.; Esposito, E.; Cuzzocrea, S. Role of Natural Antioxidants and Potential Use of Bergamot in Treating Rheumatoid Arthritis. PharmaNutrition 2015, 3, 53–59. [Google Scholar] [CrossRef]
- Shrivastava, A.K.; Singh, H.V.; Raizada, A.; Singh, S.K.; Pandey, A.; Singh, N.; Yadav, D.S.; Sharma, H. Inflammatory Markers in Patients with Rheumatoid Arthritis. Allergol. Immunopathol. 2015, 43, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Zengin, O.; Onder, M.E.; Kalem, A.; Bilici, M.; Türkbeyler, I.H.; Ozturk, Z.A.; Kisacik, B.; Onat, A.M. New Inflammatory Markers in Early Rheumatoid Arthritis. Z Rheumatol. 2018, 77, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Landewé, R.B.M.; Bijlsma, J.W.J.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; McInnes, I.B.; Sepriano, A.; van Vollenhoven, R.F.; de Wit, M.; et al. EULAR Recommendations for the Management of Rheumatoid Arthritis with Synthetic and Biological Disease-Modifying Antirheumatic Drugs: 2019 Update. Ann. Rheum. Dis. 2020, 79, 685–699. [Google Scholar] [CrossRef]
- Behl, T.; Mehta, K.; Sehgal, A.; Singh, S.; Sharma, N.; Ahmadi, A.; Arora, S.; Bungau, S. Exploring the Role of Polyphenols in Rheumatoid Arthritis. Crit. Rev. Food Sci. Nutr. 2021, 71, 104003. [Google Scholar] [CrossRef]
- Lee, C.-J.; Moon, S.-J.; Jeong, J.-H.; Lee, S.; Lee, M.-H.; Yoo, S.-M.; Lee, H.S.; Kang, H.C.; Lee, J.Y.; Lee, W.S.; et al. Kaempferol Targeting on the Fibroblast Growth Factor Receptor 3-Ribosomal S6 Kinase 2 Signaling Axis Prevents the Development of Rheumatoid Arthritis. Cell Death Dis. 2018, 9, 401. [Google Scholar] [CrossRef]
- Nakahama, T.; Kimura, A.; Nguyen, N.T.; Chinen, I.; Hanieh, H.; Nohara, K.; Fujii-Kuriyama, Y.; Kishimoto, T. Aryl Hydrocarbon Receptor Deficiency in T Cells Suppresses the Development of Collagen-Induced Arthritis. Proc. Natl. Acad. Sci. USA 2011, 108, 14222–14227. [Google Scholar] [CrossRef]
- Lina, C.; Conghua, W.; Nan, L.; Ping, Z. Combined Treatment of Etanercept and MTX Reverses Th1/Th2, Th17/Treg Imbalance in Patients with Rheumatoid Arthritis. J. Clin. Immunol. 2011, 31, 596–605. [Google Scholar] [CrossRef]
- Ramiro, S.; Sepriano, A.; Chatzidionysiou, K.; Nam, J.L.; Smolen, J.S.; van der Heijde, D.; Dougados, M.; van Vollenhoven, R.; Bijlsma, J.W.; Burmester, G.R.; et al. Safety of Synthetic and Biological DMARDs: A Systematic Literature Review Informing the 2016 Update of the EULAR Recommendations for Management of Rheumatoid Arthritis. Ann. Rheum. Dis. 2017, 76, 1101. [Google Scholar] [CrossRef]
- Ganesan, M.; Eikenberry, A.; Poluektova, L.Y.; Kharbanda, K.K.; Osna, N.A. Role of Alcohol in Pathogenesis of Hepatitis B Virus Infection. World J. Gastroenterol. 2020, 26, 883–903. [Google Scholar] [CrossRef]
- Debras, C.; Chazelas, E.; Srour, B.; Kesse-Guyot, E.; Julia, C.; Zelek, L.; Agaësse, C.; Druesne-Pecollo, N.; Galan, P.; Hercberg, S.; et al. Total and Added Sugar Intakes, Sugar Types, and Cancer Risk: Results from the Prospective NutriNet-Santé Cohort. Am. J. Clin. Nutr. 2020, 112, 1267–1279. [Google Scholar] [CrossRef] [PubMed]
- Tsigalou, C.; Konstantinidis, T.; Paraschaki, A.; Stavropoulou, E.; Voidarou, C.; Bezirtzoglou, E. Mediterranean Diet as a Tool to Combat Inflammation and Chronic Diseases. An Overview. Biomedicines 2020, 8, 201. [Google Scholar] [CrossRef] [PubMed]
- Cárdeno, A.; Sánchez-Hidalgo, M.; Alarcón-de-la-Lastra, C. An Up-Date of Olive Oil Phenols in Inflammation and Cancer: Molecular Mechanisms and Clinical Implications. Curr. Med. Chem. 2013, 20, 4758–4776. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Soto, M.; Sánchez-Hidalgo, M.; Rosillo, M.Á.; Castejón, M.L.; Alarcón-de-la-Lastra, C. Extra Virgin Olive Oil: A Key Functional Food for Prevention of Immune-Inflammatory Diseases. Food Funct. 2016, 7, 4492–4505. [Google Scholar] [CrossRef] [PubMed]
- Cárdeno, A.; Sánchez-Hidalgo, M.; Rosillo, M.A.; de la Lastra, C.A. Oleuropein, a Secoiridoid Derived from Olive Tree, Inhibits the Proliferation of Human Colorectal Cancer Cell Through Downregulation of HIF-1α. Null 2013, 65, 147–156. [Google Scholar] [CrossRef]
- Rosillo, M.Á.; Sánchez-Hidalgo, M.; Castejón, M.L.; Montoya, T.; González-Benjumea, A.; Fernández-Bolaños, J.G.; Alarcón-de-la-Lastra, C. Extra-Virgin Olive Oil Phenols Hydroxytyrosol and Hydroxytyrosol Acetate, down-Regulate the Production of Mediators Involved in Joint Erosion in Human Synovial Cells. J. Funct. Foods 2017, 36, 27–33. [Google Scholar] [CrossRef]
- Cháirez-Ramírez, M.H.; de la Cruz-López, K.G.; García-Carrancá, A. Polyphenols as Antitumor Agents Targeting Key Players in Cancer-Driving Signaling Pathways. Front. Pharmacol. 2021, 12, 710304. [Google Scholar] [CrossRef]
- Amawi, H.; Ashby, C.; Samuel, T.; Peraman, R.; Tiwari, A. Polyphenolic Nutrients in Cancer Chemoprevention and Metastasis: Role of the Epithelial-to-Mesenchymal (EMT) Pathway. Nutrients 2017, 9, 911. [Google Scholar] [CrossRef]
- Sharma, A.; Kaur, M.; Katnoria, J.K.; Nagpal, A.K. Polyphenols in Food: Cancer Prevention and Apoptosis Induction. Curr. Med. Chem. 2018, 25, 4740–4757. [Google Scholar] [CrossRef]
- Jiang, Q.; Yang, M.; Qu, Z.; Zhou, J.; Zhang, Q. Resveratrol Enhances Anticancer Effects of Paclitaxel in HepG2 Human Liver Cancer Cells. BMC Complement Altern. Med. 2017, 17, 477. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Arnan, X.; Claramunt-López, B.; Martinez Vilalta, J.; Estorach, M.; Poyatos, R. The Age of Monumental Olive Trees (Olea Europaea) in Northeastern Spain. Dendrochronologia 2012, 30, 11–14. [Google Scholar] [CrossRef]
- Finicelli, M.; Squillaro, T.; Galderisi, U.; Peluso, G. Polyphenols, the Healthy Brand of Olive Oil: Insights and Perspectives. Nutrients 2021, 13, 3831. [Google Scholar] [CrossRef] [PubMed]
- Serreli, G.; Deiana, M. Biological Relevance of Extra Virgin Olive Oil Polyphenols Metabolites. Antioxidants 2018, 7, 170. [Google Scholar] [CrossRef]
- Surachmanto, E.E.; Datau, E.A. The Role of Omega-3 Fatty Acids Contained in Olive Oil. Acta Med. Indones. 2011, 43, 138–143. [Google Scholar]
- Reboredo-Rodríguez, P.; Varela-López, A.; Forbes-Hernández, T.Y.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; Zhang, J.; Manna, P.P.; Bompadre, S.; Quiles, J.L.; et al. Phenolic Compounds Isolated from Olive Oil as Nutraceutical Tools for the Prevention and Management of Cancer and Cardiovascular Diseases. Int. J. Mol. Sci. 2018, 19, 2305. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, By-Products, and Leaf of Olea Europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef]
- de Souza, P.A.L.; Marcadenti, A.; Portal, V.L. Effects of Olive Oil Phenolic Compounds on Inflammation in the Prevention and Treatment of Coronary Artery Disease. Nutrients 2017, 9, 1087. [Google Scholar] [CrossRef]
- Rodriguez, M.G.; Caleja, C.; Nuñez-Estevez, B.; Pereira, E.; Fraga-Corral, M.; Reis, F.S.; Simal-Gandara, J.; Ferreira, I.C.F.R.; Prieto, M.A.; Barros, L. Flavonoids: A Group of Potential Food Additives with Beneficial Health Effects. In Natural Food Additives; Prieto, M.A., Otero, P., Eds.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Sun, L.; Luo, C.; Liu, J. Hydroxytyrosol Induces Apoptosis in Human Colon Cancer Cells through ROS Generation. Food Funct. 2014, 5, 1909–1914. [Google Scholar] [CrossRef]
- de Torres, A.; Espínola, F.; Moya, M.; Alcalá, S.; Vidal, A.M.; Castro, E. Assessment of Phenolic Compounds in Virgin Olive Oil by Response Surface Methodology with Particular Focus on Flavonoids and Lignans. LWT 2018, 90, 22–30. [Google Scholar] [CrossRef]
- Opara, E.; Chohan, M. Culinary Herbs and Spices: Their Bioactive Properties, the Contribution of Polyphenols and the Challenges in Deducing Their True Health Benefits. Int. J. Mol. Sci. 2014, 15, 19183–19202. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Raffoul, J.J. Potential Anticancer Properties of Grape Antioxidants. J. Oncol. 2012, 2012, 803294. [Google Scholar] [CrossRef] [PubMed]
- Karami, S.; Rahimi, M.; Babaei, A. An Overview on the Antioxidant, Anti-Inflammatory, Antimicrobial and Anti-Cancer Activity of Grape Extract. Biomed. Res. Clin. Prac. 2018, 3. [Google Scholar] [CrossRef]
- Pastrana-Bonilla, E.; Akoh, C.C.; Sellappan, S.; Krewer, G. Phenolic Content and Antioxidant Capacity of Muscadine Grapes. J. Agric. Food Chem. 2003, 51, 5497–5503. [Google Scholar] [CrossRef]
- Gitea, M.A.; Bungau, S.G.; Gitea, D.; Pasca, B.M.; Purza, A.L.; Radu, A.-F. Evaluation of the Phytochemistry–Therapeutic Activity Relationship for Grape Seeds Oil. Life 2023, 13, 178. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Q. Kaempferitrin Inhibits Proliferation, Induces Apoptosis, and Ameliorates Inflammation in Human Rheumatoid Arthritis Fibroblast-like Synoviocytes. Phytother. Res. 2019, 33, 1726–1735. [Google Scholar] [CrossRef]
- Valcarce, D.G.; Robles, V. Effect of Captivity and Cryopreservation on ROS Production in Solea Senegalensis Spermatozoa. Reproduction 2016, 152, 439–446. [Google Scholar] [CrossRef]
- Abbas, H.; El-Feky, Y.A.; Al-Sawahli, M.M.; EL-Deeb, N.M.; El-Nassan, H.B.; Zewail, M. Development and Optimization of Curcumin Analog Nano-Bilosomes Using 21.31 Full Factorial Design for Anti-Tumor Profiles Improvement in Human Hepatocellular Carcinoma: In-Vitro Evaluation, in-Vivo Safety Assay. Drug Deliv. 2022, 29, 714–727. [Google Scholar] [CrossRef]
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.R. In Vitro Assays and Techniques Utilized in Anticancer Drug Discovery: In Vitro Assays in Anticancer Drug Development. J. Appl. Toxicol. 2019, 39, 38–71. [Google Scholar] [CrossRef]
- Staton, C.A.; Reed, M.W.R.; Brown, N.J. A Critical Analysis of Current in Vitro and in Vivo Angiogenesis Assays. Int. J. Exp. Pathol. 2009, 90, 195–221. [Google Scholar] [CrossRef]
- Chang, J.-H.; Lee, K.-J.; Kim, S.-K.; Yoo, D.-H.; Kang, T.-Y. Validity of SW982 Synovial Cell Line for Studying the Drugs against Rheumatoid Arthritis in Fluvastatin-Induced Apoptosis Signaling Model. Indian J. Med. Res. 2014, 139, 117–124. [Google Scholar] [PubMed]
- Awasthi, A.; Kumar, P.; Srikanth, C.V.; Sahi, S.; Puria, R. Invitro Evaluation of Torin2 and 2, 6-Dihydroxyacetophenone in Colorectal Cancer Therapy. Pathol. Oncol. Res. 2019, 25, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Yang, Y.; Wu, W.; Wan, H.; Li, X.; Zhong, M.; Su, X.; Jia, S.; Lin, N. Triterpenoid Saponin W3 from Anemone Flaccida Suppresses Osteoclast Differentiation through Inhibiting Activation of MAPKs and NF-ΚB Pathways. Int. J. Biol. Sci. 2015, 11, 1204–1214. [Google Scholar] [CrossRef] [PubMed]
- Benton, G.; Arnaoutova, I.; George, J.; Kleinman, H.K.; Koblinski, J. Matrigel: From Discovery and ECM Mimicry to Assays and Models for Cancer Research. Adv. Drug Deliv. Rev. 2014, 79–80, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Huang, Y.; Mo, D.; Ma, N.; Gao, F.; Song, L.; Sun, X.; Xu, X.; Liu, L.; Huo, X.; et al. Tyrosol Attenuates Pro-Inflammatory Cytokines from Cultured Astrocytes and NF-ΚB Activation in in Vitro Oxygen Glucose Deprivation. Neurochem. Int. 2018, 121, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-Y.; Lee, S.; Kim, M.-J.; Kang, B.-C.; Dhakal, H.; Choi, Y.-A.; Park, P.-H.; Choi, H.; Shin, T.-Y.; Choi, H.G.; et al. Tyrosol Attenuates Lipopolysaccharide-Induced Acute Lung Injury by Inhibiting the Inflammatory Response and Maintaining the Alveolar Capillary Barrier. Food Chem. Toxicol. 2017, 109, 526–533. [Google Scholar] [CrossRef]
- Scotece, M.; Gómez, R.; Conde, J.; Lopez, V.; Gómez-Reino, J.J.; Lago, F.; Smith, A.B.; Gualillo, O. Further Evidence for the Anti-Inflammatory Activity of Oleocanthal: Inhibition of MIP-1α and IL-6 in J774 Macrophages and in ATDC5 Chondrocytes. Life Sci. 2012, 91, 1229–1235. [Google Scholar] [CrossRef]
- Castejón, M.L.; Rosillo, M.Á.; Montoya, T.; González-Benjumea, A.; Fernández-Bolaños, J.M.; Alarcón-de-la-Lastra, C. Oleuropein Down-Regulated IL-1β-Induced Inflammation and Oxidative Stress in Human Synovial Fibroblast Cell Line SW982. Food Funct. 2017, 8, 1890–1898. [Google Scholar] [CrossRef]
- Hormozi, M.; Salehi Marzijerani, A.; Baharvand, P. Effects of Hydroxytyrosol on Expression of Apoptotic Genes and Activity of Antioxidant Enzymes in LS180 Cells. Cancer Manag. Res. 2020, 12, 7913–7919. [Google Scholar] [CrossRef]
- Francesco, A.D.; Falconi, A.; Germanio, C.D.; Bonaventura, M.V.M.D.; Costa, A.; Caramuta, S.; Carlo, M.D.; Compagnone, D.; Dainese, E.; Cifani, C.; et al. Extravirgin Olive Oil Up-Regulates CB1 Tumor Suppressor Gene in Human Colon Cancer Cells and in Rat Colon via Epigenetic Mechanisms. J. Nutr. Biochem. 2015, 26, 250–258. [Google Scholar] [CrossRef]
- Calahorra, J.; Martínez-Lara, E.; Granadino-Roldán, J.M.; Martí, J.M.; Cañuelo, A.; Blanco, S.; Oliver, F.J.; Siles, E. Crosstalk between Hydroxytyrosol, a Major Olive Oil Phenol, and HIF-1 in MCF-7 Breast Cancer Cells. Sci. Rep. 2020, 10, 6361. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.-W.; Zhang, H.-D.; Mao, L.; Mao, C.-F.; Chen, W.; Cui, M.; Ma, R.; Cao, H.-X.; Jing, C.-W.; Wang, Z.; et al. Luteolin Inhibits Breast Cancer Development and Progression In Vitro and In Vivo by Suppressing Notch Signaling and Regulating MiRNAs. Cell Physiol. Biochem. 2015, 37, 1693–1711. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.W.; Ahn, Y.E.; Chung, W.S.; Choi, H.J.; Suh, Y.J. Synergistic Effect between Celecoxib and Luteolin Is Dependent on Estrogen Receptor in Human Breast Cancer Cells. Tumor Biol. 2015, 36, 6349–6359. [Google Scholar] [CrossRef]
- Park, S.-H.; Kim, J.-H.; Lee, D.-H.; Kang, J.-W.; Song, H.-H.; Oh, S.-R.; Yoon, D.-Y. Luteolin 8-C-β-Fucopyranoside Inhibits Invasion and Suppresses TPA-Induced MMP-9 and IL-8 via ERK/AP-1 and ERK/NF-ΚB Signaling in MCF-7 Breast Cancer Cells. Biochimie 2013, 95, 2082–2090. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Woo, J.S.; Kwon, C.H.; Kim, J.H.; Kim, Y.K.; Kim, K.H. Luteolin Induces Apoptotic Cell Death through AIF Nuclear Translocation Mediated by Activation of ERK and P38 in Human Breast Cancer Cell Lines. Cell. Biol. Int. 2012, 36, 339–344. [Google Scholar] [CrossRef]
- Cai, X.; Lu, W.; Ye, T.; Lu, M.; Wang, J.; Huo, J.; Qian, S.; Wang, X.; Cao, P. The Molecular Mechanism of Luteolin-Induced Apoptosis Is Potentially Related to Inhibition of Angiogenesis in Human Pancreatic Carcinoma Cells. Oncol. Rep. 2012, 28, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Pratheeshkumar, P.; Son, Y.-O.; Budhraja, A.; Wang, X.; Ding, S.; Wang, L.; Hitron, A.; Lee, J.-C.; Kim, D.; Divya, S.P.; et al. Luteolin Inhibits Human Prostate Tumor Growth by Suppressing Vascular Endothelial Growth Factor Receptor 2-Mediated Angiogenesis. PLoS ONE 2012, 7, e52279. [Google Scholar] [CrossRef]
- Kloesch, B.; Becker, T.; Dietersdorfer, E.; Kiener, H.; Steiner, G. Anti-Inflammatory and Apoptotic Effects of the Polyphenol Curcumin on Human Fibroblast-like Synoviocytes. Int. Immunopharmacol. 2013, 15, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H. Mechanistic Insight into Osteoclast Differentiation in Osteoimmunology. J. Mol. Med. 2005, 83, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.T.; Araki, Y.; Sato, K.; Aizaki, Y.; Yokota, K.; Kim, Y.T.; Oda, H.; Kurokawa, R.; Mimura, T. Aberrant Histone Acetylation Contributes to Elevated Interleukin-6 Production in Rheumatoid Arthritis Synovial Fibroblasts. Biochem. Biophys. Res. Commun. 2014, 444, 682–686. [Google Scholar] [CrossRef]
- Lian, Y.-T.; Yang, X.-F.; Wang, Z.-H.; Yang, Y.; Yang, Y.; Shu, Y.-W.; Cheng, L.-X.; Liu, K. Curcumin Serves as a Human Kv1.3 Blocker to Inhibit Effector Memory T Lymphocyte Activities: INHIBITION OF KV1.3 CHANNEL AND T EM CELL BY CURCUMIN. Phytother. Res. 2013, 27, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Kronski, E.; Fiori, M.E.; Barbieri, O.; Astigiano, S.; Mirisola, V.; Killian, P.H.; Bruno, A.; Pagani, A.; Rovera, F.; Pfeffer, U.; et al. MiR181b Is Induced by the Chemopreventive Polyphenol Curcumin and Inhibits Breast Cancer Metastasis via Down-Regulation of the Inflammatory Cytokines CXCL1 and -2. Mol. Oncol. 2014, 8, 581–595. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-T.; Ho, Y.-S. Anticancer Effect of Curcumin on Breast Cancer and Stem Cells. Food Sci. Hum. Wellness 2018, 7, 134–137. [Google Scholar] [CrossRef]
- Abraham, J. PI3K/AKT/MTOR Pathway Inhibitors: The Ideal Combination Partners for Breast Cancer Therapies? Expert Rev. Anticancer Ther. 2015, 15, 51–68. [Google Scholar] [CrossRef]
- Hu, S.; Xu, Y.; Meng, L.; Huang, L.; Sun, H. Curcumin Inhibits Proliferation and Promotes Apoptosis of Breast Cancer Cells. Exp. Med. 2018, 16, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Gao, Q.; Chen, K.; Wang, Y.; Chen, L.; Li, X. Curcumin Suppresses Migration and Invasion of Human Endometrial Carcinoma Cells. Oncol. Lett. 2015, 10, 1297–1302. [Google Scholar] [CrossRef]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A.M. Gingerols and Shogaols: Important Nutraceutical Principles from Ginger. Phytochemistry 2015, 117, 554–568. [Google Scholar] [CrossRef]
- de Lima, R.M.T.; dos Reis, A.C.; de Menezes, A.-A.P.M.; de Oliveira Santos, J.V.; de Oliveira Filho, J.W.G.; de Oliveira Ferreira, J.R.; de Alencar, M.V.O.B.; da Mata, A.M.O.F.; Khan, I.N.; Islam, A.; et al. Protective and Therapeutic Potential of Ginger (Zingiber Officinale) Extract and [6]-Gingerol in Cancer: A Comprehensive Review: Ginger Extract and [6]-Gingerol as Anticancer Agents. Phytother. Res. 2018, 32, 1885–1907. [Google Scholar] [CrossRef]
- Fan, J.; Yang, X.; Bi, Z. 6-Gingerol Inhibits Osteosarcoma Cell Proliferation through Apoptosis and AMPK Activation. Tumor Biol. 2015, 36, 1135–1141. [Google Scholar] [CrossRef]
- Radhakrishnan, E.; Bava, S.V.; Narayanan, S.S.; Nath, L.R.; Thulasidasan, A.K.T.; Soniya, E.V.; Anto, R.J. [6]-Gingerol Induces Caspase-Dependent Apoptosis and Prevents PMA-Induced Proliferation in Colon Cancer Cells by Inhibiting MAPK/AP-1 Signaling. PLoS ONE 2014, 9, e104401. [Google Scholar] [CrossRef]
- Tsai, Y.; Xia, C.; Sun, Z. The Inhibitory Effect of 6-Gingerol on Ubiquitin-Specific Peptidase 14 Enhances Autophagy-Dependent Ferroptosis and Anti-Tumor in Vivo and in Vitro. Front. Pharmacol. 2020, 11, 598555. [Google Scholar] [CrossRef] [PubMed]
- Elmali, N.; Baysal, O.; Harma, A.; Esenkaya, I.; Mizrak, B. Effects of Resveratrol in Inflammatory Arthritis. Inflammation 2007, 30, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, G.; Wang, T.; Cao, W.; Zhang, L.; Chen, X. Nrf2–Keap1 Pathway–Mediated Effects of Resveratrol on Oxidative Stress and Apoptosis in Hydrogen Peroxide–Treated Rheumatoid Arthritis Fibroblast-like Synoviocytes. Ann. N. Y. Acad. Sci. 2019, 1457, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Yaguchi, T.; Yoshiya, S.; Nishizaki, T. Resveratrol Induces Apoptosis MH7A Human Rheumatoid Arthritis Synovial Cells in a Sirtuin 1-Dependent Manner. Rheumatol. Int. 2012, 32, 151–157. [Google Scholar] [CrossRef]
- Yoon, H.-Y.; Lee, E.-G.; Lee, H.; Cho, I.J.; Choi, Y.J.; Sung, M.-S.; Yoo, H.-G.; Yoo, W.-H. Kaempferol Inhibits IL-1β-Induced Proliferation of Rheumatoid Arthritis Synovial Fibroblasts and the Production of COX-2, PGE2 and MMPs. Int. J. Mol. Med. 2013, 32, 971–977. [Google Scholar] [CrossRef]
- Yoon, C.-H.; Chung, S.-J.; Lee, S.-W.; Park, Y.-B.; Lee, S.-K.; Park, M.-C. Gallic Acid, a Natural Polyphenolic Acid, Induces Apoptosis and Inhibits Proinflammatory Gene Expressions in Rheumatoid Arthritis Fibroblast-like Synoviocytes. Jt. Bone Spine 2013, 80, 274–279. [Google Scholar] [CrossRef]
- Sung, M.-S.; Lee, E.-G.; Jeon, H.-S.; Chae, H.-J.; Park, S.J.; Lee, Y.C.; Yoo, W.-H. Quercetin Inhibits IL-1β-Induced Proliferation and Production of MMPs, COX-2, and PGE2 by Rheumatoid Synovial Fibroblast. Inflammation 2012, 35, 1585–1594. [Google Scholar] [CrossRef]
- Kitamura, A.; Nishida, K.; Komiyama, T.; Doi, H.; Kadota, Y.; Yoshida, A.; Ozaki, T. Increased Level of Heme Oxygenase-1 in Rheumatoid Arthritis Synovial Fluid. Mod. Rheumatol. 2011, 21, 150–157. [Google Scholar] [CrossRef]
- Devesa, I.; Ferrándiz, M.L.; Terencio, M.C.; Joosten, L.A.B.; van den Berg, W.B.; Alcaraz, M.J. Influence of Heme Oxygenase 1 Modulation on the Progression of Murine Collagen-Induced Arthritis. Arthritis Rheum. 2005, 52, 3230–3238. [Google Scholar] [CrossRef]
- Devesa, I.; Ferrándiz, M.L.; Guillén, I.; Cerdá, J.M.; Alcaraz, M.J. Potential Role of Heme Oxygenase-1 in the Progression of Rat Adjuvant Arthritis. Lab. Investig. 2005, 85, 34–44. [Google Scholar] [CrossRef][Green Version]
- Funes, S.C.; Rios, M.; Fernández-Fierro, A.; Covián, C.; Bueno, S.M.; Riedel, C.A.; Mackern-Oberti, J.P.; Kalergis, A.M. Naturally Derived Heme-Oxygenase 1 Inducers and Their Therapeutic Application to Immune-Mediated Diseases. Front. Immunol. 2020, 11, 1467. [Google Scholar] [CrossRef] [PubMed]
- Saw, C.L.L.; Guo, Y.; Yang, A.Y.; Paredes-Gonzalez, X.; Ramirez, C.; Pung, D.; Kong, A.-N.T. The Berry Constituents Quercetin, Kaempferol, and Pterostilbene Synergistically Attenuate Reactive Oxygen Species: Involvement of the Nrf2-ARE Signaling Pathway. Food Chem. Toxicol. 2014, 72, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Drummond, E.M.; Harbourne, N.; Marete, E.; Martyn, D.; Jacquier, J.; O’Riordan, D.; Gibney, E.R. Inhibition of Proinflammatory Biomarkers in THP1 Macrophages by Polyphenols Derived From Chamomile, Meadowsweet and Willow Bark: ANTIINFLAMMATORY POLYPHENOLS FROM HERBS. Phytother. Res. 2013, 27, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.-G.; Go, R.-E.; Hwang, K.-A.; Choi, K.-C. Resveratrol Inhibits DHT-Induced Progression of Prostate Cancer Cell Line through Interfering with the AR and CXCR4 Pathway. J. Steroid Biochem. Mol. Biol. 2019, 192, 105406. [Google Scholar] [CrossRef]
- Aires, V.; Limagne, E.; Cotte, A.K.; Latruffe, N.; Ghiringhelli, F.; Delmas, D. Resveratrol Metabolites Inhibit Human Metastatic Colon Cancer Cells Progression and Synergize with Chemotherapeutic Drugs to Induce Cell Death. Mol. Nutr. Food Res. 2013, 57, 1170–1181. [Google Scholar] [CrossRef]
- Feng, M.; Zhong, L.-X.; Zhan, Z.-Y.; Huang, Z.-H.; Xiong, J.-P. Resveratrol Treatment Inhibits Proliferation of and Induces Apoptosis in Human Colon Cancer Cells. Med. Sci. Monit. 2016, 22, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.-C.; Syu, J.-J.; Chen, J.-C.; Wang, T.-J.; Chang, P.-Y.; Chen, C.-Y.; Jian, Y.-T.; Jian, Y.-J.; Lin, Y.-W. Resveratrol Enhances Etoposide-Induced Cytotoxicity through Down-Regulating ERK1/2 and AKT-Mediated X-Ray Repair Cross-Complement Group 1 (XRCC1) Protein Expression in Human Non-Small-Cell Lung Cancer Cells. Basic Clin. Pharmacol. Toxicol. 2015, 117, 383–391. [Google Scholar] [CrossRef]
- Miki, H.; Uehara, N.; Kimura, A.; Sasaki, T.; Yuri, T.; Yoshizawa, K.; Tsubura, A. Resveratrol Induces Apoptosis via ROS-Triggered Autophagy in Human Colon Cancer Cells. Int. J. Oncol. 2012, 40, 1020–1028. [Google Scholar] [CrossRef]
- Colin, D.J.; Limagne, E.; Ragot, K.; Lizard, G.; Ghiringhelli, F.; Solary, É.; Chauffert, B.; Latruffe, N.; Delmas, D. The Role of Reactive Oxygen Species and Subsequent DNA-Damage Response in the Emergence of Resistance towards Resveratrol in Colon Cancer Models. Cell Death Dis. 2014, 5, e1533. [Google Scholar] [CrossRef]
- Demoulin, B.; Hermant, M.; Castrogiovanni, C.; Staudt, C.; Dumont, P. Resveratrol Induces DNA Damage in Colon Cancer Cells by Poisoning Topoisomerase II and Activates the ATM Kinase to Trigger P53-Dependent Apoptosis. Toxicol. Vitr. 2015, 29, 1156–1165. [Google Scholar] [CrossRef]
- Yeh, C.-B.; Hsieh, M.-J.; Lin, C.-W.; Chiou, H.-L.; Lin, P.-Y.; Chen, T.-Y.; Yang, S.-F. The Antimetastatic Effects of Resveratrol on Hepatocellular Carcinoma through the Downregulation of a Metastasis-Associated Protease by SP-1 Modulation. PLoS ONE 2013, 8, e56661. [Google Scholar] [CrossRef] [PubMed]
- García-Zepeda, S.P.; García-Villa, E.; Díaz-Chávez, J.; Hernández-Pando, R.; Gariglio, P. Resveratrol Induces Cell Death in Cervical Cancer Cells through Apoptosis and Autophagy. Eur. J. Cancer Prev. 2013, 22, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Rosillo, M.Á.; Alcaraz, M.J.; Sánchez-Hidalgo, M.; Fernández-Bolaños, J.G.; Alarcón-de-la-Lastra, C.; Ferrándiz, M.L. Anti-Inflammatory and Joint Protective Effects of Extra-Virgin Olive-Oil Polyphenol Extract in Experimental Arthritis. J. Nutr. Biochem. 2014, 25, 1275–1281. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, M.; Benfante, V.; Stefano, A.; Yezzi, A.; Di Raimondo, D.; Tuttolomondo, A.; Comelli, A. Anti-Arthritic and Anti-Cancer Activities of Polyphenols: A Review of the Most Recent In Vitro Assays. Life 2023, 13, 361. https://doi.org/10.3390/life13020361
Ali M, Benfante V, Stefano A, Yezzi A, Di Raimondo D, Tuttolomondo A, Comelli A. Anti-Arthritic and Anti-Cancer Activities of Polyphenols: A Review of the Most Recent In Vitro Assays. Life. 2023; 13(2):361. https://doi.org/10.3390/life13020361
Chicago/Turabian StyleAli, Muhammad, Viviana Benfante, Alessandro Stefano, Anthony Yezzi, Domenico Di Raimondo, Antonino Tuttolomondo, and Albert Comelli. 2023. "Anti-Arthritic and Anti-Cancer Activities of Polyphenols: A Review of the Most Recent In Vitro Assays" Life 13, no. 2: 361. https://doi.org/10.3390/life13020361
APA StyleAli, M., Benfante, V., Stefano, A., Yezzi, A., Di Raimondo, D., Tuttolomondo, A., & Comelli, A. (2023). Anti-Arthritic and Anti-Cancer Activities of Polyphenols: A Review of the Most Recent In Vitro Assays. Life, 13(2), 361. https://doi.org/10.3390/life13020361











