Divergence between Hemichannel and Gap Junction Permeabilities of Connexin 30 and 26
Abstract
:1. Introduction
2. Materials, Methods and Analyses
2.1. DNA Constructs Preparation
2.2. Xenopus oocytes Preparation
2.3. Recording Hemichannel Current in Xenopus oocytes
2.4. Whole Cell Recording in N2A Cells
2.5. Constant Field Equations to Calculate Relative Ion Permeabilities
2.6. Dye Release and ATP Release Assay from Xenopus oocytes
2.7. ATP Transfer Assay in Xenopus oocyte Pairs
3. Results
3.1. Cx30 Forms Voltage- and Ca++-Sensitive Hemichannels in Xenopus oocytes and N2A Cells
3.2. Permeability of Cx30 Hemichannels to Ions
3.3. Permeability of Cx26 and Cx30 Hemichannels to Fluorescent Dyes and ATP
3.4. Comparison of Cx26 and Cx30 Hemichannel Permeability to ATP-γ-S
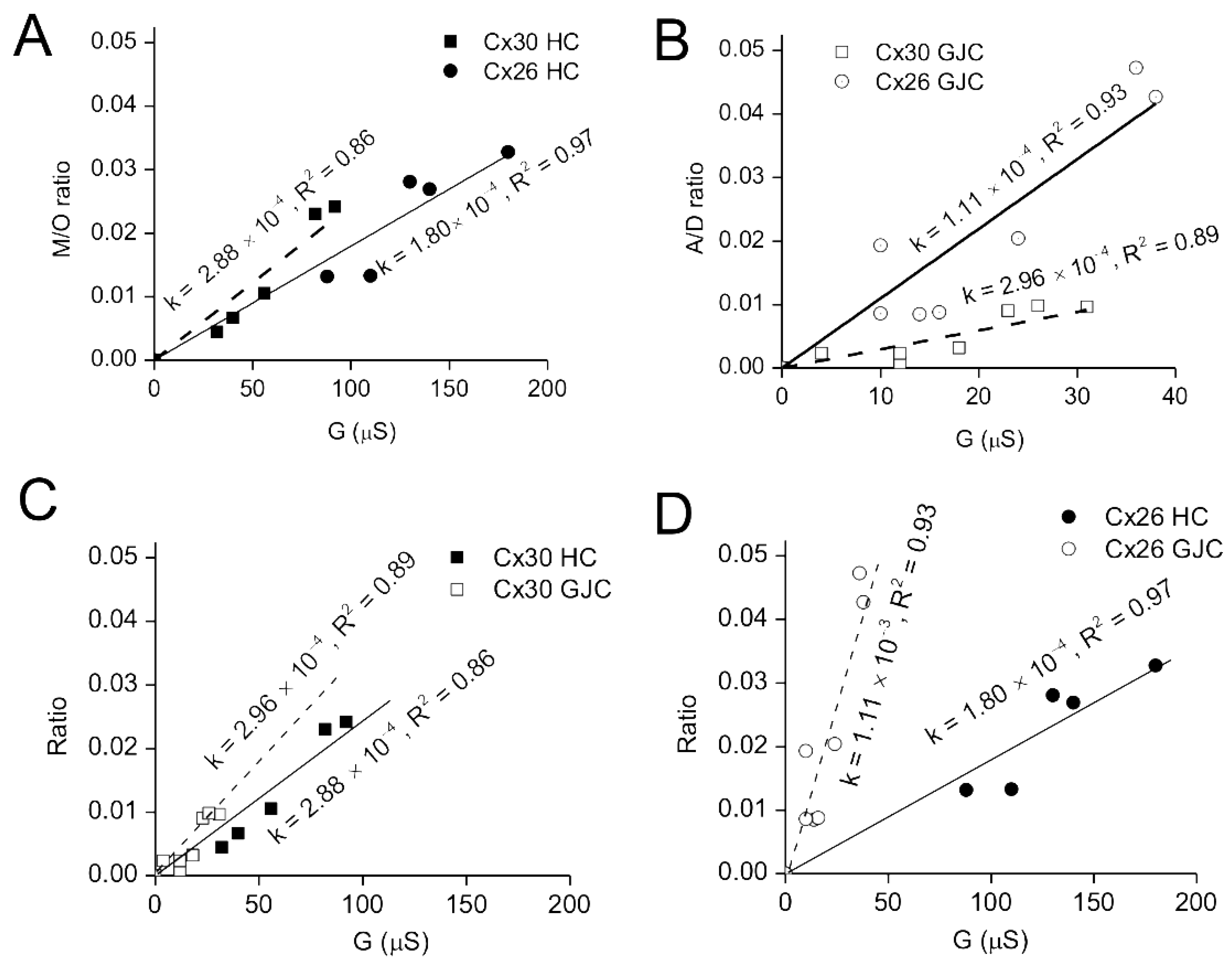
3.5. Comparisons of Gap Junction and Hemichannel Permeabilities of Cx26 and 30
4. Discussion
4.1. Permeability of Connexin Hemichannels to Large Molecules
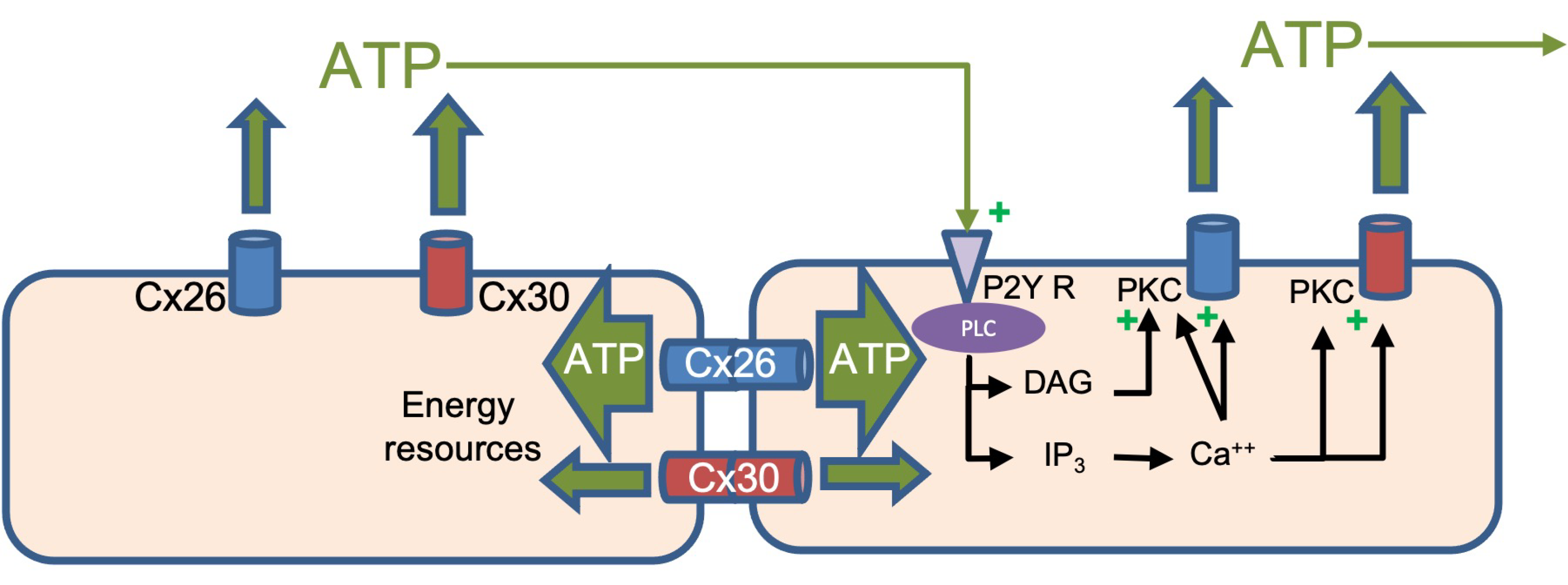
4.2. Is a Hemichannel Just Half of a Gap Junction Channel?
- (1)
- They reside in different lipid environments, as gap junction plaques have specialized lipid contents resembling lipid rafts [34] that are unlikely to be preserved around unclustered hemichannels;
- (2)
- They could also be subject to different post-translational modifications, particularly if some kinases are specifically clustered at gap junctional contacts;
- (3)
- The docking process between hemichannels when they form gap junctions is likely to induce protein conformational changes, as very large energies are involved in this docking of the extracellular loops that involve many electrostatic and hydrogen-bonding interactions [17]. Certainly, changes would occur in the extracellular domains that mediate docking and also form part of the pore in gap junctions. This region has been shown in structures of Cx46 and 50 gap junctions to contribute significantly to the energy profile of permeant transit [18]. In MD simulations of Cx26 and 30 gap junctions [35], a single charge difference exposed in this region (Lys41 in Cx26 and Glu49 in Cx30) likely explains the differences in ion selectivity of their gap junctions. Propagated conformational changes might also occur within the membrane upon docking of hemichannels to form a gap junction, consistent with the differences seen in the hemichannel structure of Cx31.3 [16] compared with all prior structures of gap junctions [17,18,19].
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Riquelme, M.A.; Gu, S.; Hua, R.; Jiang, J.X. Mechanotransduction via the coordinated actions of integrins, PI3K signaling and Connexin hemichannels. Bone Res. 2021, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Bruzzone, S.; Guida, L.; Zocchi, E.; Franco, L.; De Flora, A. Connexin 43 hemi channels mediate Ca2þ-regulated transmembrane NADþ fluxes in intact cells. FASEB J. 2001, 15, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Kang, N.; Lovatt, D.; Torres, A.; Zhao, Z.; Lin, J.; Nedergaard, M. Connexin 43 hemichannels are permeable to ATP. J. Neurosci. 2008, 28, 4702–47011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Nicholson, B.J. The role of connexins in ear and skin physiology—Functional insights from disease-associated mutations. Biochim. Biophys. Acta 2013, 1828, 167–178. [Google Scholar] [CrossRef] [Green Version]
- McCulloch, F.; Chambrey, R.; Eladari, D.; Petri-Perterdi, J. localization of connexin 30 in the luminal membrane of cells in the dustal nephron. J. Physiol. 2005, 289, F1304–F1312. [Google Scholar]
- Sipos, A.; Vargas, S.L.; Toma, I.; Hanner, F.; Willecke, K.; Peti-Peterdi, J. Connexin 30 Deficiency Impairs Renal Tubular ATP Release and Pressure Natriuresis. J. Am. Soc. Nephrol. 2009, 20, 1724–1732. [Google Scholar] [CrossRef] [Green Version]
- Sveningson, P.; Burfod, J.L. and Peti-Peterdi, J. ATP releasing connexin 30 hemichannles mediate flow-induced calcium signaling in the collecting duct. Front. Physiol. 2013, 4, 292. [Google Scholar]
- Anselmi, F.; Hernandez, V.H.; Crispino, G.; Seydel, A.; Ortolano, S.; Roper, S.D.; Kessaris, N.; Richardson, W.; Rickheit, G.; Filippov, M.A.; et al. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc. Natl. Acad. Sci. USA 2008, 105, 18770–18775. [Google Scholar] [CrossRef] [Green Version]
- Ceriani, F.; Pozzan, T.; Mammano, F. Critical role vo ATP-induced release for Ca2+ signaling in non-sensory cell networks of the developing conchlea. Proc. Natl. Acad. Sci. USA 2016, 113, E7194–E7201. [Google Scholar] [CrossRef] [Green Version]
- Beltramello, M.; Bicego, M.; Piazza, V.; Ciubotaru, C.D.; Mammano, F.; D’Andrea, P. Permeability and gating properties of human connexins 26 and 30 expressed in HeLa cells. Biochem. Biophys. Res. Commun. 2003, 305, 1024–1033. [Google Scholar] [CrossRef]
- Manthey, D.; Banach, K.; Desplantez, T.; Lee, C.G.; Kozak, C.A.; Traub, O.; Weingart, R.; Willecke, K. Intracellular domains of mouse connexin26 and -30 affect diffusional and electrical properties of gap junction channels. J. Membr. Biol. 2001, 181, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.B.; Ye, Z.C.; Calloe, K.; Braunstein, T.H.; Hofgaard, J.P.; Ransom, B.R.; Nielsen, M.S.; MacAulay, N. Activation, permeability, and inhibition of astrocytic and neuronal large pore (hemi)channels. J. Biol. Chem. 2014, 289, 26058–26073. [Google Scholar] [CrossRef] [PubMed]
- Essenfelder, G.M.; Bruzzone, R.; Lamartine, J.; Charollais, A.; Blanchet-Bardon, C.; Barbe, M.T.; Meda, P.; Waksman, G. Connexin30 mutations responsible for hidrotic ectodermal dysplasia cause abnormal hemichannel activity. Hum. Mol. Genet. 2004, 13, 1703–1714. [Google Scholar] [CrossRef] [PubMed]
- Verselis, V.K.; Trexler, E.B.; Bukauskas, F.F. Connexin hemichannels and cell-cell channels: Comparison of properties. Braz. J. Med. Biol. Res. 2000, 33, 379–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, B.S.; Nansen, D.B.; Ranson, B.R.; Nielsen, M.S. and MacAulay, N, Connexin hemichannels in Aastrocytes: An assessment of controversies regarding their functional chanarcteristcs. Neurochem. Res. 2017, 42, 2537–2550. [Google Scholar] [CrossRef]
- Lee, H.-J.; Jeong, H.; Hyun, J.; Ryu, B.; Park, K.; Lim, H.-H.; Yoo, J.; Woo, J.-S. Cryo-EM structure of hunman Cx31.3/GJC3 connexin hemichannel. Sci. Adv. 2020, 6, eaba4996. [Google Scholar] [CrossRef]
- Maeda, S.; Nakagawa, S.; Suga, M.; Yamashita, E.; Oshima, A.; Fujiyoshi, Y.; Tsukihara, T. Structure of the connexin 26 gap junction channel at 3.5 angstrom resolution. Nature 2009, 458, 597–602. [Google Scholar] [CrossRef]
- Myers, J.B.; Haddad, B.G.; O’Neill, S.E.; Chorev, D.S.; Yoshioka, C.C.; Robinson, C.V.; Zuckerman, D.M.; Reichow, S.L. Structure of native lens connexin-46/50 intercellular channels by CryoEM. Nature 2018, 564, 372–377. [Google Scholar] [CrossRef]
- Khan, A.K.; Jagielnicki, M.; Bennett, B.C.; Purdy, M.D.; Yeager, M. Cryo-EM structure of an open conformation of a gap junction hemichannel in lipid bilayer nanodiscs. Structure 2021, 29, 1040–1047. [Google Scholar] [CrossRef]
- Suchyna, T.M.; Nitsche, J.M.; Chilton, M.; Harris, A.L.; Veenstra, R.D.; Nicholson, B.J. Different ionic selectivities for connexins 26 and 32 produce rectifying gap junction channels. Biophys. J. 1999, 77, 2968–2987. [Google Scholar] [CrossRef] [Green Version]
- Barrio, L.C.; Suchyna, T.; Bargiello, T.; Xu, L.X.; Roginski, R.S.; Bennett, M.V.; Nicholson, B.J. Gap junctions formed by connexins 26 and 32 alone and in combination are differently affected by applied voltage. Proc. Natl. Acad. Sci. USA 1991, 88, 8410–8414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, P.A.; Chang, H.C.; Spaeth, K.E.; Nitsche, J.M.; Nicholson, B.J. The permeability of gap junction channels to probes of different size is dependent on connexin composition and permeant-pore affinities. Biophys. J. 2004, 87, 958–973. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; McBride, D.W., Jr.; Hamill, O.P. The ion selectivity of a membrane conductance inactivated by extracellular calcium in Xenopus oocytes. J. Physiol. 1998, 508, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Carette, D.; Gilleron, J.; Chevallier, D.; Segretain, D.; Pointis, G. Connexin a check-point component of cell apoptosis in normal and physiopathological conditions. Biochimie 2014, 101, 1–9. [Google Scholar] [CrossRef]
- Retamal, M.A.; Reyes, E.P.; Garcia, I.E.; Pinto, B.; Martinez, A.D.; Gonzalez, C. Diseases associated with leaky hemichannels. Front. Cell. Neurosci. 2015, 9, 267. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Davidson, J.O.; Gunn, K.C.; Phillips, A.R.; Green, C.R.; Gunn, A.J. Role of Hemichannels in CNS Inflammation and the Inflammasome Pathway. Adv. Protein Chem. Struct. Biol. 2016, 104, 1–37. [Google Scholar]
- Willebrords, J.; Crespo Yanguas, S.; Maes, M.; Decrock, E.; Wang, N.; Leybaert, L.; Kwak, B.R.; Green, C.R.; Cogliati, B.; Vinken, M. Connexins and their channels in inflammation. Crit. Rev. Biochem. Mol. Biol. 2016, 51, 413–439. [Google Scholar] [CrossRef] [Green Version]
- Batra, N.; Kar, R.; Jiang, J.X. Gap junctions and hemichannels in signal transmission, function and development of bone. Biochim. Biophys. Acta 2012, 1818, 1909–1918. [Google Scholar] [CrossRef] [Green Version]
- Srinivas, M.; Kronengold, J.; Bukauskas, F.F.; Bargiello, T.A.; Verselis, V.K. Correlative studies of Gating in Cx46 and Cx50 hemichannels and gap junction channels. Biophys. J. 2005, 88, 1725–1739. [Google Scholar] [CrossRef] [Green Version]
- Hansen, D.B.; Braunstein, T.H.; Nielsen, M.S.; MacAulay, N. Distinct permeation profiles of the connexin 30 and 43 hemichannels. FEBS Lett. 2014, 588, 1446–1457. [Google Scholar] [CrossRef]
- Valiunas, V.; Manthey, D.; Vogel, R.; Willecke, K.; Weingart, R. Biophysical properties of mouse connexin30 gap junction channels studied in transfected human HeLa cells. J. Physiol. 1999, 519, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Valiunas, V.; Weingart, R. Electrical properties of gap junction hemichannels identified in transfected HeLa cells. Pflug. Arch. 2000, 440, 366–379. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, D.; Gomez-Hernandez, J.M.; Barrio, L.C. Species specificity of mammalian connexin-26 to form open voltage-gated hemichannels. FASEB J. 2006, 20, 2329–2338. [Google Scholar] [CrossRef]
- Defamie, N.; Mesnil, M. The modulation of gap-junctional intercellular communication by lipid rafts. Biochim. Biophys. Acta 2012, 1818, 1866–1869. [Google Scholar] [CrossRef] [PubMed]
- Zonta, F.; Polles, G.; Zanotti, G.; Mammano, F. Permeation Pathway of Homomeric Connexin 26 and Connexin 30 Channels Investigated by Molecular Dynamics. J. Biomol. Struct. Dyn. 2012, 29, 985–998. [Google Scholar] [CrossRef] [Green Version]
- Apps, S.A.; Rankin, W.A.; Kurmis, A.P. Connexin 26 mutations in autosomal recessive deafness disorders: A review. Int. J. Audiol. 2007, 46, 75–81. [Google Scholar] [CrossRef]
- Mammano, F. Inner Ear Connexin Channels: Roles in Development and Maintenance of Cochlear Function. Cold Spring Harb. Perspect. Med. 2019, 9, a033233. [Google Scholar] [CrossRef]
- Boulay, A.C.; del Castillo, F.J.; Giraudet, F.; Hamard, G.; Giaume, C.; Petit, C.; Avan, P.; Cohen-Salmon, M. Hearing is normal without connexin30. J. Neurosci. 2013, 33, 430–434. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Tang, W.; Ahmad, S.; Sipp, J.A.; Chen, P.; Lin, X. Gap junction-mediated intercellular biochemical coupling in cochlear supporting cells is required for normal cochlear functions. Proc. Natl. Acad. Sci. USA 2005, 102, 15201–15206. [Google Scholar] [CrossRef]



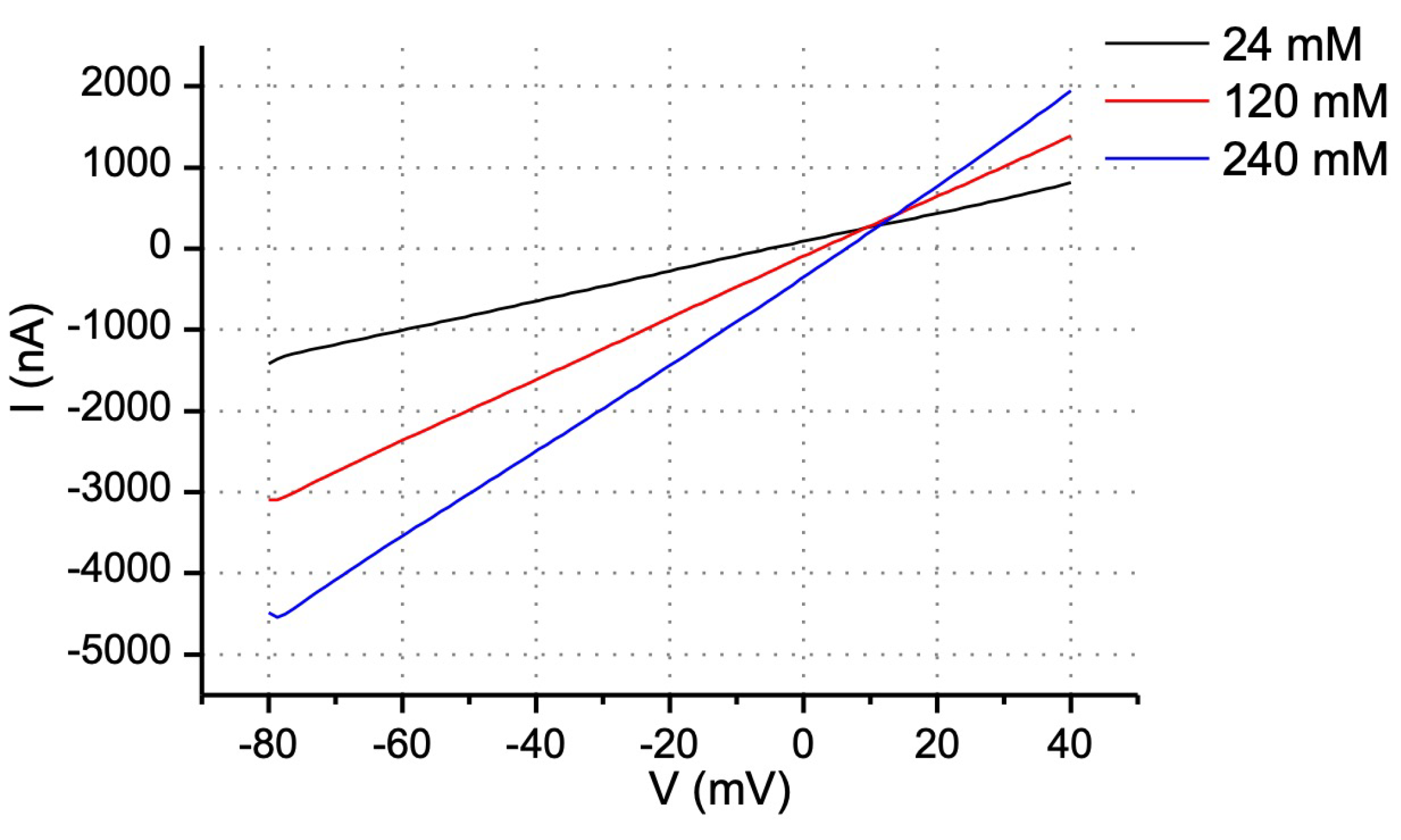

| NaCl Concentration (mM) | RP (mV) |
|---|---|
| 24 | −8.2 ± 0.7 |
| 120 | 3.2 ± 0.4 |
| 240 | 10 ± 2.5 |
| % Substitution | TEA+ (MW: 130) | Gluconate− (MW: 196) |
|---|---|---|
| 0 | −0.3 ± 0.7 | −0.2 ± 1.8 |
| 50 | −6.6 ± 0.4 | −2.5 ± 0.7 |
| 100 | −15.3 ± 2.5 | −5.1 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Nicholson, B.J. Divergence between Hemichannel and Gap Junction Permeabilities of Connexin 30 and 26. Life 2023, 13, 390. https://doi.org/10.3390/life13020390
Xu J, Nicholson BJ. Divergence between Hemichannel and Gap Junction Permeabilities of Connexin 30 and 26. Life. 2023; 13(2):390. https://doi.org/10.3390/life13020390
Chicago/Turabian StyleXu, Ji, and Bruce J. Nicholson. 2023. "Divergence between Hemichannel and Gap Junction Permeabilities of Connexin 30 and 26" Life 13, no. 2: 390. https://doi.org/10.3390/life13020390





