Revealing the Underlying Mechanism of Acacia Nilotica against Asthma from a Systematic Perspective: A Network Pharmacology and Molecular Docking Study
Abstract
:1. Introduction
2. Results
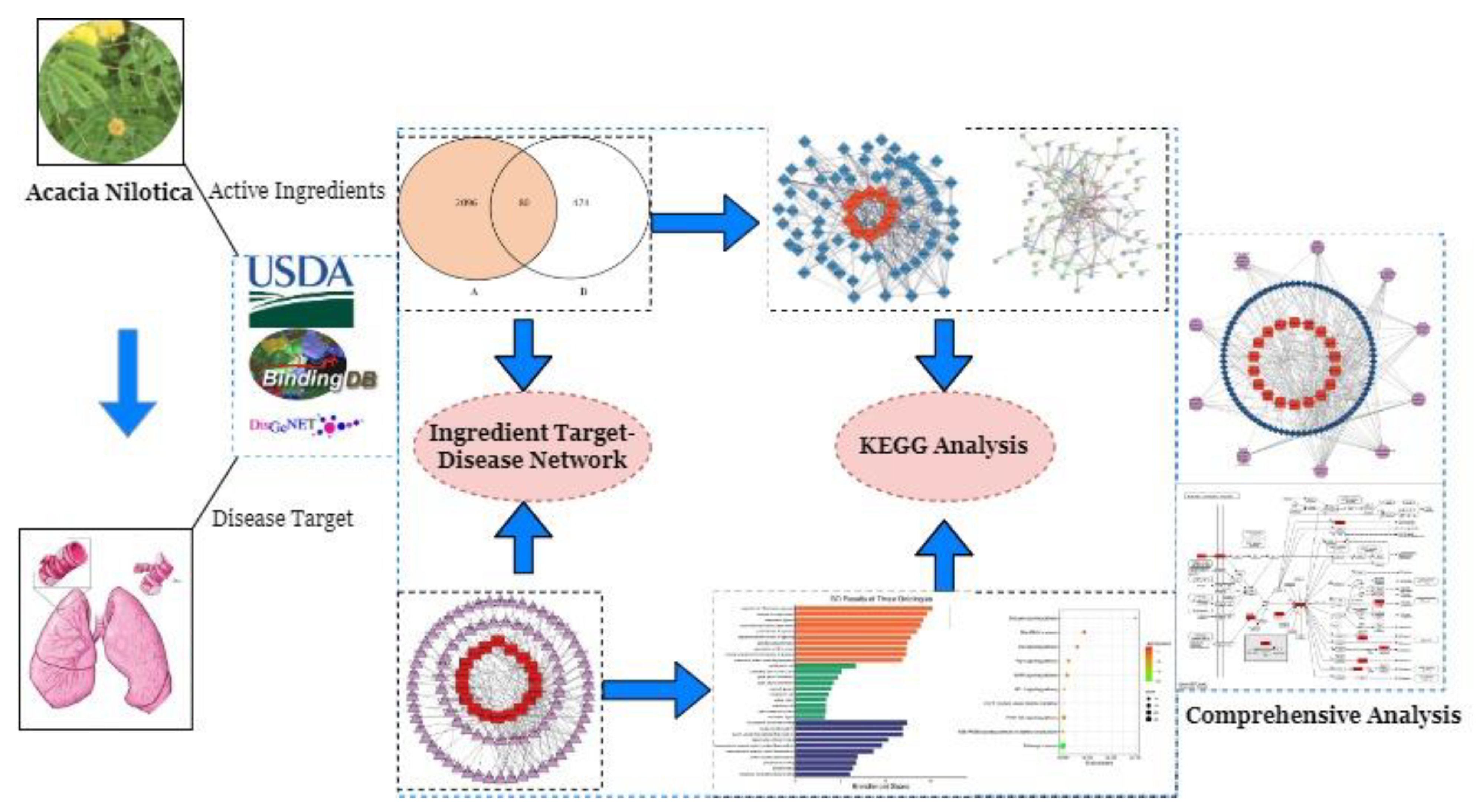
2.1. Active Compound’s Screening of AN
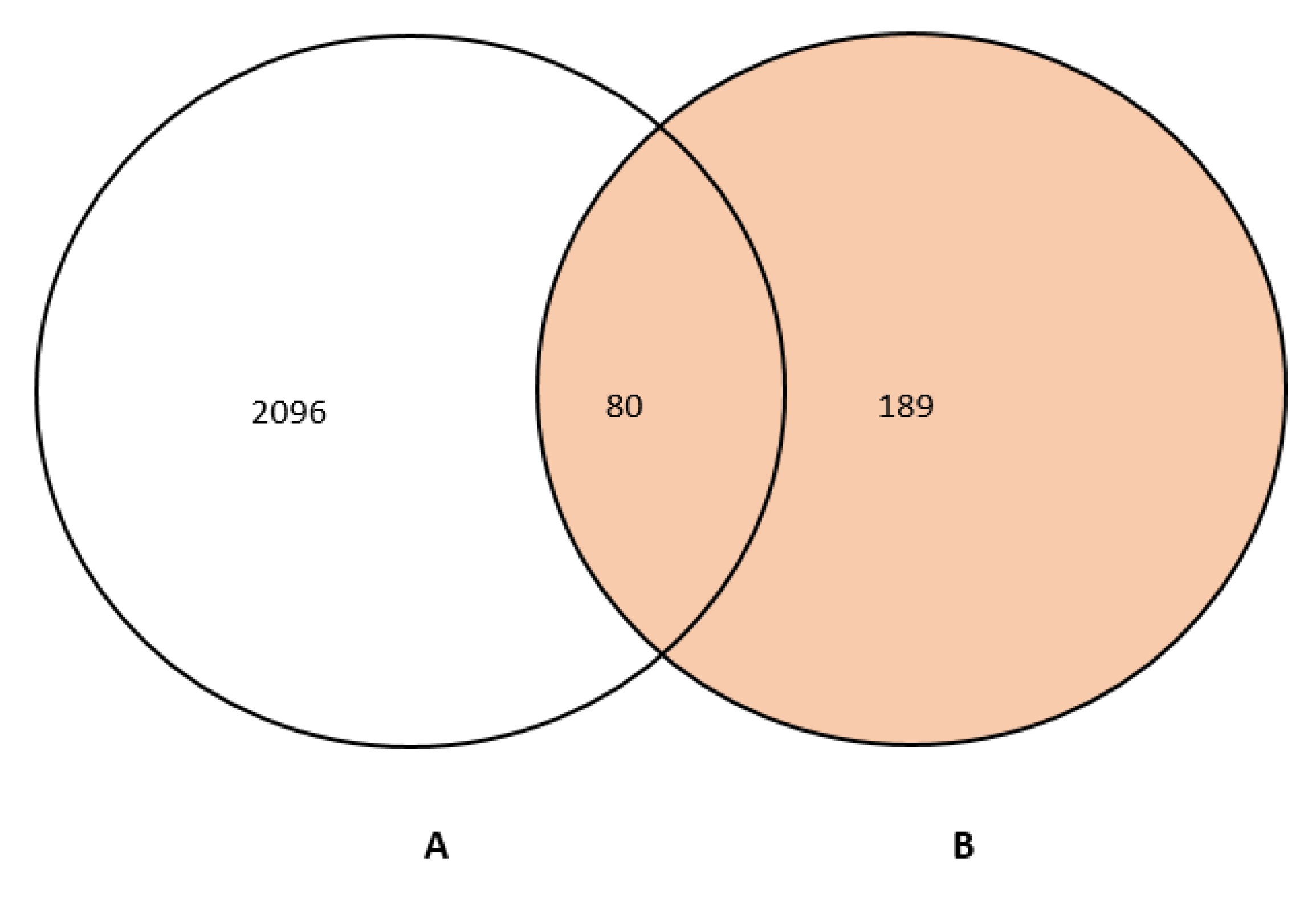
2.2. Potential Targets Identification
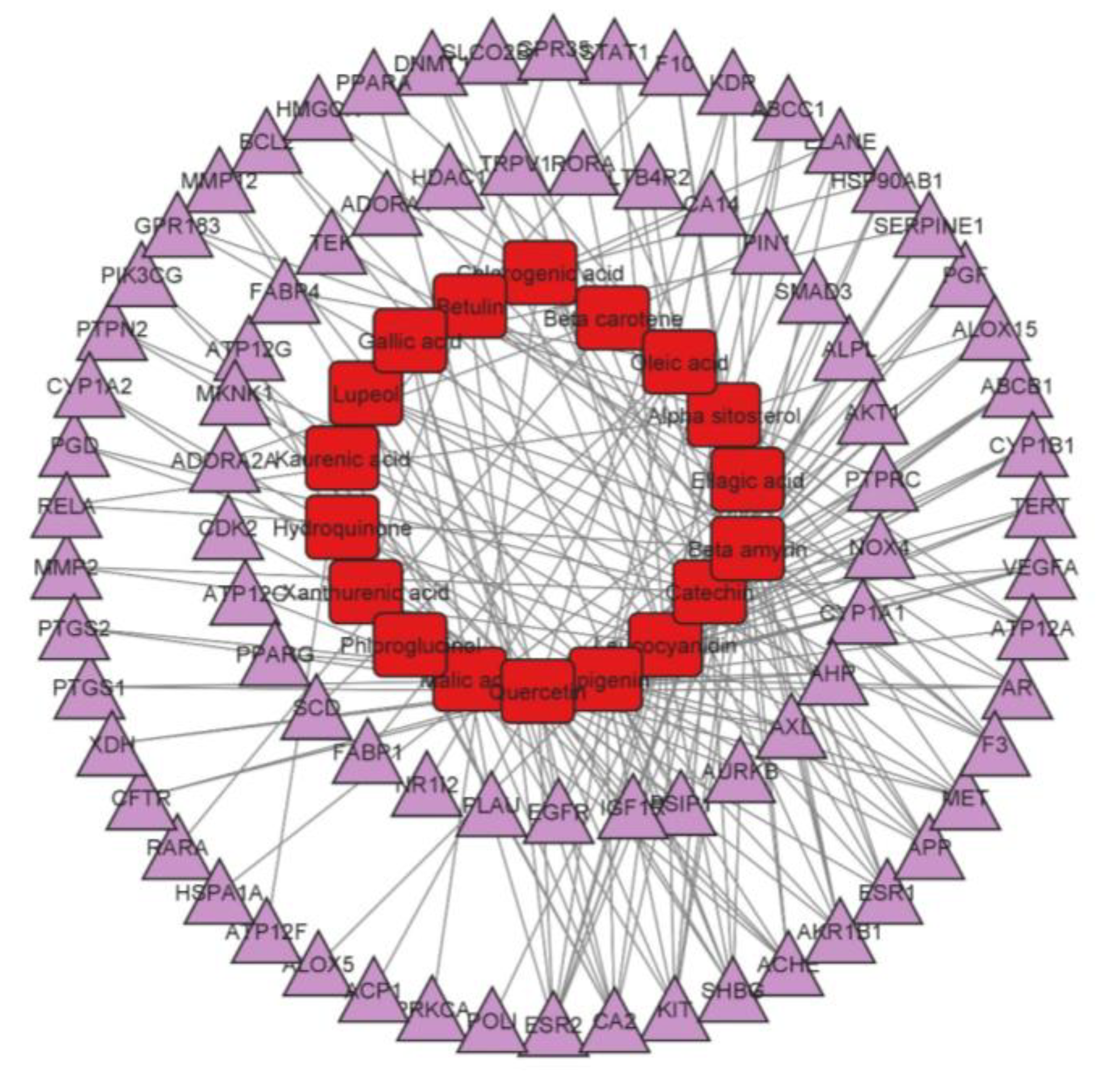
2.3. Phytoconstituents-Target Network
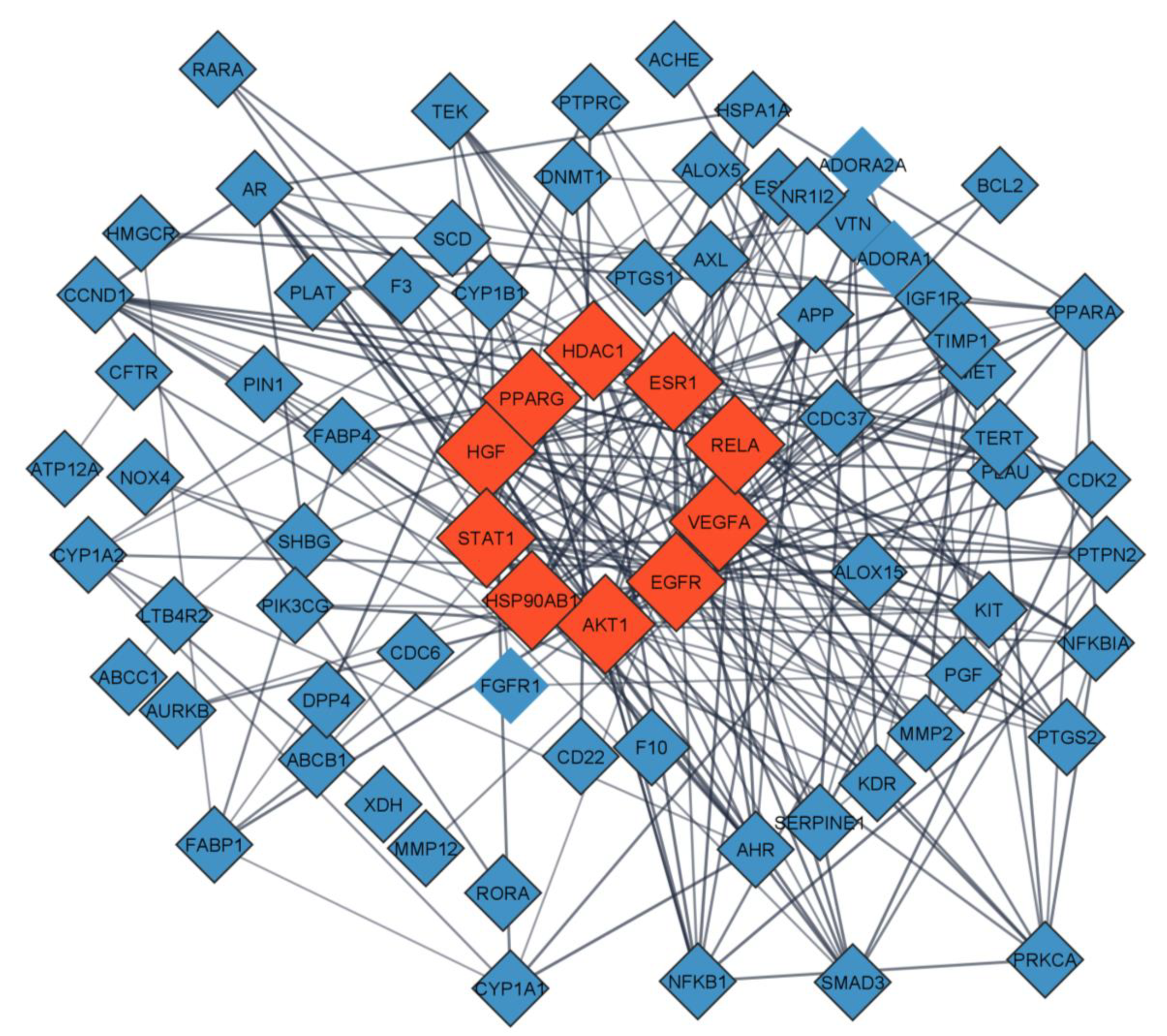
2.4. PPI Network Analysis
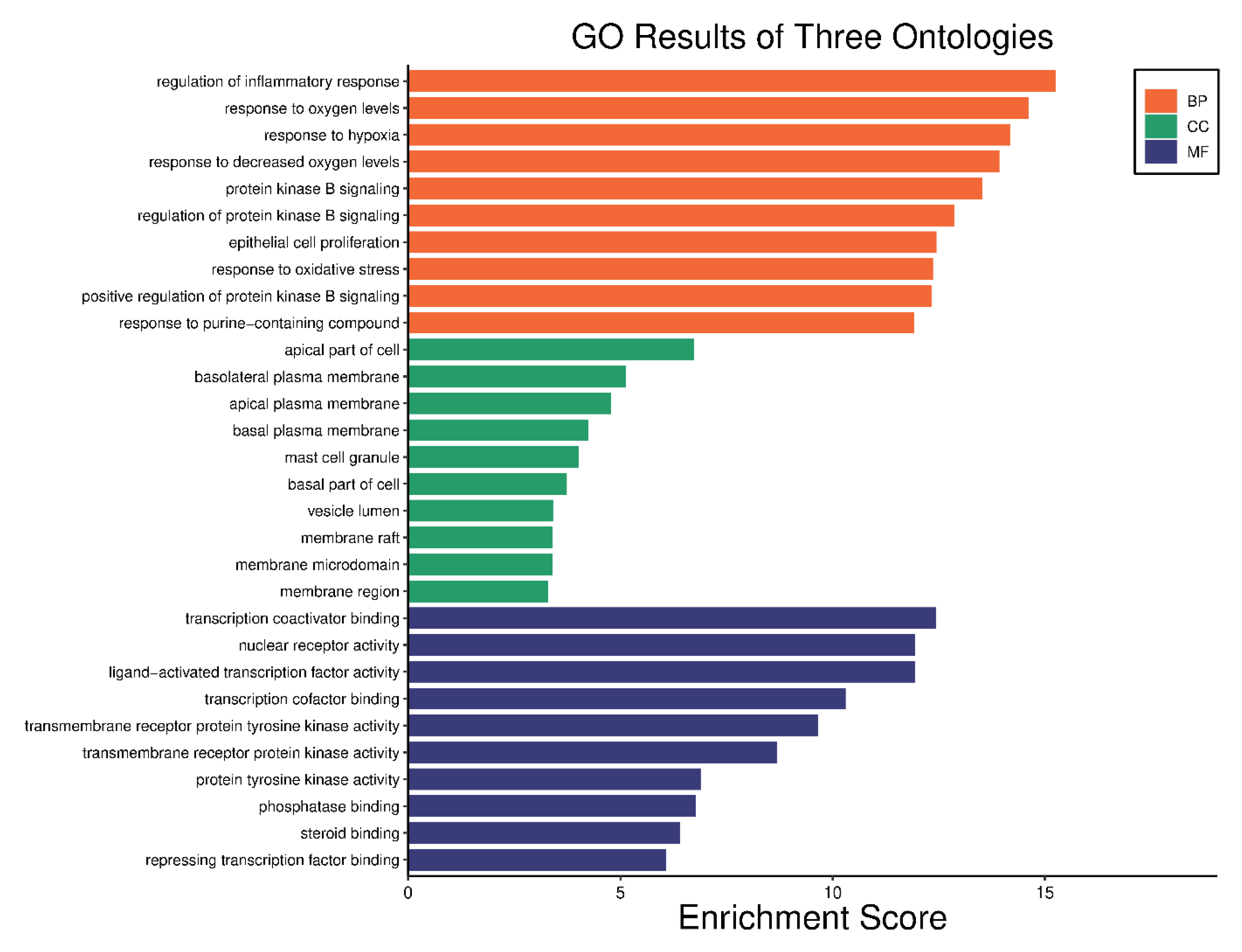
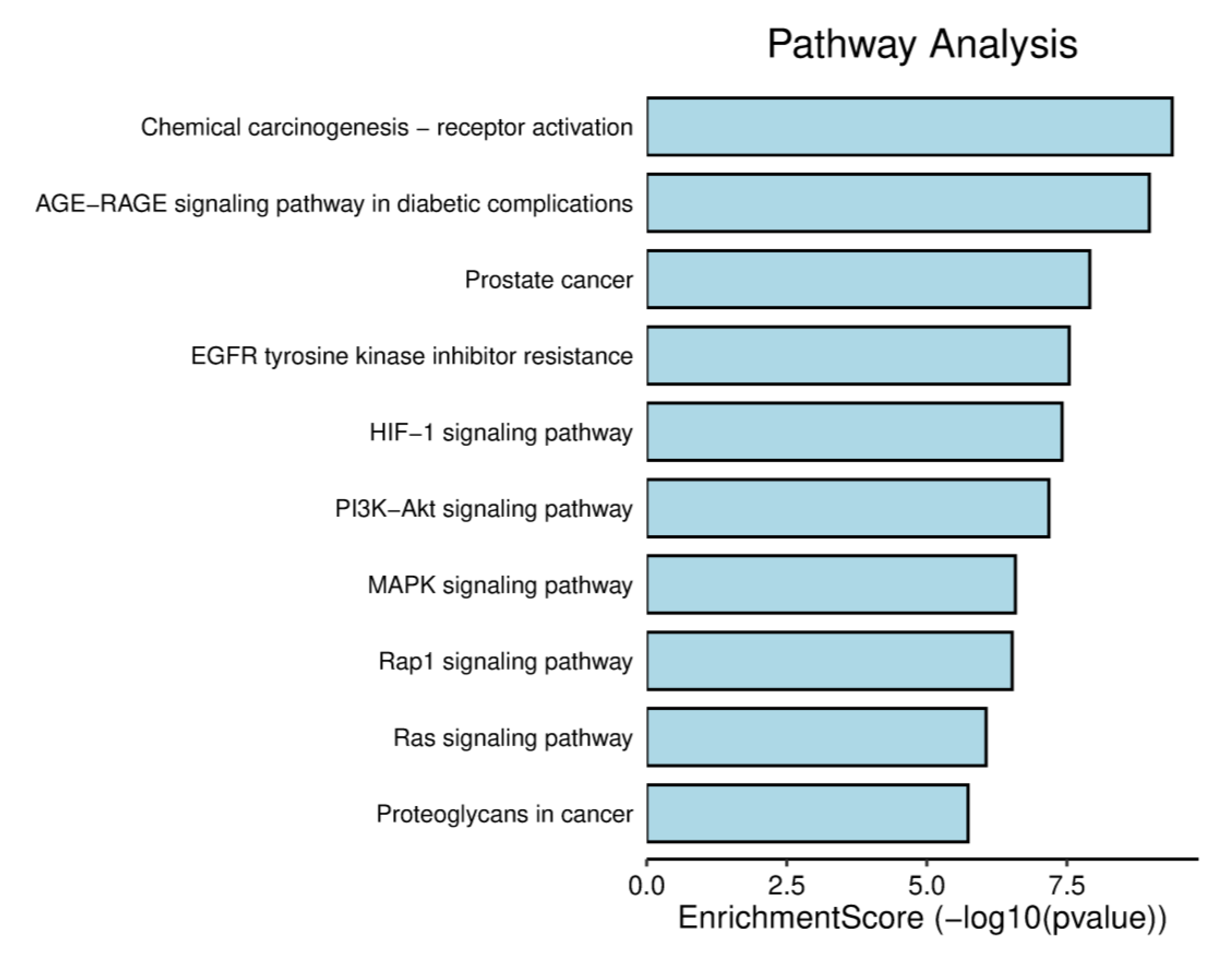
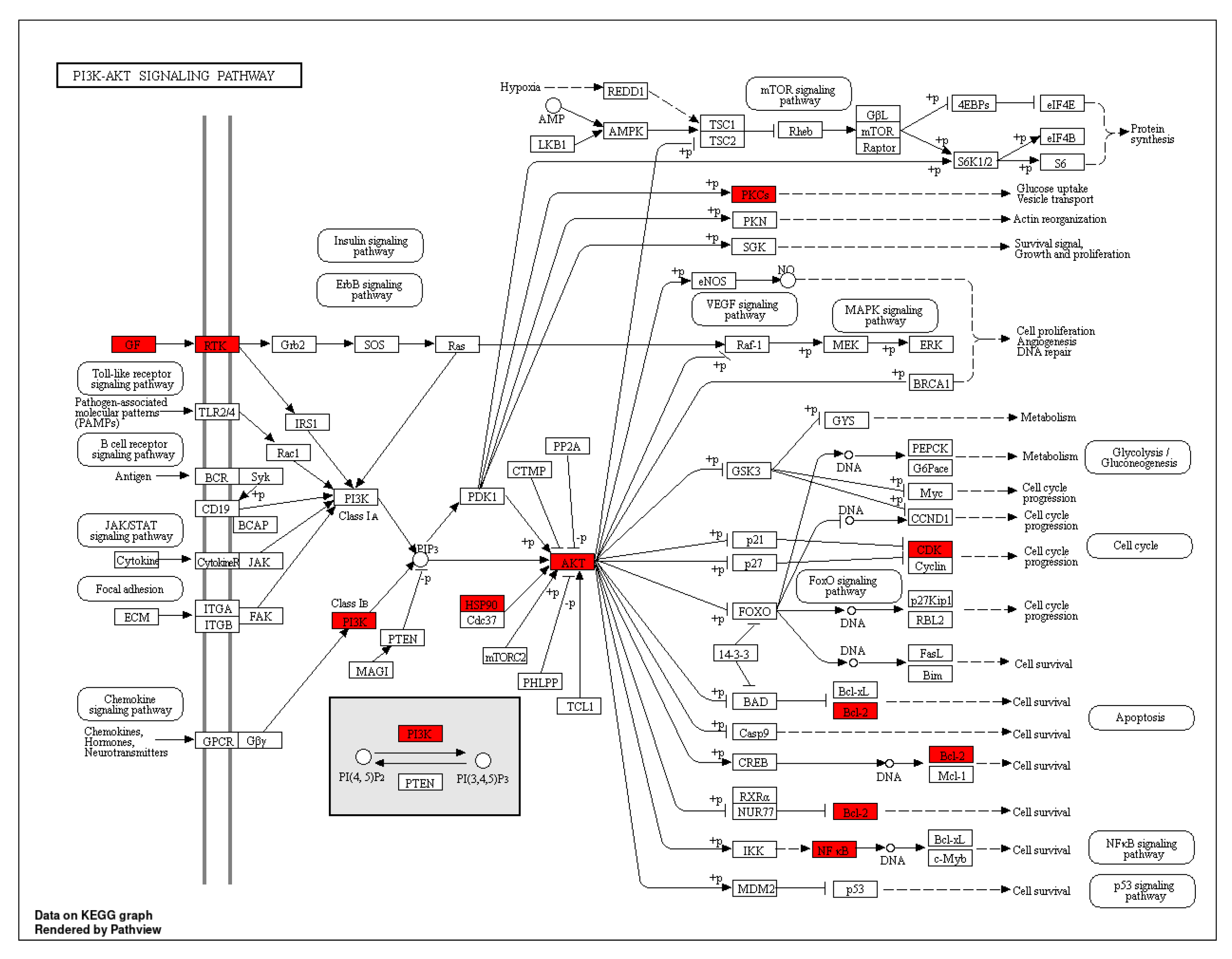
2.5. GO Analysis and KEGG Pathway
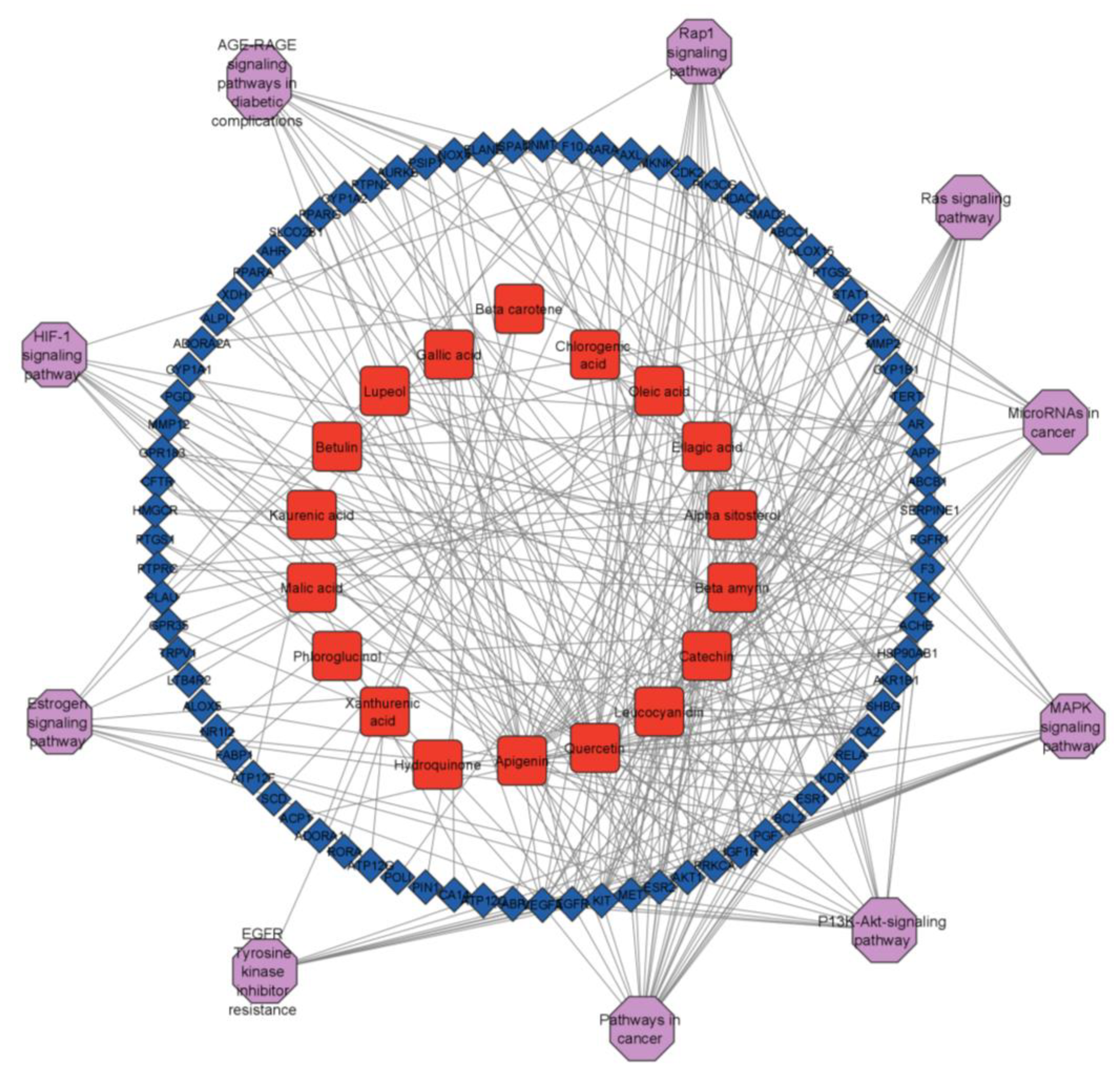
2.6. Analysis of Target-Pathway-Compound Network
2.7. Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Database Construction and ADMET Analysis of AN Phytoconstituents
4.2. Target Genes Associated with Asthma and Selected Compounds
4.3. Interactions between Compounds and Overlapping Genes: Network Construction
4.4. Building a Protein-Protein Interaction Network
4.5. Target Protein Gene Ontology (GO) and KEGG Enrichment Analysis
4.6. Target-Pathway-Compound Network Construction
4.7. Molecular Docking
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bi, J.; Lin, Y.; Sun, Y.; Zhang, M.; Chen, Q.; Miu, X.; Tang, L.; Liu, J.; Zhu, L.; Ni, Z.; et al. Investigation of the Active Ingredients and Mechanism of Polygonum cuspidatum in Asthma Based on Network Pharmacology and Experimental Verification. Drug Des. Devel. Ther. 2021, 15, 1075–1089. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Chen, X.; Xia, Q.; Zhang, S.; Yao, C. Network pharmacology-based study on the mechanism of pinellia ternata in asthma treatment. Evid. Based Complement. Altern. Med. 2020, 2020, 9732626. [Google Scholar] [CrossRef] [PubMed]
- Oyebode, O.; Kandala, N.B.; Chilton, P.J.; Lilford, R.J. Use of traditional medicine in middle-income countries: A WHO-SAGE study. Health Policy Plan. 2016, 8, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Jame, R. Phytochemical and pharmacological uses of Acacia nilotica—A review. Seeds 2018, 1, 15–21. [Google Scholar]
- Abduljawad, E.A. Review of some evidenced medicinal activities of Acacia Nilotica. Arch. Pharma. Pract. 2020, 11, 20–25. [Google Scholar]
- Younis, W.; Asif, H.; Sharif, A.; Riaz, H.; Bukhari, I.A.; Assiri, A.M. Traditional medicinal plants used for respiratory disorders in Pakistan: A review of the ethno-medicinal and pharmacological evidence. Chin. Med. 2018, 13, 1–29. [Google Scholar]
- Huang, X.F.; Cheng, W.B.; Jiang, Y.; Liu, Q.; Liu, X.H.; Xu, W.F.; Huang, H.T. A network pharmacology-based strategy for predicting anti-inflammatory targets of ephedra in treating asthma. Int. Immunopharmacol. 2020, 1, 106423. [Google Scholar] [CrossRef]
- Lan, H.; Luo, L.; Chen, Y.; Wang, M.; Yu, Z.; Gong, Y. MIF signaling blocking alleviates airway inflammation and airway epithelial barrier disruption in a HDM-induced asthma model. Cell. Immunol. 2020, 347, 103965. [Google Scholar] [CrossRef]
- Aslam, M.S.; Ahmad, M.S. Worldwide importance of medicinal plants: Current and historical perspectives. Recent Adv. Biol. Med. 2016, 2, 909. [Google Scholar] [CrossRef]
- Jung, C.H.; Lee, J.Y.; Cho, C.H.; Kim, C.J. Anti-asthmatic action of quercetin and rutin in conscious guinea-pigs challenged with aerosolized ovalbumin. Arch. Pharmacal Res. 2007, 30, 1599–1607. [Google Scholar] [CrossRef]
- Li, R.R.; Pang, L.L.; Du, Q.; Shi, Y.; Dai, W.J.; Yin, K.S. Apigenin inhibits allergen-induced airway inflammation and switches immune response in a murine model of asthma. Immunopharmacol. Immunotoxicol. 2010, 32, 364–370. [Google Scholar] [CrossRef]
- Li, J.; Zhang, B. Apigenin protects ovalbumin-induced asthma through the regulation of Th17 cells. Fitoterapia 2013, 91, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wang, H.; Zhang, J.; Yu, C.; Liu, C.; Sun, H.; Wu, Y.; Wang, Y.; Lin, X. Antiasthmatic activity of quercetin glycosides in neonatal asthmatic rats. 3 Biotech. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Brown, M.; Kogut, P.; Serban, K.; Li, X.; McConville, J.; Chen, B.; Bentley, J.K.; Hershenson, M.B.; Dulin, N.; et al. Akt activation induces hypertrophy without contractile phenotypic maturation in airway smooth muscle. Am. J. Physiol. Lung Cell Mol. 2011, 300, L701–L709. [Google Scholar] [CrossRef]
- Cui, H.; Cheng, Y.; He, Y.; Cheng, W.; Zhao, W.; Zhao, H.; Zhou, F.H.; Wang, L.; Dong, J.; Cai, S. The AKT inhibitor MK2206 suppresses airway inflammation and the pro remodeling pathway in a TDI induced asthma mouse model. Mol. Med. Rep. 2020, 22, 3723–3734. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, M.; Takahashi, M.; Aoike, N. Expression of vascular endothelial growth factor, basic fibroblast growth factor, and angiogenin immunoreactivity in asthmatic airways and its relationship to angiogenesis. J. Allergy Clin. Immunol. 2001, 107, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Burgel, P.R.; Nadel, J.A. Epidermal growth factor receptor-mediated innate immune responses and their roles in airway diseases. Eur. Respir. J. 2008, 32, 1068–1081. [Google Scholar] [CrossRef] [PubMed]
- Le Cras, T.D.; Acciani, T.H.; Mushaben, E.M.; Kramer, E.L.; Pastura, P.A.; Hardie, W.D.; Korfhagen, T.R.; Sivaprasad, U.; Ericksen, M.; Gibson, A.M.; et al. Epithelial EGF receptor signaling mediates airway hyperreactivity and remodeling in a mouse model of chronic asthma. Am. J. Physiol. Lung Cell Mol. 2011, 300, L414–L421. [Google Scholar] [CrossRef]
- Inoue, H.; Akimoto, K.; Homma, T.; Tanaka, A.; Sagara, H. Airway epithelial dysfunction in asthma: Relevant to epidermal growth factor receptors and airway epithelial cells. J. Clin. Med. 2020, 9, 3698. [Google Scholar] [CrossRef]
- Haase, M.; Fitze, G. HSP90AB1: Helping the good and the bad. Gene 2016, 574, 171–186. [Google Scholar] [CrossRef]
- Liu, R.; Mao, Y.; Gu, Z.; He, J. Network Pharmacology-Based Analysis of the Underlying Mechanism of Hyssopus cuspidatus Boriss. for Antiasthma: A Characteristic Medicinal Material in Xinjiang. Evid.-Based Complement. Altern. Med. 2021, 2021, 7671247. [Google Scholar] [CrossRef] [PubMed]
- Chandran, U.; Patwardhan, B. Network ethnopharmacological evaluation of the immunomodulatory activity of Withania somnifera. J. Ethnopharmacol. 2017, 197, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Noor, F.; Rehman, A.; Ashfaq, U.A.; Saleem, M.H.; Okla, M.K.; Al-Hashimi, A.; AbdElgawad, H.; Aslam, S. Integrating Network Pharmacology and Molecular Docking Approaches to Decipher the Multi-Target Pharmacological Mechanism of Abrus precatorius L. Acting on Diabetes. Pharmaceuticals 2022, 15, 414. [Google Scholar] [CrossRef] [PubMed]
- Gilson, M.K.; Liu, T.; Baitaluk, M.; Nicola, G.; Hwang, L.; Chong, J. BindingDB in 2015: A public database for medicinal chemistry, computational chemistry and systems pharmacology. Nucleic Acids Res. 2016, 44, D1045–D1053. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Guo, L.; Ma, H.; Wu, H.; Fan, X. Network pharmacology and molecular docking suggest the mechanism for biological activity of rosmarinic acid. Evid. Based Complement. Altern. Med. 2021, 2021, 5190808. [Google Scholar] [CrossRef]
- Alzarea, S.I.; Qasim, S.; Uttra, A.M.; Khan, Y.H.; Aljoufi, F.A.; Ahmed, S.R.; Alanazi, M.; Malhi, T.H. Network Pharmacology and Molecular Docking Based Prediction of Mechanism of Pharmacological Attributes of Glutinol. Processes 2022, 10, 1492. [Google Scholar] [CrossRef]
- Alzarea, S.I.; Qasim, S.; Afzal, M.; Alsaidan, O.A.; Alhassan, H.H.; Alharbi, M.; Alqinyah, M.; Alenazi, F.S. Anandamide Reuptake Inhibitor (VDM11) as a Possible Candidate for COVID 19 Associated Depression; A Combination of Network Pharmacology, Molecular Docking and In Vivo Experimental Analysis. Processes 2023, 11, 143. [Google Scholar] [CrossRef]
| Compound | Pubchem ID | Absorption | Distribution | Metabolism | Excretion | Toxicity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WS (log mol/L) | IS (% abs) | SP (log Kp) | BBB (log BB) | CNSP (log PS) | CYP3A4 Inhibitor | CYP2C19 Inhibitor | TC (log mL/min/kg) | Max Tolerated Dose | ORAT (LD50) | HT | SS | AMES | ||
| Catechin | 9064 | −3.11 | 68.82 | −2.37 | −1.054 | −3.298 | No | No | 0.183 | 0.438 | 2.428 | No | No | No |
| Leucocyanidin | 71629 | −2.98 | 56.71 | −2.37 | −0.91 | −3.213 | No | No | −0.072 | 0.446 | 2.394 | No | No | No |
| Alpha sitosterol | 9548595 | −6.66 | 94.87 | −2.61 | 0.782 | −1.554 | Yes | No | 0.585 | −0.578 | 2.56 | No | No | No |
| Apigenin | 5280443 | −3.32 | 93.25 | −2.37 | −1.734 | −2.061 | No | Yes | 0.566 | 0.328 | 2.45 | No | No | No |
| Beta amyrin | 73145 | −6.531 | 93.73 | −2.11 | 0.667 | −1.773 | Yes | No | −0.044 | −0.56 | 2.478 | No | No | No |
| Beta carotene | 5280489 | −7.39 | 91.73 | −2.21 | 0.938 | −1.094 | Yes | No | 1.061 | −0.379 | 2.073 | No | No | No |
| Betulin | 72326 | −5.446 | 94.53 | −2.32 | −1.295 | −2.035 | Yes | No | 0.236 | −0.794 | 2.699 | Yes | No | No |
| Chlorogenic acid | 1794427 | −2.449 | 36.37 | −2.35 | −1.407 | −3.856 | No | No | 0.307 | −0.134 | 1.973 | No | No | No |
| Ellagic acid | 5281855 | −3.181 | 86.68 | −2.35 | −1.272 | −3.533 | No | No | 0.537 | 0.476 | 2.399 | No | No | No |
| Gallic acid | 370 | −2.56 | 43.37 | −2.35 | −1.102 | −3.74 | No | No | 0.518 | 0.7 | 2.218 | No | No | No |
| Hydroquinone | 785 | −0.762 | 86.85 | −2.18 | −0.318 | −2.076 | No | No | 0.52 | 0.707 | 2.008 | No | Yes | No |
| Kaurenic acid | 73062 | −3.096 | 100 | −2.35 | 0.05 | −1.602 | Yes | No | 0.506 | 0.046 | 2.031 | Yes | No | No |
| Lupeol | 259846 | −5.861 | 95.78 | −2.44 | 0.726 | −1.714 | Yes | No | 0.153 | −0.502 | 2.563 | No | No | No |
| Malic acid | 222656 | −1.381 | 13.83 | −2.35 | −1.788 | −3.523 | No | No | 0.81 | 1.212 | 1.818 | No | No | No |
| Oleic acid | 445639 | −5.924 | 91.82 | −2.25 | −1.168 | −1.654 | Yes | No | 1.884 | −0.81 | 1.417 | No | Yes | No |
| Phloroglucinol | 359 | −1.408 | 83.54 | −2.51 | −0.466 | −3.252 | No | No | 0.581 | 0.107 | 1.958 | No | No | No |
| Quercetin | 5280343 | −2.925 | 77.20 | −2.35 | −1.098 | −3.065 | No | Yes | 0.407 | 0.499 | 2.471 | No | No | No |
| Xanthurenic acid | 5699 | −2.621 | 75.57 | −2.35 | −0.853 | −3.313 | No | No | 0.553 | 1.039 | 2.805 | Yes | No | No |
| Compounds | Class | Degree | Compounds | Class | Degree |
|---|---|---|---|---|---|
| Catechin | Flavonoids | 24 | Gallic acid | Phenolic acids | 5 |
| Leucocyanidin | Flavonoids | 24 | Hydroquinone | Phenols | 2 |
| Alpha sitosterol | Sterol | 14 | Kaurenic acid | Diterpene | 4 |
| Apigenin | Flavonoid | 35 | Lupeol | Terpenoids | 5 |
| Beta amyrin | Terpenoids | 14 | Malic acid | Organic acids | 2 |
| Beta carotene | Carotenoid | 6 | Oleic acid | Fatty acids | 7 |
| Betulin | Terpenoids | 5 | Phloroglucinol | Phenols | 2 |
| Chlorogenic acid | Phenolic acids | 6 | Quercetin | Flavonoid | 35 |
| Ellagic acid | Phenolic acids | 14 | Xanthurenic acid | Quinoline carboxylic acid | 2 |
| Compounds | Compound-Target | Docking Score (kJ/mol) | Interaction | |||
|---|---|---|---|---|---|---|
| H-Bond Interactions | Arene-π | |||||
| Distance (ºA) | Score (%) | Amino Acid | ||||
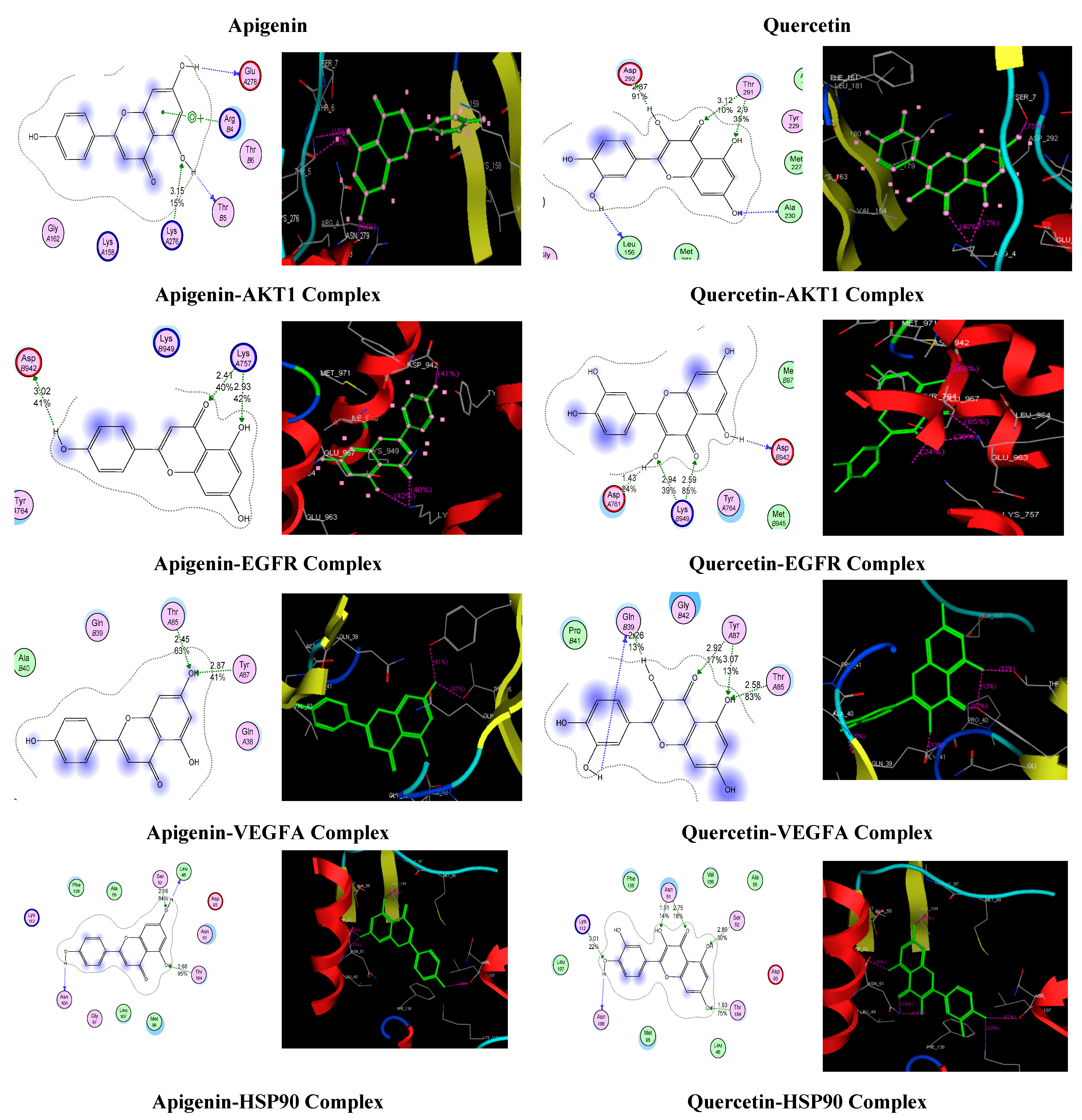
| Apigenin | Apigenin-AKT1 Complex | −12.4640 | 3.15 | 15 | LysA276 | ArgB4 |
| Apigenin-EGFR Complex | −12.0665 | 3.02 2.41 2.93 | 41 40 42 | AspB942 LysA757 LysA757 | ---- | |
| Apigenin-VEGFA Complex | −12.8896 | 2.45 2.87 | 63 41 | ThrA85 TyrA87 | ---- | |
| Apigenin-Hsp90AB1 Complex | −14.7315 | 2.16 2.68 | 84 95 | Ser52 Thr184 | ---- | |
| Quercetin | Quercetin-AKT1 Complex | −13.6098 | 2.87 3.12 2.9 | 91 10 35 | Asp292 Thr291 Thr291 | ---- |
| Quercetin-EGFR Complex | −14.6961 | 1.43 2.94 2.59 | 24 39 85 | AspA761 LysB949 LysB949 | ---- | |
| Quercetin-VEGFA Complex | −15.1991 | 2.26 2.92 3.07 2.58 | 13 17 13 83 | GlnB39 TyrA87 TyrA87 ThrA85 | ||
| Quercetin-Hsp90AB1 Complex | −15.3669 | 3.01 1.91 2.75 2.89 1.93 | 22 14 18 30 75 | Lys112 Asn51 Asn51 Ser52 Thr184 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alnusaire, T.S.; Qasim, S.; Al-Sanea, M.M.; Hendawy, O.; Uttra, A.M.; Ahmed, S.R. Revealing the Underlying Mechanism of Acacia Nilotica against Asthma from a Systematic Perspective: A Network Pharmacology and Molecular Docking Study. Life 2023, 13, 411. https://doi.org/10.3390/life13020411
Alnusaire TS, Qasim S, Al-Sanea MM, Hendawy O, Uttra AM, Ahmed SR. Revealing the Underlying Mechanism of Acacia Nilotica against Asthma from a Systematic Perspective: A Network Pharmacology and Molecular Docking Study. Life. 2023; 13(2):411. https://doi.org/10.3390/life13020411
Chicago/Turabian StyleAlnusaire, Taghreed S., Sumera Qasim, Mohammad M. Al-Sanea, Omnia Hendawy, Ambreen Malik Uttra, and Shaimaa R. Ahmed. 2023. "Revealing the Underlying Mechanism of Acacia Nilotica against Asthma from a Systematic Perspective: A Network Pharmacology and Molecular Docking Study" Life 13, no. 2: 411. https://doi.org/10.3390/life13020411
APA StyleAlnusaire, T. S., Qasim, S., Al-Sanea, M. M., Hendawy, O., Uttra, A. M., & Ahmed, S. R. (2023). Revealing the Underlying Mechanism of Acacia Nilotica against Asthma from a Systematic Perspective: A Network Pharmacology and Molecular Docking Study. Life, 13(2), 411. https://doi.org/10.3390/life13020411







